More Information
Submitted: April 19, 2022 | Approved: May 03, 2022 | Published: May 04, 2022
How to cite this article: Yadeta AT. Food applications of Aloe species: A review. J Plant Sci Phytopathol. 2022; 6: 024-032.
DOI: 10.29328/journal.jpsp.1001070
Copyright License: © 2022 Yadeta AT. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Food applications; Nutritional substances; Aloe species; Phytochemicals screening; Proximate analysis; Minerals analysis
Food applications of Aloe species: A review
Adamu Tizazu Yadeta*
Department of Chemistry, College of Natural and Computational Sciences, Mekdela Amba University, Tulu Awuliya, Ethiopia
*Address for Correspondence: Adamu Tizazu Yadeta, Department of Chemistry, College of Natural and Computational Sciences, Mekdela Amba University, Tulu Awuliya, Ethiopia, Email: adamutizazu1@gmail.com
Plants have a high concentration of biologically active molecules. Aloe plants tend to store water and important chemical constituents in their swollen and succulent leaves due to their ability to survive in hot and dry conditions, which makes them a unique source of phytochemicals. The Aloe leaf contains more than 200 nutritional substances, including vitamins, minerals, amino acids, and active enzymes. These constituents are analyzed as phytochemical screening (qualitative analysis) or proximate and mineral content analyses (quantitative analysis). Aloe is used as a food product and beverage ingredient. Functional and nutraceutical foods, edible coatings/films, Aloe species as cooked vegetables, and raw eating of Aloe species are how the Aloe plant is considered in food applications. The researchers reported edible Aloes for several species. However, it is not mean that all species of Aloe are edible. It is not only the leaves of Aloe that have nutritional values also other parts of the plant do. The study evaluated the nutritional value of Aloe flowers and their possible use as edible flowers. Aloe species are increasingly being incorporated into different health drinks, foods, and beverages due to the beneficial biological activities of the phytochemicals.
Natural products are sources of bioactive compounds with a wide molecular and functional diversity. Plants have a high concentration of biologically active molecules [1]. The physical-chemical properties and activity of natural products are a function of the type of extract, the plant species, the post-harvest conditions, and the raw materials utilized [2]. In addition to that, the plant age, and growth conditions [3], such as geographical distribution, soil quality, water availability, solar radiation, and temperature determine the chemical composition of plants [4]. Aloe plants tend to store water and important chemical constituents in their swollen and succulent leaves due to their ability to survive in hot and dry conditions, which makes them a unique source of phytochemicals [5]. Combinations of active molecules extracted from Aloe species have been indicated to confer a variety of biological effects with different mechanisms of action [6]. The nutrients and ant-nutrient that have been identified in Aloe plants include vitamins, minerals, enzymes, simple and complex polysaccharides, fatty acids, indoles, alkanes, pyrimidines, aldehydes, dicarboxylic acids, ketones, phenolic compounds, phytosterols, and alkaloids with potential biological and toxicological activities [7-9].
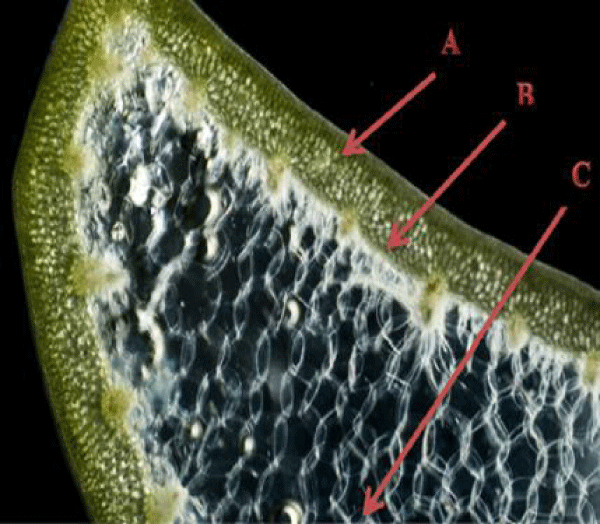
Aloe plant leaves, which are the most commonly used medicinal parts, are heterogeneous and can be divided into three major parts, namely: (i) the outer green epidermis, which majorly consists of structural components; (ii) the outer pulp region below the epidermis, consists of vascular bundles where the bitter latex or sap is derived; and (iii) the inner leaf pulp, consists of Aloe gel and containing parenchyma cells. Regarding the different compositions of these leaf portions, they are also likely to have distinct classes of bioactive compounds, which are believed to contribute to the different biological properties of leaves [10]. The outer thick layer (rind) consists of 15-20 cells which give protection to the gel matrix and helps in the synthesis of carbohydrates and proteins [11]. The middle layer (latex), a bitter yellow sap contains anthraquinones and glycosides. The inner layer (gel) consists of soft, clear, moist, and slippery tissues having large parenchyma cells [12]. This is a transparent mucilaginous jelly-like material. It contains water (99%), glucomannans, amino acids, lipids, sterols, and vitamins [13]. Although leaves are the most used part of the plant, recently some studies have reported the bioactive roots [14] and flowers [15] of the plant (Figures 1,2).
Figure 1: Aloe elegans. (Photo taken by Mudin) [16].
Figure 2: A cross-section illustration of Aloe leaf; A: epidermis or the outer rind, B: the outer leaf pulp, sap/exudate (latex) C: mesophyll or the inner leaf pulp (gel) [17].
Aloe species are increasingly being incorporated into different health drinks, foods, and beverages due to the beneficial biological activities of the phytochemicals [18]. Several constituents from various phytochemical classes such as alkaloids, anthrones, chromones, flavonoids, glycoproteins, naphthalenes, and pyrones have been isolated from different Aloe species [19]. Interest in the antioxidant properties of Aloe species is mainly related to their therapeutic and curative uses, however, nowadays there is also growing interest in the use of health-promoting food additives and natural antioxidants for extending the shelf life without the need for synthetic antioxidants [20].
The Aloe leaf contains more than 200 nutritional substances, including vitamins, minerals, amino acids, and active enzymes, which work in synergy to bring about these biological and healing effects. Aloe is also used as a food product and beverage ingredient [21]. The literature includes references to several species of edible Aloes. However, it is not mean that all species of Aloe are edible [22]. Nowadays, many commercial food-product producers have pushed up the usage of A. vera gel or juice in one form or the other due to its nutrient ingredients [23]. It is not only the leaves of Aloe that have nutritional values also other parts of the plant do. The study evaluated the nutritional value of Aloe flowers and their possible use as edible flowers [24]. For instance, the overall nutritional content of A. greatheadii var. davyana pollen appears to be a good source [25]. But most of the works of literature state the nutritional value of A. vera, due to it is widely known and available everywhere.
