More Information
Submitted: 24 July 2020 | Approved: 13 August 2020 | Published: 14 August 2020
How to cite this article: Abd El-Aziz MH, Khalil MS. Antiviral and Antinematodal potentials of chitosan: Review. J Plant Sci Phytopathol. 2020; 4: 055-059.
DOI: 10.29328/journal.jpsp.1001051
Copyright License: © 2020 Abd El-Aziz MH, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Chitosan; Chitin; Virus and plant parasitic nematodes
Antiviral and Antinematodal potentials of chitosan: Review
Mahmoud Hamdy Abd El-Aziz1 and Mohamed Salah Khalil2*
1Plant Pathology Institute, Agricultural. Research Center, Alexandria, Egypt
2Central Agricultural Pesticides Lab., Agricultural Research Center, Alexandria, Egypt
*Address for Correspondence: Dr. Mohamed Salah Khalil, Researcher, Agricultural Research Center, Central Agricultural Pesticides Laboratory, Fungicides, Bactericides and Nematicides Department, Tel: 002/0100-929-7847; Email: melonema@gmail.com
For many years, chemical pesticides have been performed to control different pests and diseases and this may be due to their broad spectrum of action, easy of application and the relatively low cost. But these chemicals have environmental risks, thus alternative control agents are needed. Chitosan is one of the novel suggested solutions to reduce the economic losses associated with chemical pesticides. Chitosan is naturally-occurring compound, as well as safe and biodegradable which obtained from certain natural sources. Chitosan have unique properties which help to control viruses, bacteria, fungi, insects, plant nematodes and other pests locally and systemically.
Chitin and/or chitosan molecules are largely used as safe and environmental-friendly tools to ameliorate crop productivity and conservation of agronomic commodities [1]. In particular, chitosan involved in the production of drugs, cosmetics, biotechnological items, and food have achieved better results using these particular molecules. However, in recent years, the use of modified biopolymer molecules based on chitin and/or chitosan has magnificent advantages for many users [2].
Chitosan has a broad spectrum of unique biological activities especially against viral infections in plants [2,3]. The ability of chitosan and/or its derivatives to suppress viral infections is mediated by its effect on the plant and is most probably determined by its ability to cause plant resistance to infection, in addition to desirable changes in the metabolism of plants and fruits, as well as improving germination and crop yields [4].
