You are here
A comprehensive study of the high temperature pyrolysis of sewage sludge: kinetics, energy analysis and products formation
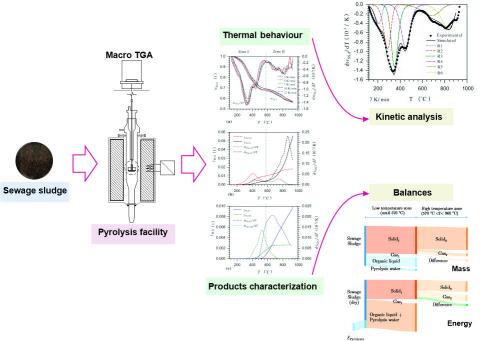
This study evaluates the pyrolysis of sewage sludge until 960 °C using heating rates between 3 K/min and 12 K/min in a macro TG/EGA. Mass and energy balances and kinetic parameters are determined. Thermal decomposition is divided into a low temperature zone (until 550 °C to 590 °C), for decomposition of organic matter, and a high temperature zone, for decomposition of inorganic matter and secondary reactions of the residual organic matter. In dry basis at 570 °C solid, liquid and gaseous products amount to 69.2 wt.-%, 29.2 wt.-% and 1.6 wt.-%, respectively. An increment in the final temperature to 960 °C causes a successive decrease of the solid residue to 56.9 wt.-%. The solid product contains more than 80 wt.-% mineral matter with high amounts of valuable elements, such as Ca and P. An energy requirement of 2.18 MJ/kg of dry sewage sludge is calculated for the pyrolysis until 570 °C. At this temperature, 58.5 % of the energy entering the process is concentrated in the liquid product and 40.0 % in the solid. A suitable set of kinetic parameters is determined through a formal independent parallel reactions model with six-pseudo components, using a combination of isoconversional and fitting methods.