2.1 Qualitative analysis
2.1.1. Carbohydrates: Several researchers have tried the separation of the A. arborescens gel carbohydrate polymers into their polysaccharide components [26]. However, the types and molecular sizes of the polysaccharides extracted from Aloe gel appear very diverse which makes to separate. The difference in the previous reports may be due to plant species or different geographical origins, seasonal and cultivar variation, technical differences used to isolate the polysaccharide, or degradation of polysaccharides by endogenous enzyme activity [27-28]. The investigators isolated crude water-soluble polysaccharides from gel juice, skin juice, and flowers of A. arborescens by ethanol precipitation and their result indicates that skin juice contained 2.4 times the level of polysaccharides than gel juice from one plant [26].
2.1.2. Protein and amino acids: Glycoproteins have been isolated from Aloe species that possess different bioactivities. Lectins and lectin-like substances having hemagglutinating activity were isolated from A. vera gel [29]. The proteins aloctin A (molecular weight of 18 kDa consisting of 7.5 and 10.5 kDa subunits having 18% carbohydrate fraction) and aloctin B (molecular weight of 24 kDa having two 12 kDa subunits and 50% carbohydrate fraction) have also been isolated [30]. Among the eight amino acids essential for humans, seven of them are present in the gel of Aloe. Among 22 amino acids, 20 are present [29].
2.1.3. Lipids: Among the commonly known lipids, fatty acids are bioactive compounds and an important part of the phytochemical content of Aloe, being widely used as feedstocks in the food industry [31] Dried A. vera (L.) Burm f. flowers are a valuable source of lipid constituents. This study evaluated the nutritional value of Aloe flowers and their possible use as edible flowers [24].
2.1.4. Vitamins: A. vera gel contains ascorbic acid, carotenoids, tocopherols, vitamin B1 (thiamin), vitamin B2 (riboflavin), vitamin B6, niacin, and folic acid. The majority of these possess antioxidant potential. Minute quantities of vitamin B12 have also been detected in the gel [32].
2.1.5. Enzymes: A. vera gel includes at least six different enzymes: cellulose, carboxypeptidase, amylase, bradykinase, oxidase, and catalase. These enzymes help in digestion and aid in the nutrient absorption of the food by decomposition of fats and sugars [29].
2.1.6. Minerals and trace elements: Magnesium, calcium, iron, copper, zinc, and chromium are existent in A. vera gel. The antiallergic properties of A. vera gel are attributed to magnesium lactate is present. As compared to most other plants, A. vera contains greater amounts of potassium and chloride, but a lesser quantity of sodium [29]. Elements in biological sources are more efficient than pure element status [33]. Minerals are nutritive elements that are present in the tissues and fluids of all bodies. Minerals may be classified as Macro and Micronutrient. The macronutrients are Ca, P, Na, and Cl while micronutrients are Cu, Co, K, Mg, I, Zn, Mn, Mo, F, Cr, Se, and S [34].
2.2.Quantitative analysis
2.2.1. Proximate analysis: Proximate analysis, also known as Weende analysis is a quantitative chemical method of assessing and expressing the nutritional value of a feed. It reports the moisture, ash (minerals), crude fiber, crude fat, and crude protein (total nitrogen) present in the fuel as a percentage of dry fuel weight [35]. Proximate analysis is a system of investigation of nutrients also termed “conventional analysis”, which determines the gross components rather than the individual nutrients amino acids, fatty acids, monosaccharides e.t.c. [36]. Generally, proximate analyses give the overall nutritional composition of the sample in question, which is briefly complemented by the antinutrient, and mineral composition of the sample [37].
Moisture content: Moisture determination satisfies the technological, analytical, commercial, and regulatory necessities in the processing, testing, and storage of food products and is an index of economic value, stability, and nutritional quality of food products [38]. The dry matter that remains after moisture removal is commonly referred to as total solids [39]. Therefore, moisture content has great economic importance to a food manufacturer because water is an inexpensive filler. The main feature of all the Aloe leaves was their high moisture content [40]. Too much moisture in any sample has been proved to cause caking, especially in flour, and can also determine the storage/shelve life and the viability of microorganisms’ growth [37].
Ash content: Ash refers to the inorganic residue remaining after ignition or complete oxidation of organic matter in a biological material [41]. Ash content is a measure of total minerals and is a reliable index of nutritional value for many foods and feed and is recognized as a useful tool in determining the nature and distribution of mineral constituents of the sample [38]. Ash is known as the residue left after all the moisture and organic matter has been removed at high temperatures. Therefore, the high ash content of the plants is generally a measure of mineral richness [42]. The well-studied Aloe spp., A. vera has high ash content [35].
Carbohydrate content: Carbohydrates biomolecules are present in all foods (grains, vegetables, fruits, and milk), and are classified into simple monosaccharides (fructose, glucose, galactose, sorbitol), oligosaccharides (maltose, sucrose, lactose, raffinose, stachyose, verbascose), and more complex polysaccharides (starch, cellulose, etc.) [38]. These molecules are important in foods as a major source of energy, to impart crucial textural properties, and as dietary fiber which influences physiological processes [39]. Carbohydrates also have contributions, including bulk, body, viscosity, stability to emulsions and foams, water-holding capacity, freeze-thaw stability, browning, flavors, aromas, and a range of desirable textures. They also provide satiety. A. vera leaves are a rich source of fibers [40]. Aloe species have a high content of carbohydrates. Carbohydrates provide readily accessible fuel for physical performance and regulate nerve tissue [37]. Due to these actions, Aloe species have such applications in nutritional values.
Protein content: Nitrogen is the most distinguishing element present in proteins. However, nitrogen content in various proteins ranges due to the variation in the specific amino acid composition of proteins. Generally, proteins rich in basic amino acids contain more nitrogen [39]. Aloe species have protein, which would be serving as an enzymatic catalyst, mediate cell responses, and control growth and cell differentiation [43].
Lipid/fat content: Analysis of lipids in foods is important for accurate nutritional knowledge [39]. The analysis of lipids in food has three distinctive objectives: to determine (1) total lipid content, (2) the composition, and (3) the quality of lipids [38]. In living organisms, fats are the usually stored form of energy. They are the main structural element of phospholipids and sterol [44].
Vitamins: Vitamins are defined as relatively low-molecular-weight compounds required in small quantities for normal metabolism. With few exceptions, humans cannot synthesize most of the vitamins [39]. Vitamin analysis of biological samples has played a critical role in determining human nutritional requirements. The presence of important antioxidant vitamin C in A. vera has been reported [40].