Chitosan, which imitates the phytopathogen contact, is known to induce a broad spectrum of defense responses in various plant species [5]. Chitosan and derivatives are not – toxic agent and act as a powerful elicitor for plant responses towards plant diseases locally and systemically to alert healthy parts of the plant [6]. Therefore, in this review we just aimed to throw a light on the importance of chitosan or its derivatives in plant protection fields especially against plant virus and plant parasitic nematodes.
Chitosan processing
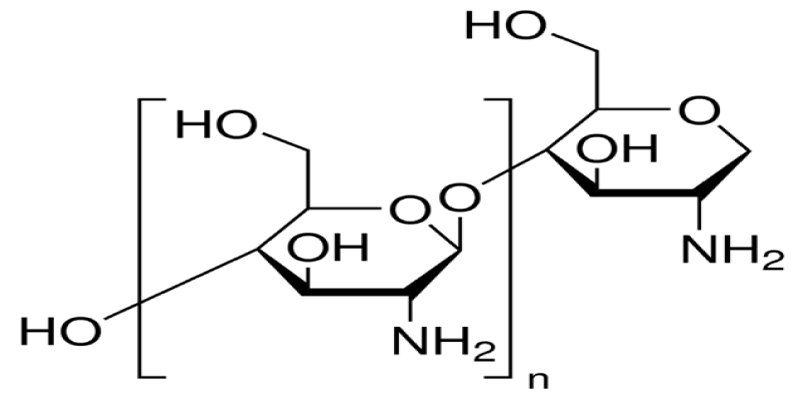
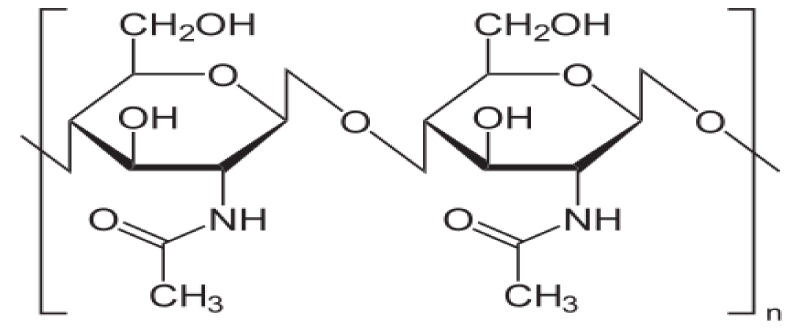
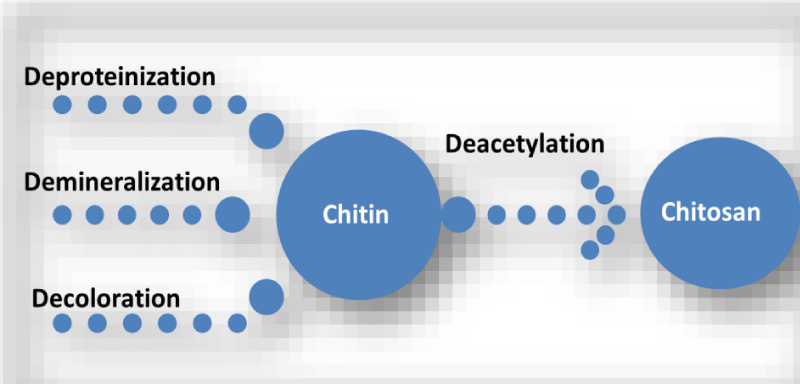
The row material of chitosan (Figure 1) is chitin (Figure 2) which is a biodegradable polymer produced from crab or shrimp shells [7-9]. Recently, chitin or chitosan produced from mycelia of Zygomycetes fungi as alternative sources (Krishnaveni and Ragunathan, 2015 and Khalil, 2016) [9]. The treatment of crustacean shells with alkali solution (NaOH) is mainly remove proteins and this process called Deproteinization, while to remove CaCO3 (Demineralization) from crustacean shells it will treat with acidic solution (HCL), moreover, to eliminate pigments (Decoloration) it was done by using NaOH or potassium permanganate or hydrogen peroxide or sodium hypochlorite [9-13]. After chitin production (Figure 3), the purity must be tested because any remaining impurities might cause problems related to produce products. The using of NaOH again produce chitosan and this treatment is expected to produce 70% of deacetylated chitosan [14].
Figure 1: Chitosan.
Figure 2: Chitin.
Figure 3: The processes of chitosan production from crustacean shells.
Dissolving of chitosan
Normally chitosan is insoluble in water, but soluble in some aqueous acidic solutions. The most widespread solvent to dissolve chitosan is 1% acetic acid (as a reference) at a pH near 4. Also, chitosan is soluble in 1% hydrochloric acid and dilute nitric acid, but is insoluble in sulfuric and phosphoric acids. Acetic acid solution with high concentration at elevated temperature can provide depolymerization of chitosan [15]. There are many important factors that have vital effects on chitosan solubility. These factors can include temperature, alkali concentration, time of deacetylation, prior treatments applied to chitin isolation, ratio of chitin to alkali solution, particle size, etc. [16].
Chitosan significantly has found applications in various fields such as cosmetics, paper, textile and food processing industries, medicine, agriculture, photography, chromatographic separations, wastewater treatment, and solid state batteries [17]. The versatility chitosan in various fields is due to its enviable properties such as biodegradability, biocompatibility, functional groups, low toxicity, renewability, large molecular weight, particle size, density, viscosity, etc. [17]. Chitosan has been recognized as a promising adsorbent [18].