There are many data that show the total contents of moisture, ash, carbohydrate, protein, and lipid. Many factors affect the values of these parameters even within the same species. Table 1 indicates such values. From the table, it should be considered as leaf powder for both works. That is why moisture content is too minimum than leaf gel.
| Table 1: Proximate parameters of Aloe leaves. | |||||||
| Aloe species | Part of Aloe analyzed | Proximate parameters’ values (%) | Ref. | ||||
| Moisture content | Ash content | Carbohydrate content | Protein content | Lipid (fat) content | |||
| A. barbadensis | leaf extracts powder | 11.71 + 0.02 | 2.36 + 0.01 | 73.08 + 0.04 | 4.73 + 0.01 | 0.27 + 0.01 | [37] |
| A.vera (A. barbadensis) | leaf extracts powder | 10.51 | 0.5 | 78.88 | 0.613 | 3.5 | [44] |
| Values were reported as Mean Triplicate + Standard Error, in the first row. | |||||||
2.2.2. Minerals analysis: The determination of ash/minerals in food contributes to an assessment of a food’s nutritional value and refers to elements and verifying if the food contains some minerals in quantities dangerous to the health of the consumer, whether their presence is natural or adulteration of certain foodstuff/processed or stored food products [38]. Minerals are essential for the proper functioning of tissues and act as second messengers in some biochemical cascade mechanisms [45]. As the mineral analysis showed, A. barbadensis is rich in minerals like potassium, sodium, phosphorus, magnesium, zinc, iron, manganese, copper, and lead [37]. The high percent content of potassium and magnesium indicates the ability of A. barbadensis to help lower blood pressure [46]. The high sodium content and calcium in A. barbadensis indicate its importance in the formation of bones and teeth as well as muscle contraction by calcium. The high value of phosphorus content in this study which is very vital in bone formation indicates the importance of the A. barbadensis in our body system [44].
Selenium has been described as an indispensable element with the unique anti-oxidative potential required to sustain the antioxidant defense mechanism in the human body [47]. The reports indicate the presence of Se in Aloe gel which makes it effective for nutraceutical applications [48]. Generally, the values of some minerals analyzed from A. vera (A. barbadensis) are given in Table 2. In both literatures, the value of potassium (K) is high.
| Table 2: In both literatures, the value of potassium (K) is high. | ||||||||||||
| Minerals value | Ref. | |||||||||||
| Mg | Ca | Zn | Fe | Mn | Cu | Pb | N | P | Na | K | ||
| Minerals (%) | 0.033 | - | 0.007 | 0.003 | 0.013 | 0.002 | 0.00017 | - | 0.665 | 0.517 | 1.062 | [37] |
| Minerals (mg/kg) | 1.00 | 0.45 | - | - | - | - | - | 0.01 | 5.69 | 10.00 | 55.00 | [44] |
| (-) indicates unanalyzed value | ||||||||||||
In addition to the biological effects on the human body, the antioxidant activity of Aloe makes it a safe natural ingredient in food processing to increase the shelf life and nutritional value of food [49]. Literature state several species of edible Aloes, for their uses in snack foods, famine foods, as a cooked vegetable, and as an ingredient in preserves [22].
The cultivated species A. arborescens and A. vera have been used commonly in foodstuffs, especially in dairy products such as yogurt and ice cream, in Asia and the United States. A perceptible increase in the different manufactured food products containing A. ferox, such as fruit juice blends and confectionary mirrors the global rise in popularity of A. vera leaf mesophyll in food products in South Africa [50]. A. vera food and supplement market has grown rapidly in recent years, likely owing to increasing consumer awareness of these purported health benefits [51]. The A. vera juice contributes wide application in food and beverage products like production of ready to serve the drink, soft drink, laxative drink, sherbet, Aloe sports drink with electrolyte, A. vera lemon juice, diet drink with soluble fiber, health drink, hangover drink with B vitamin, amino acids and acetaminophen, healthy vegetable juice mix, tropical fruit juice with A. vera, A. vera yogurts, A. vera mix for whiskey and white bread, cucumber juice with the addition of A. vera [52].
3.1. Functional and nutraceutical foods
In the nutraceutical industry, A. vera gel is being used as a mineral source for different functional foods and as a supplement in other food products for the production of various health drinks and beverages [48]. The food industry uses A. vera in the production of functional products, especially health drinks, yogurt, beverages of orange, grape, raspberry, pineapple, cranberry, strawberry, jam, and jelly [53]. Other food products including ice cream, milk, confectionery, etc. are also prepared using A. vera gel as a flavoring component and food preservative [54]. Despite the bitter test of the latex of Aloe species, some of them have sweet test flowers that attract insects, birds, and other animals including human beings. A. greatheadii var. davyana, is used for migratory beekeepers, who move their hives to the aloe fields to utilize the strong nectar and pollen flow to build up their colonies and obtain a substantial honey crop [55].
Among the Aloe species, the most studied A. vera has become one of the most important raw materials in the food industry since it represents an emerging source of bioactive components which have different applications [56]. One of the active components of Aloe, polysaccharides have prebiotic potential. Therefore, it has been used for the preparation of prebiotic foods [57]. Conversely, the addition of small doses of A. vera pulp not only enhanced the nutritional values but also does not affect the taste or appearance of cheese products. In addition to this, it may improve the activity of probiotic bacteria as it serves as a good prebiotic functional material in cheese production [58].
Nowadays, new and modified food and beverages are prepared from A. vera. A fermented South Asian dairy product (dahi) was formulated by replacing skim milk with A. vera gel [59]. A. vera gel enriched beverages (sweetened A. vera juice, ready-to-serve juices, and squashes) have also been reported which are claimed to have the potential to maintain good health [60]. Other health foods developed from A. vera include ice cream, lassi (a traditional fermented dairy beverage of South Asia), mango nectar, and carbonated beverages [61]. Aloe species have a different processed form to use as food and drinks. For instance, A.vera has been processed as A.vera juice, A.vera concentrate, and A.vera powder in many food applications [52].
In the United States, the Food and Drug Administration (FDA) has approved the internal use of gel as a “dietary supplement”. In addition to this, according to Annex I of Regulation No. 1831/2003 of the European Commission (EC), A.vera can be used by the feed industries as sensory additive functional group “flavoring compounds”, to increase the smell or palatability of feedings stuff [62].
3.2. Edible coatings/films
One of the simple, non-toxic and biodegradable methods that have been recently tested is the use of edible coatings from natural sources such as plant extracts [63]. Edible coatings are defined as a thin layer of an edible material that can act as a barrier to restrain the exchange of moisture, oxygen, and the solute movement of horticultural products, thus reducing water loss, respiration, and oxidation reaction rates [64]. This means plants such as Aloes are used to prepare edible coatings/films due to they are simple, non-toxic, and biodegradable and these coatings/films are used as a barrier to restrain the exchange of moisture, oxygen, and the solute movement of horticultural products, thus reducing water loss, respiration, and oxidation reaction rates, especially in fruits. Aloe species such as A. vera gel is considered among one the best edible and biologically safe coatings for different food applications due to their film-forming properties and biodegradability. The polysaccharides of A. vera act as a natural barrier to moisture and oxygen that are the main agents of the deterioration of fruits and vegetables [11]. As investigated, the post-harvest quality of sweet cherries coated with A. vera gel-based coating. The characteristics such as respiration rate, weight loss, color changes, softening, ripening, and stem browning were reduced as compared to uncoated fruits while maintaining the taste, aroma, and flavor [65].
In addition to Table 3, other previous studies have also noted that the use of an A. vera gel coating in products such as apple fruit [75], Table grape [76], Nectarine [77], Apricot fruit [78], pomegranate arils [65], Stonefruits [79], Blueberry [80], Tomato fruit [81], Litchi fruit [82], White button mushroom [83] and Fresh-cut guava [84] to reduce respiration rates, moisture loss, softening, microbial decay and maintaining the other quality characteristics which could extend the fruits shelf-life. In addition to the contribution to food safety, the incorporation of A. vera gel also prevents them from microbial spoilage. A variety of antimicrobial compounds are present in A. vera gel and antimicrobial activity is exhibited due to their synergistic effect [85]. A. vera gel can efficiently inhibit the growth of food-borne spoilage and pathogenic microorganisms [86].
Table 3: A.vera edible coatings on foods and drinks. |
|||
| Aloe spp. coating | Coated products | Coating function | Ref. |
| A.vera gel | kiwifruit slices | Retarding the yellowing process, reducing microbial growth, and improving total pectin and texture retention | [66] |
| A.vera gel | Papaya fruit | Increase the post-harvest storage life of papaya | [67] |
| A.vera gel | Sweet cherry fruit | Physical barrier and thus reduced the weight loss and lowered the respiration rate during postharvest storage. delayed color changes, softening and TA losses, maintaining fruit quality. |
[68] |
| A.vera or A.arborescens |
Peach and plum fruits | Retarding postharvest ripening, which could be attributed to their effect on delaying climacteric ethylene production and quality losses. | [69] |
| salicylic acid and A.vera gel | Orange fruit | Maintained the qualitative characteristics of fruit during storage, Salicylic acid ameliorated chilling injury by fortification of antioxidant systems which reduced the malondialdehyde content and A. vera gel suppresses the microbial growth and reduces the deterioration due to bioactive components such as aleonin and aloe-emodin | [70] |
| A.vera gel | Fish gelatin films | Lowers the solubility of the films significantly without causing a significant change in the thickness, color, and surface microstructure of the films. | [71] |
| A.vera gel and Spirulina platensis | Mango fruit | Reduced the respiration rate and the weight loss of the mango fruits | [72] |
| A.vera gel | Pomegranate arils | Maintaining quality parameters of minimally processed arils such as firmness, color, and bioactive compounds. | [65] |
| A.vera gel with gum arabic, garlic extract, and ginger extract | Puava fruits | Postharvest quality and storability | [73] |
| A. vera gel with ferulic acid | fresh-cut apples | Delaying quality changes and ensuring the safety | [74] |
3.3 Aloe species as cooked vegetables
Flowers that can be consumed by a human being safely are known as edible flowers. The analysis revealed that the nutritional and bioactive attributes make the edible flowers a complete form of nutrition available for human beings and need further exploration for value-added product development [87]. The Aloe species have edible flowers [24]. These flowers are either eaten cooked or uncooked. Like vegetables, some Aloe species are eaten by cooking their flowers. The flowers of Aloe boylei Baker, Aloe cooperi Baker, and other species are cooked as a vegetable. The flowers of Aloe macrocarpa Tod are eaten by various tribes, and used as a seasoning herb in cooking in West Africa [88].
3.4. Raw eating of Aloe species
In addition to functional and nutraceutical foods, edible coating/films, and antimicrobial agent food applications of Aloe species, some Aloe species are eaten without incorporating them with any other substances. For instance, the gel of Aloe zebrina Baker and Aloe maculata All., however, is used as famine food in case of an emergency, and the flowers of several species, including A. ferox, contain nectar that is eaten by children [89]. In different parts of Africa, flowers of various species are eaten. Young flowering shoots of Aloe kraussii Baker and Aloe minima Baker are eaten as raw vegetables by Zulus [88]. Most Aloe species produce diurnal and tubular brightly colored flowers, usually yellow or red [90]. These flowers produce nectars [91], which are eaten by different living things including humans, especially children due to the sweetness of the flowers [89].
The scientific community is divided into two groups regarding the safety of A. vera products. One group warns to use A. vera with caution and utmost care to avoid contamination of its components due to some components being hazardous, while another group advocates that the A. vera is quite safe for human consumption [52]. Although natural products and plants are a good candidate to use in Aloe species, it should be considered that they are not as safe as the public thinks, and may cause problems in long-term use. Some species of this genus such as A. ferox are potentially toxic [17]. However, most Aloe species are not toxic but a few are extremely poisonous. Therefore, it is necessary to use medicinal plants by evaluating their possible adverse effects [92].
There is insufficient data available to properly evaluate the safety of aloe products [93]. Reports of allergic conditions and hypersensitivity to aloe preparations have been noted and several single-case reports are available [94]. The oral using of A.vera with furosemide or digoxin treatment for irregular heart rhythms and congestive heart failure can lower the level of potassium in the body. Hence, it should not be consumed with these drugs. Use of Aloe latex or Aloe juice for longer periods or in high doses can cause an imbalance of electrolytes – loss of sodium can result in secondary hyperaldosteronism, and loss of potassium can result in hypokalemia leading to fatigue, muscular weakness, weight loss, mental problems and dis-functioning of kidneys [95]. During pregnancy, lactation, or childhood, and for persons suffering from abdominal pain, appendicitis, or intestinal obstruction, Aloe should not be used internally [52].