Agricultural applications of chitosan
Chitin and/or chitosan has fungistatic or fungicidal activity against many plant fungal diseases. In soil applications, chitosan was successfully reduced the incidence of Fusarium wilt in several plant species [19-21]. Similarly, chitosan proved efficacy against Cylindrocladium floridanum [22], Alternaria solani [23] and Aspergillus flavus infections [24].
Otherwise, chitosan or its derivatives prevents the growth of several pathogenic bacteria including Xanthomonas [25], Pseudomonas syringae [26], Agrobacterium tumefaciens and Erwinia carotovora [11]. However, the antimicrobial activity of chitosan seems to be higher against fungi than bacteria [27], and among bacteria often been higher against Gram-positive than Gram-negative ones.
The chitinase and chitosanase activity in seeds protected by films of chitin and its derivatives has also been reported by Zargar, et al. [28]. The antimicrobial properties of chitosan and its outstanding film-creating aptitude have been exploited in the post-harvest preservation of fruits and vegetables. Covering fruits and vegetables with a chitosan film grants them antimicrobial protection and enhanced shelf life [29].
Therefore, using of chitin or chitosan in agriculture has four main directions: (1) plant protection against pests and diseases, as well as in preened post-harvest, (2) as microbial biocide (bio-agent) and increase the antagonist microorganism action, (3) support of beneficial plant microorganism symbiotic relationships and (4) plant growth regulation and development. Moreover, biogenic elicitors of natural oligosaccharide are the key of signal molecules in plants [30]. They occur in vivo upon the destruction of cell walls as a result of trauma or phytopathogen invasion and trigger a cascade of local and systemic responses preventing further reproduction of a fungus, bacterium, or virus [31,32].
The antiviral activity of chitosan
Different studies clarified that chitosan prevents the plant viral infection [1,19,33]. Because deamination at the site of polymer chain breakage causes the formation of a new 2, 5-anhydromannose structure, which led to suggest that it is precisely this terminal residue that determines the high activity of deaminated derivatives [34]. Also, the anionic derivatives of chitosan such as chitosan 6-O-sulfate-N-succinate, carboxymethyl chitosan and chitosan sulfate were recorded the lowest activity. Thus, the polycationic properties of chitosan are probably important to its antiviral activity not only toward phage infections but also toward viral infections of plants [34-36]. However, chitosan recorded structurally changes in the particles of phage and damage in their integrity [1].
Chitosan recorded decrement in the number of tobacco mosaic virus particles (Hu, et al. 2009).
Certain studies showed important results about the antiviral impact of chitosan against alfalfa mosaic virus (AMV) on bean [37,38]. It was found that, treatment of the lower surface of a bean leaf with chitosan caused resistance to AMV of its upper surface, the treatment of lower leaves caused resistance in upper leaves, and the treatment of one half of a leaf caused resistance on the other, untreated half. These data suggest that chitosan induces systemic acquired resistance in plants. Chitosan was found to be inhibit the number of local necrosis caused by tobacco mosaic virus especially in low-molecular (Davydova, et al. 2011) and alfalfa mosaic virus [37].
Probably that using chitosan is suppressing the infection irrespective of the type of the virus. Furthermore, plants carrying a dominant gene of resistance to a certain virus are known to respond to inoculation by the formation of local lesions, and the virus usually cannot move beyond them. Such a response of the plant is called the hypersensitivity response.
Chitosan was found to induce the most important molecular markers of systemic acquired resistance in plants such as salicylic acid which is an essential component of distant signaling [39] and pathogenesis related proteins (PR-proteins) [40], as well as, β-1,3-glucanases and chitinases particularly [5].
The induction of antiviral resistance action may not require the penetration of chitosan into plant cells or the vascular system. However, radiolabeled chitosan oligomers with a polymerization degree of ˃ 6 can't move along plant vessels (Pena-Cortes, et al. 1995). On the other hand, insoluble microcrystalline chitosan was as effective as its soluble analogues in inducing antiviral resistance in bean [38].