The European Commission presented a report on the advisability of incorporating additional categories of substances into the existing legal provisions allowing the use of herbal substances and preparations in medicines as well as in food supplements by July 2007 [96]. In that report, the dried juice and concentration of leaves were evaluated. Lastly, it was concluded that in view of existing possible risks, such traditional use cannot be recommended and referred to in the “Community list of herbal substances, preparations, and combinations thereof for use of traditional herbal medicinal products [92].” The Aloe gel is expensive, therefore it is not surprising that some producers try to increase their business profits by adding water to the Aloe components [97].
The food industry has developed Management quality (ISO 9000:2000) and safety systems (HACCP) to certify the biological activity, sensorial constancy, and value of ultimate produce made from Aloe [98]. Safety control points include a pasteurization step and addition of vitamin C and citric acid whereas the quality control points include the collection of raw material, filleting step, homogenization, and the addition of pectolytic enzymes, filtration, deaeration, sterilization, flash cooling, and storage [99].
The analyses carried out on Aloe plants indicate their nutritional and phytochemical composition. The miraculous medicinal plant Aloe has been proved to be a good source of water, carbohydrate, protein, lipids, vitamins, enzymes, and minerals. All of them are good indications of high nutritive value. It could, therefore, be used as an important dietary source of nutrients in a food-based approach for combating nutrient deficiency. Despite the presence of some Anti-nutrient that could serve as mineral inhibitors, Aloe species can still be used as sources of these minerals. The Phytochemical content is also an indication as the plant has a potential protective agent against degenerative diseases.
The Aloe plant owing to its beneficial therapeutic effects has found wide applications in a variety of products including food applications. Its consumption in various fields can be maximized by developing appropriate processing techniques. It is expected that its applications will increase with time. However, there are some complications linked to the use of Aloe that needs to be addressed precautions need to be considered while using aloe in some specific conditions and with some specific compounds. It is recommended that its continuous use for an extended period should be avoided to avoid any possible complications.
- Roumita SS, Namrita L, Bianca F, Analike Blom van S, Fawzi M, Antibiotic-potentiating activity, phytochemical profile, and cytotoxicity of Acalypha integrifolia Willd (Euphorbiaceae). J Herb Med. 2018; 11:53–59.
- Fanali S, Aturki Z, D'Orazio G, Rocco A, Ferranti A, Mercolini L, Raggi MA. Analysis of Aloe-based phytotherapeutic products by using nano-LC-MS. J Sep Sci. 2010 Sep;33(17-18):2663-70. doi: 10.1002/jssc.201000408. PMID: 20806241.
- Luigi L, Marco P, Gian P, Anthraquinones M, and β-polysaccharides content and distribution in Aloe plants grown under different light intensities, Biochem. Syst. and Eco. 2013; 51:264–268.
- Muñoz OM, Leal X, Quitral V, Cardemil L, Extraction, characterization and properties of the gel of Aloe vera (Aloe barbadensis Miller) cultivated in Chile Med Arom. Plants. 2015; 4(3):1-7.
- Majumder R, Das CK, Mandal M. Lead bioactive compounds of Aloe vera as potential anticancer agent. Pharmacol Res. 2019 Oct;148:104416. doi: 10.1016/j.phrs.2019.104416. Epub 2019 Aug 27. PMID: 31470079.
- Sánchez M, González-Burgos E, Iglesias I, Gómez-Serranillos MP. Pharmacological Update Properties of Aloe Vera and its Major Active Constituents. Molecules. 2020 Mar 13;25(6):1324. doi: 10.3390/molecules25061324. PMID: 32183224; PMCID: PMC7144722.
- Boudreau MD, Beland FA. An evaluation of the biological and toxicological properties of Aloe barbadensis (miller), Aloe vera. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2006 Apr;24(1):103-54. doi: 10.1080/10590500600614303. PMID: 16690538.
- Nejatzadeh-Barandozi F. Antibacterial activities and antioxidant capacity of Aloe vera. Org Med Chem Lett. 2013 Jul 19;3(1):5. doi: 10.1186/2191-2858-3-5. PMID: 23870710; PMCID: PMC3729540.
- Boudreau MD, Mellick PW, Olson GR, Felton RP, Thorn BT, Beland FA. Clear evidence of carcinogenic activity by a whole-leaf extract of Aloe barbadensis miller (aloe vera) in F344/N rats. Toxicol Sci. 2013 Jan;131(1):26-39. doi: 10.1093/toxsci/kfs275. Epub 2012 Sep 11. PMID: 22968693; PMCID: PMC3537128.
- Cock IE. The Genus Aloe: Phytochemistry and Therapeutic Uses Including Treatments for Gastrointestinal Conditions and Chronic Inflammation. Prog Drug Res. 2015;70:179-235. doi: 10.1007/978-3-0348-0927-6_6. PMID: 26462368.
- Misir J, Brishti FH, Hoque, M.M. Aloe vera gel as a novel edible coating for fresh fruits: a review. Am. J. Food Sci. Technol. 2014, 2 (3), 93–97.
- Hamman JH. Composition and applications of Aloe vera leaf gel. Molecules. 2008 Aug 8;13(8):1599-616. doi: 10.3390/molecules13081599. PMID: 18794775; PMCID: PMC6245421.
- Benítez S, Achaerandio I, Pujolà M, Sepulcre F. Aloe vera as an alternative to traditional edible coatings used in fresh-cut fruits: a case of study with kiwifruit slices. LWT – Food Sci. Technol. 2015, 61 (1), 184–193.
- Abdissa N, Gohlke S, Frese M, Sewald N. Cytotoxic Compounds from Aloe megalacantha. Molecules. 2017 Jul 7;22(7):1136. doi: 10.3390/molecules22071136. PMID: 28686200; PMCID: PMC6152336.
- Martínez-Sánchez A, López-Cañavate ME, Guirao-Martínez J, Roca MJ, Aguayo E. Aloe vera Flowers, a Byproduct with Great Potential and Wide Application, Depending on Maturity Stage. Foods. 2020 Oct 26;9(11):1542. doi: 10.3390/foods9111542. PMID: 33114533; PMCID: PMC7693977.
- Mudin J, Etana D, Salah H, Dagne A, Milkyas E. Anthraquinones from the roots of Aloe gilbertii and Aloe eleganis. J. Natur. Sci. Res.2018 8 (1) 1-7.
- Akaberi M, Sobhani Z, Javadi B, Sahebkar A, Emami SA. Therapeutic effects of Aloe spp. in traditional and modern medicine: A review. Biomed Pharmacother. 2016 Dec;84:759-772. doi: 10.1016/j.biopha.2016.09.096. Epub 2016 Oct 4. PMID: 27716590.
- Nalimu F, Oloro J, Kahwa I, Ogwang PE. Review on the phytochemistry and toxicological profiles of Aloe vera and Aloe ferox. Futur J Pharm Sci. 2021;7(1):145. doi: 10.1186/s43094-021-00296-2. Epub 2021 Jul 21. PMID: 34307697; PMCID: PMC8294304.
- Amoo SO, Aremu AO, Van Staden J. Unraveling the medicinal potential of South African Aloe species. J Ethnopharmacol. 2014 Apr 11;153(1):19-41. doi: 10.1016/j.jep.2014.01.036. Epub 2014 Feb 5. PMID: 24509153.
- Lucini L, Pellizzoni M, Pellegrino R, Molinari GP, Colla G. Phytochemical constituents and in vitro radical scavenging activity of different Aloe species. Food Chem. 2015 Mar 1;170:501-7. doi: 10.1016/j.foodchem.2014.08.034. Epub 2014 Aug 27. PMID: 25306376.
- Sun YN, Kim JH, Li W, Jo AR, Yan XT, Yang SY, Kim YH. Soluble epoxide hydrolase inhibitory activity of anthraquinone components from Aloe. Bioorg Med Chem. 2015 Oct 15;23(20):6659-65. doi: 10.1016/j.bmc.2015.09.003. Epub 2015 Sep 5. PMID: 26372074.
- Steenkamp V, Stewart MJ. Medicinal applications and toxicological activities of Aloe products. Pharm. Bio. 2007, 45, 411–420.
- Lad VN, Murthy ZVP. Rheology of Aloe barbadensis Miller: A naturally available material of high therapeutic and nutrient value for food applications. Journal of Food Engineering. 2013, 115, 279–284.
- López-Cervantes J, Dalia ISM, Paola CF, María F, Mariscal D, Gabriela S, de la ML, Olga NCB. Antioxidant capacity, proximate composition, and lipid constituents of A. vera flowers. J Appl Res Med Aromatic Plants. 2018, 10, 93-98.
- Human H, Nicolson SW. Nutritional content of fresh, bee-collected and stored pollen of Aloe greatheadii var. davyana (Asphodelaceae). Phytochemistry. 2006 Jul;67(14):1486-92. doi: 10.1016/j.phytochem.2006.05.023. Epub 2006 Jun 30. PMID: 16808932.
- Chang XL, Feng YM, Wang WH. Comparison of the polysaccharides isolated from skin juice, gel juice and flower of A. arborescens tissues. J Taiwan Institute Chem Eng. 2011, 42, 13–19.
- Chang XL, Feng YM, Wang CH, Liu ZP. Effects of heat treatments on the stabilities of polysaccharides substances and barbaloin in gel juice from A. vera Miller. J Food Eng. 2006, 75, 245-251.
- Reynolds T, Dweck AC. Aloe vera leaf gel: a review update. J Ethnopharmacol. 1999 Dec 15;68(1-3):3-37. doi: 10.1016/s0378-8741(99)00085-9. PMID: 10624859..
- Shabnam J, Atta-ur R. Food and nutritional analysis. Elsevier. 2005. 202-211.
- Akev N, Turkay G, Can A, Gurel A, Yildiz F, Yardibi H, Ekiz EE, Uzun H. Tumour preventive effect of Aloe vera leaf pulp lectin (Aloctin I) on Ehrlich ascites tumours in mice. Phytother Res. 2007 Nov;21(11):1070-5. doi: 10.1002/ptr.2215. PMID: 17685385..
- Andrea B, Dumitrița R, Florina C, Francisc D, Anastasia V, Socaci S, Adela P. Comparative analysis of some bioactive compounds in leaves of different Aloe species. BMC Chem. 2020 Oct 31;14(1):67. doi: 10.1186/s13065-020-00720-3. PMID: 33292458; PMCID: PMC7602344..
- Lawless J, Allan JA. Vera-Natural Wonder Cure, Harper Collins Publishers, Hammersmith, London. 2006.
- Sahito SR, Kazi TG, Kazi GH, Memon MA, Shaikh Q, Jakhrani MA, Shar GQ. Comparison of sample preparation methods for the determination of essential and toxic elements in important indigenous medicinal plant Aloe barbandensis. J Chem Soc Pak. 2003;25:201–205.
- Dinesh KS, Swapnil R, Arora SS, Gupta PM, Renu S, Chopra AK. Study of the trace elements in A. vera L. (A. barbandensis Miller) viz. Liliaceae and its biological and environmental importance. J Chem Pharm Res. 2011;3(3); 64-68.
- Haque MZ, Islam MB, Jalil MA, Shafique MZ. Proximate Analysis of Aloe vera leaves. IOSR Journal of Applied Chemistry (IOSR-JAC). 2014;7(6):36-40.
- Onyeike EN, Osuji JO. Research Techniques in biological and chemical sciences. Springer field Publishers Ltd., Owerri, Nigeria. 2003;pp 403.
- Adesuyi AO, Awosanya OA, Adaramola FB, Omeonu AI. Nutritional and phytochemical screening of Aloe barbadensis. Curr Res J Biol Sci. 2012;4(1):4-9.
- Jain V, Gupta K. Food and nutritional analysis / overview. Elsevier. 2005, 202-211.
- Nielsen SS. Food analysis, 4th Ed. Springer New York Dordrecht Heidelberg London. 2009;pp:48-55.
- Muaz A, Fatma H. Chemical composition and biochemical Activity of A. vera (A. barbadensis Miller) leaves. IJCBS. 2013;3:29-33.
- Keshun L. Effects of sample size, dry ashing temperature and duration on determination of ash content in algae and other biomass. 2019;40:101486.
- Lai PK, Roy J. Antimicrobial and chemopreventive properties of herbs and spices. Curr Med Chem. 2004 Jun;11(11):1451-60. doi: 10.2174/0929867043365107. PMID: 15180577.
- Whimey EN, Rolfes SR. Understanding nutrition, 10th Edn. Thomson/Wadsworth Publishing Company, Belmont, C.A. 2005;132-137.
- Usman RB, Adamu M, Isyaku IM, Bala HA. Quantitative and qualitative phytochemicals and proximate analysis of A. vera (A. barbadensis Miller), Inter J Advan Acad Res. | Sci Tec Eng. 2020;6(1):95-109.
- Anitia B, Akpan EJ, Okon PA, Umoren IU. Nutritive and anti-nutritive evaluation of sweet potatoes (Ipomoea batatas) leaves. Pak. J Nutrit. 2006, 5, 166-168.