Nematicidal activity of chitosan
The availability of chemical nematicides commercially have environmental limitations and high toxic for humans. Hence, it was necessary to find new nematicides derived from natural substances or to develop new strategies for managing PPNs [41].
Currently, chitosan is a novel trend which considered a bioactive polymer and widely applied in agricultural systems. Chitosan has precedence due to its functional properties such as anti-bacterial, anti-fungal, anti-viral, and anti-protozoal activities, as well as non-toxic, easy to modify and biodegradation [42,43].
The nematicidal or nematostatic activity of chitosan were proved in crop protection fields. The nematicidal activity of chitosan and its derivatives hasn'tbeen studied enough. However, there are certain investigations confirmed the activity of chitosan against plant parasitic nematodes [41,44-47].
Chitinous materials (included chitosan) were effective in reducing egg hatching and larvae viability of the root-knot nematodes species such as Meloidogyne incognita [46], Meloidogyne javanica [45] and Meloidogyne arenaria [1], in addition to cyst nematode; Heterodera glycines [48].
The molecular weight and concentration of chitosan are considered specified factors for nematicidal activity. Khalil and Badawy [46] were succeeded to prove that low molecular weight of chitosan was more effective than high molecular weight as a natural nematicide. Also, it was found that chitosan combined with some agricultural wastes e.g. mentha, Brassica, onion, groundnut, urad, coconut and corn cob was more effective against Meloidogyne incognita, on eggplant than chitosan alone Asif, et al. 2017. All treatments decreased egg masses, eggs and soil population per 250 g soil.
In spite of the action of chitosan in reducing plant disease are currently not fully understood, but several investigations have shown that chitosan can induce plant resistance to several pathogens by restricting pathogen growth and/or by eliciting several defense mechanisms [19]. There are many reports on combined applications of chitin/chitosan with biotic or abiotic agents. Combining the use of chitin or chitosan with bio-control agents might result in synergistic, additive or antagonistic effects against root-knot nematodes. Also, addition of chitin or chitosan to the soil enhanced the population of chitinolytic microorganisms (relating to the enzyme converting chitin (a polysaccharide) into chitobiose (a disaccharide) that ruin the eggs and cuticles of young nematodes which have chitin in their composition [49].
The indirect effect of chitosan was proved by Asif, et al. (2017) who found that chlorophyll, carotenoid, phenolic content, peroxidase and catalase were induced with chitosan alone or in combination with the agricultural wastes. While, Radwan, et al. [12] reported that chitosan displayed elicitor activity by inducing local and systemic resistance mechanisms of tomato plants against the root-knot nematode, M. incognita.
Finally it could be concluded that chitosan is effective tool against plant virus and nematodes with environmental friendly properties. Also, chitosan is considered to be a promising antimicrobial agent owing to its antibacterial and antifungal activities.
- Jethro Tull. Horse hoeing husbandary, Berkshire. MDCC, 33; 1731.
- Crawley MJ. Biodiversity. In: Crawley, M.J., (ed.) Plant Ecology, 2nd Edn. Blackwell Scientific, Oxford; 1997.
- Aldrich RJ, Kremer RJ. Principles in weed management. Iowa State University Press; 1997.
- Rao VS. Principles of Weed Science, 2nd Edn, Science Publishers, Enfield, New Hampshire, USA; 2000.
- Oudhia P. Medicinal weeds in rice fields of Chhattisgarh, India. International Rice Research Notes. 1999; 24:40.
- Gibbons DW, Bohan DA, Rothery P, Stuart RC, Haughton AJ, et al. Weed seed resources for birds in fields with contrasting conventional and genetically modified herbicide-tolerant crops. Proceedings of the Royal Society B: Biological Sciences. 2006; 273: 1921-1928. PubMed: PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16822753
- Hawes C, Haughton AJ, Bohan DA, Squire GR. Functional approaches for assessing plant and invertebrate abundance patterns in arable systems. Basic and Applied Ecology. 2009; 10: 34-42.