- Otsuki N, Dang NH, Kumagai E, Kondo A, Iwata S, Morimoto C. Aqueous extract of Carica papaya leaves exhibits anti-tumor activity and immunomodulatory effects. J Ethnopharmacol. 2010 Feb 17;127(3):760-7. doi: 10.1016/j.jep.2009.11.024. Epub 2009 Dec 2. PMID: 19961915..
- Burk RF. Selenium, an antioxidant nutrient. Nutr Clin Care. 2002 Mar-Apr;5(2):75-9. doi: 10.1046/j.1523-5408.2002.00006.x. PMID: 12134713.
- Anirban R, Gupta SD, Sampad G, Shashaank M, Aswatha BK. Chemometric studies on mineral distribution and microstructureanalysis of freeze-dried A. vera L. gel at different harvesting regimens. Industrial Crops and Products. 2013;51:194– 201.
- Hęś M, Dziedzic K, Górecka D, Jędrusek-Golińska A, Gujska E. Aloe vera (L.) Webb.: Natural Sources of Antioxidants - A Review. Plant Foods Hum Nutr. 2019 Sep;74(3):255-265. doi: 10.1007/s11130-019-00747-5. PMID: 31209704; PMCID: PMC6684795..
- Grace OM. Current perspectives on the economic botany of the genus Aloe L. (Xanthorrhoeaceae), South African J Botany. 2011;77: 980–987.
- Hu J, Lloyd M, Hobbs C, Cox P, Burke K, Pearce G, Streicker MA, Gao Q, Frankos V. Absence of genotoxicity of purified Aloe vera whole leaf dry juice as assessed by an in vitro mouse lymphoma tk assay and an in vivo comet assay in male F344 rats. Toxicol Rep. 2021 Mar 9;8:511-519. doi: 10.1016/j.toxrep.2021.03.007. PMID: 33747796; PMCID: PMC7973126.
- Ahlawat KS, Khatkar BS. Processing, food applications and safety of aloe vera products: a review. J Food Sci Technol. 2011 Oct;48(5):525-33. doi: 10.1007/s13197-011-0229-z. Epub 2011 Jan 18. PMID: 23572784; PMCID: PMC3551117.
- Samah ME, Hoda SE. Production of UF-soft cheese using probiotic bacteria and A. vera pulp as a good source of nutrients. Ann Agricul Sci. 2020;65:13–20.
- Chowdhury MAH, Sultana T, Rahman MA, Saha BK, Chowdhury T, Tarafder S. Sulphur fertilization enhanced yield, its uptake, use efficiency and economic returns of Aloe vera L. Heliyon. 2020 Dec 18;6(12):e05726. doi: 10.1016/j.heliyon.2020.e05726. PMID: 33364495; PMCID: PMC7753130..
- Symes CT, Human H, Nicolson SW. Appearances can be deceiving; Pollination in two sympatric winter-flowering Aloe species. South African J Botany. 2009;75:668–674.
- Minjares-Fuentes R, Femenia A, Comas-Serra F, Rossello C, Rodríguez-Gonzalez VM, Gonzalez-Laredo RF, Gallegos-Infante JA, Medina-Torres L. Effect of different drying procedures on physicochemical properties and flow behavior of A. vera (A. barbadensis Miller) gel. LWT - Food Sci Technol. 2016;74:378-386.
- Basannavar S, Pothuraju R, Sharma RK. Effect of Aloe vera (Aloe barbadensis Miller) on survivability, extent of proteolysis and ACE inhibition of potential probiotic cultures in fermented milk. J Sci Food Agric. 2014 Oct;94(13):2712-7. doi: 10.1002/jsfa.6615. Epub 2014 Mar 21. PMID: 24733678.
- Christaki EV, Florou-Paneri PC. A. vera: A plant for many uses. J Food Agric Environ. 2010;8(2):245–249.
- Pushkala R, Srividya N. Influence of Aloe gel enrichment on the physicochemical, textural and sensory characteristics of dahi. J Food Sci Eng. 2011;1;141–153.
- Sharma R, Tandon D, Joshi VK, Attri S. Development and evaluation of different beverages from A. vera (L.) Burm f. for their nutritional, functional and sensory qualities. Indial J Nat Products Resour. 2015; (4):278–282.
- Abid AM, Akmal N, Muhammad KIK, Tahir A, Rabia Z, Misbah, M, Muhammad, A. The therapeutic properties and applications of A. vera: A review. J Herb Med. 2018;12:1–10.
- Franz C, Bauer R, Carle R, Tedesco D, Tubaro A, Zitterl-Eglseer K. Study of the assessment of plants/herbs, plant/herb extracts and their naturally or synthetically produced components as “additives” for use in animal production CFT/EFSA/FEEDAP/2005/ 01. 2005.
- Šuput DZ, Lazić VL, Popović SZ, Hromiš NM. Edible films and coatings: sources, properties and application. Food Feed Res. 2015;42:11-22.
- Falguera V, Quintero JP, Jiménez A, Muñoz JA, Ibarz A. Edible films and coatings: structures, active functions and trends in their use. Trends Food Sci Technol. 2011;22:292–303.
- Martínez-Romero D, Castillo S, Guillén F, Díaz-Mula HM, Zapata PJ, Valero D, Serrano M. A. vera gel coating maintains quality and safety of ready to eat Pomegranate arils. Postharvest Biol Technol. 2013;86:107–112.
- Benítez S, Achaerandio I, Sepulcre F, Pujolà M. A. vera based edible coatings improve the quality of minimally processed Hayward’ kiwifruit. Postharvest Biol Technol. 2013;81:29–36.
- Aney PM, Rezwan S, Mallavarapu M, Islam MM. Prolonging the shelf life of papaya (Carica papaya L.) using A. vera gel at ambient temperature. Scientia Horticulturae. 2002;265:109228.
- Mart´ınez-Romero D, Alburquerque N, Valverde JM, Guill´en F, Castillo S, Valero D, Serrano M. Postharvest sweet cherry quality and safety maintenance by A. vera treatment: A new edible coating. Postharvest Biol Technol. 2006;39:93–100.
- Fabián G, Huertas MDM, Pedro JZ, Daniel V, María S, Salvador C, Domingo MR. A. arborescens and A. vera gels as coatings in delaying postharvest ripening in peach and plum fruit. Postharvest Biol Technol. 2013;83:54–57.
- Masoud R, Mahmoud KS, Asghar R. Inhibitory effect of salicylic acid and A. vera gel edible coating on microbial load and chilling injury of orange fruit. Scientia Horticulturae. 2019; 247:27–34.
- Sui Chin S, Han Lyn F, Nur HZA. Effect of A. vera (A. barbadensis Miller) gel on the physical and functional properties of fish gelatin films as active packaging. Food Packag Shelf Life. 2017;12:128–134.