- Kromp B. Carabid beetles in sustainable agriculture: a review on pest control efficacy, cultivation impacts and enhancement. Agriculture, Ecosystems & Environment. 1999; 74: 187-228.
- Evans CL. The war on weeds in the Prairie west: an environmental history. University of Calgary Press; 2002.
- Popay I, Champion P, James T. An Illustrated Guide to Common Weeds of New Zealand. 3rd Edn. New Zealand Plant Protection Society. 2010.
- Hsieh YS, Harris PJ. Structures of xyloglucans in primary cell walls of gymnosperms, monilophytes (ferns sensu lato) and lycophytes. Phytochemistry. 2012; 79: 87-101.
- Rasanen RM, Hieta JP, Immanen J, Nieminen K, Haavikko R, et al. Chemical profiles of birch and alder bark by ambient mass spectrometry. Anal Bioanal Chem. 2019; 411: 7573-7583.
- Collett MG, Taylor SM. Photosensitising toxins in alligator weed (Alternanthera philoxeroides) likely to be anthraquinones. Toxicon. 2019; 167: 172-173. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31226258
- Alipieva K, Evstatieva L, Handjieva N, Popova S. Comparative analysis of the composition of flower volatiles from Lamium L. species and Lamiastrum galeobdolon Heist. ex Fabr. Zeitschrift fur Naturforschung C. 2003; 58: 779-782.
- Masi M, Di Lecce R, Tuzi A, Linaldeddu BT, Montecchio L, et al. Hyfraxinic Acid, a Phytotoxic Tetrasubstituted Octanoic Acid, Produced by the Ash (Fraxinus excelsior L.) Pathogen Hymenoscyphus fraxineus Together with Viridiol and Some of Its Analogues. J Agric Food Chem. 2019; 67: 13617-13623. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31661270
- Metlicar V, Vovk I, Albreht A. Japanese and Bohemian Knotweeds as Sustainable Sources of Carotenoids. Plants. 2019; 8: 384. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6843863/
- Alamzeb M, Omer M, Ur-Rashid M, Raza M, Ali S, et al. NMR, Novel Pharmacological and In Silico Docking Studies of Oxyacanthine and Tetrandrine: Bisbenzylisoquinoline Alkaloids Isolated from Berberis glaucocarpa Roots. J Anal Methods Chem. 2018; 7692913. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29888027
- Kitryte V, Narkeviciute A, Tamkute L, Syrpas M, Pukalskiene M, et al. Consecutive high-pressure and enzyme assisted fractionation of blackberry (Rubus fruticosus L.) pomace into functional ingredients: Process optimization and product characterization. Food Chem. 2020; 312: 126072. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31893552
- Weretilnyk EA, Bednarek S, McCue KF, Rhodes D, Hanson AD. Comparative biochemical and immunological studies of the glycine betaine synthesis pathway in diverse families of dicotyledons. Planta. 1989; 178: 342-352.