- Fariba E, Somayeh R, Preservation of mango fruit with guar-based edible coatings enriched with Spirulina platensis and A. vera extract during storage at ambient temperature. Scientia Horticulturae. 2020;265:109258.
- Muhammad AA, Hira A, Maryem Z, Ali S. Effect of gum arabic and A. vera gel based edible coatings in combination with plant extracts on postharvest quality and storability of ‘Gola’ guava fruits. Scientia Horticulturae. 2020;271:109506.
- Iolanda NL, Ingrid AA, Bernd K, Maribel A, Inmaculada V, Peter M. Combination of ferulic acid with A. vera gel or alginate coatings for shelf-life prolongation of fresh-cut apples. Food Packag Shelf Life. 2021;27:1-11.
- Ergun M, Satici F. Use of A. vera gel as biopreservative for ‘Granny Smith and ‘Red Chief’ apples. J Anim Plant Sci. 2012;22:363–368.
- Valverde JM, Valero D, Martínez-Romero D, Guillén F, Castillo S, Serrano M. Novel edible coating based on aloe vera gel to maintain table grape quality and safety. J Agric Food Chem. 2005 Oct 5;53(20):7807-13. doi: 10.1021/jf050962v. PMID: 16190634..
- Navarro D, Díaz-Mula HM, Guillén F, Zapata PJ, Castillo S, Serrano M, Valero D, Martínez-Romero D. Reduction of nectarine decay caused by Rhizopus stolonifer, Botrytis cinerea and Penicillium digitatum with Aloe vera gel alone or with the addition of thymol. Int J Food Microbiol. 2011 Dec 2;151(2):241-6. doi: 10.1016/j.ijfoodmicro.2011.09.009. Epub 2011 Sep 17. PMID: 21974979.
- Nourozi F, Sayyari M. Enrichment of A. vera gel with basil seed mucilage preserve bioactive compounds and postharvest quality of apricot fruits. Sci Hortic. 2020;262:109041.
- Paladines D, Valero D, Valverde JM, Díaz-Mula H, Serrano M, Martínez-Romero D. The addition of rosehip oil improves the beneficial effect of A. vera gel on delaying ripening and maintaining postharvest quality of several stonefruit. Postharvest Biol Technol. 2014;92:23–28.
- Vieira JM, Flores-López ML, de Rodríguez DJ, Sousa MC, Vicente AA, Martins JT. Effect of chitosan-A. vera coating on postharvest quality of blueberry (Vaccinium corymbosum) fruit. Postharvest Biol Technol. 2016;116: 88–97.
- Chrysargyris A, Nikou A, Tzortzakis N. Effectiveness of A. vera gel coating for maintaining tomato fruit quality. New Zeal. J Crop Hortic Sci. 2016.
- Ali S, Khan AS, Nawaz A, Anjum MA, Naz S, Ejaz S, Hussain S. A. vera gel coating delays postharvest browning and maintains quality of harvested litchi fruit, Postharvest Biol Technol. 2019;157:110960.
- Mirshekari A, Madani B, Golding JA. vera gel treatment delays postharvest browning of white button mushroom (Agaricus bisporus). J Food Meas Charact Published. 2019;13:1250-1256.
- Nasution Z, Ye JNW, Hamzah YY. Characteristics of fresh-cut guava coated with A. vera gel as affected by different additives Kasetsart. J Nat Sci. 2015;49:111–121.
- Lawrence R, Tripathi P, Jeyakumar E. Isolation, Purification and Evaluation of Antibacterial Agents from Aloe vera. Braz J Microbiol. 2009 Oct;40(4):906-15. doi: 10.1590/S1517-838220090004000023. Epub 2009 Dec 1. PMID: 24031440; PMCID: PMC3768575.
- Kedarnath KKM, Chimkod VB, Patil CS. Antimicrobial activity of A. vera leaf extract. Int J Appl Biol Pharm Technol. 2013;4(4):286–290.
- Soumya RP, Sandeep SR, Rubeka I, Vasudha S, Payel G. A review on nutritional, bioactive, toxicological properties and preservation of edible flowers. Future Foods. 2021;4:100078.
- Tom R. Aloes: the genus Aloe. CRC Press LLC. 2004, 24-27.
- O'Brien C, Van Wyk BE, Van Heerden FR. Physical and chemical characteristics of A. ferox leaf gel. South African J Botany. 2011;77:988–995.
- López-Cervantes J. Journal of Applied Research on Medicinal and Aromatic Plants (2018), https://doi.org/10.1016/j.jarmap.2018.02.004.
- Human H, Nicolson SW. Flower structure and nectar availability in Aloe greatheadii var. davyana: an evaluation of a winter nectar source for honeybees. Int J Plant Sci. 2008;169:263–269.
- Rodríguez Rodríguez E, Darias Martín J, Díaz Romero C. Aloe vera as a functional ingredient in foods. Crit Rev Food Sci Nutr. 2010 Apr;50(4):305-26. doi: 10.1080/10408390802544454. PMID: 20301017.
- Food and Drug Administration, HHS. Status of certain additional over-the-counter drug category II and III active ingredients. Final rule. Fed Regist. 2002 May 9;67(90):31125-7. PMID: 12001972.
- Weiyang C, Ben-Erik VW, Ilze V, Alvaro M, Viljoen. Cape Aloes—A review of the phytochemistry, pharmacology and commercialisation of A. ferox: Invited mini review, Phytochemistry Lett. 2012;5:1–12.
- Mulay S. Aloe vera–a review. Int J Pharm. Phytopharmacol Res. 2013;3(3):203-211.
- Committee Herbal Medicional Products (HMPC). Assessment report on A. barbadensis Miller and Aloe (various species, mainly A. ferox Miller and its hybrids). (Doc. Ref: EMEA/HMPC/76313/2006). Evaluation of medicines for human use. European Medicines Agency (EMEA), London. 2007.
- Cordella C, Moussa I, Martel AC, Sbirrazzuoli N, Lizzani-Cuvelier L. Recent developments in food characterization and adulteration detection: technique-oriented perspectives. J Agric Food Chem. 2002 Mar 27;50(7):1751-64. doi: 10.1021/jf011096z. PMID: 11902909.
- Hernadez RJ, Giacin JR. Factors affecting permeation, sorption, and migration processes in package-product systems. CRC Press, Boca Raton, FL. 1998;269–329.
- He Q, Changhong L, Kojo E, Tian Z. Quality and safety assurance in the processing of A. vera gel juice, Food Control. 2005;16:95–104.