- Mithofer A, Reichelt M, Nakamura Y. Wound and insect‐induced jasmonate accumulation in carnivorous Drosera capensis: two sides of the same coin. Plant Biol. 2014; 16: 982-987. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24499476
- Małajowicz J, Kusmirek S. Structure and properties of ricin–the toxic protein of Ricinus communis. Postepy Biochemii. 2019; 65: 03-108. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31642648
- Cruz-Salas CN, Prieto C, Calderon-Santoyo M, Lagarón JM, Ragazzo-Sanchez JA. Micro-and Nanostructures of Agave Fructans to Stabilize Compounds of High Biological Value via Electrohydrodynamic Processing. Nanomaterials. 2019; 9:1659. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31766573
- Sendker J, Ellendorff T, Holzenbein A. Occurrence of benzoic acid esters as putative catabolites of prunasin in senescent leaves of Prunus laurocerasus. J Nat Prod. 2016; 79: 1724-1729. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27331617
- Bridi R, Giordano A, Penailillo MF, Montenegro G. Antioxidant Effect of Extracts from Native Chilean Plants on the Lipoperoxidation and Protein Oxidation of Bovine Muscle. Molecules. 2019; 24: 3264. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31500282
- Ouyang MA, He ZD, Wu CL. Anti-oxidative activity of glycosides from Ligustrum sinense. Natural product research. 2003; 17: 381-387. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/14577686
- Ben Ammar R, Miyamoto T, Chekir-Ghedira L, Ghedira K, Lacaille-Dubois MA. Isolation and identification of new anthraquinones from Rhamnus alaternus L and evaluation of their free radical scavenging activity. Natural product research. 2019; 33: 280-286. PubMed: https://pubmed.ncbi.nlm.nih.gov/29533086/
- Matouskova M, Jurova J, Grulova D, Wajs-Bonikowska A, Renco M, Sedlak V, Poracova J, Gogalova Z, Kalemba D. Phytotoxic Effect of Invasive Heracleum mantegazzianum Essential Oil on Dicot and Monocot Species. Molecules. 2019; 24: 425. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6384721/
- Lachowicz S, Oszmianski J. Profile of Bioactive Compounds in the Morphological Parts of Wild Fallopia japonica (Houtt) and Fallopia sachalinensis (F. Schmidt) and Their Antioxidative Activity. Molecules. 2019; 24: 1436. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30979044
- Liu QR, Li J, Zhao XF, Xu B, Xiao XH, et al. Alkaloids and phenylpropanoid from Rhizomes of Arundo donax L. Natural Product Res. 2019: 1-6.
- Ikbal C, Habib B, Hichem BJ, Monia BH, Habib BH, et al. Purification of a natural insecticidal substance from Cestrum parqui (Solanaceae). Pak J Biol Sci. 2007; 10: 3822-3828. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/19090236
- Mandim F, Barros L, Heleno SA, Pires TC, Dias MI, Alves MJ, Santos PF, Ferreira IC. Phenolic profile and effects of acetone fractions obtained from the inflorescences of Calluna vulgaris (L.) Hull on vaginal pathogenic and non-pathogenic bacteria. Food & Function. 2019; 10: 2399-2407. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31049501
- Zhang T, Liu H, Bai X, Liu P, Yang Y, et al. Fractionation and antioxidant activities of the water-soluble polysaccharides from Lonicera japonica Thunb. International journal of biological macromolecules. 2019. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31739015
- Matthaus B, Ozcan MM. Fatty acid, tocopherol and squalene contents of Rosaceae seed oils. Botanical studies. 2014; 55:48. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5432826/
- Jo MS, Yu JS, Lee JC, Lee S, Cho YC, et al. Lobatamunsolides A–C, Norlignans from the Roots of Pueraria lobata and their Nitric Oxide Inhibitory Activities in Macrophages. Biomolecules. 2019; 9:755. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31757072
- Fu Y, Li F, Ding Y, Li HY, Xiang XR, et al. Polysaccharides from loquat (Eriobotrya japonica) leaves: Impacts of extraction methods on their physicochemical characteristics and biological activities. Int J Biol Macromolecules. 2020. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31923490
- Chu MJ, Du YM, Liu XM, Yan N, Wang FZ, et al. Extraction of proanthocyanidins from chinese wild rice (zizania latifolia) and analyses of structural composition and potential bioactivities of different fractions. Molecules. 2019; 24:1681. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31052148
- Dong LM, Zhang M, Xu QL, Zhang Q, Luo B, et al. Two new thymol derivatives from the roots of Ageratina adenophora. Molecules. 2017; 22: 592. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6154539/
- Zhang ZP, Shen CC, Gao FL, Wei H, Ren DF, et al. Isolation, purification and structural characterization of two novel water-soluble polysaccharides from Anredera cordifolia. Molecules. 2017; 22: 1276. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28769023
- Priolo N, Del Valle SM, Arribere MC, López L, Caffini N. Isolation and characterization of a cysteine protease from the latex of Araujia hortorum fruits. Journal of Protein Chemistry. 2000; 19: 39-49.
- Akihara Y, Kamikawa S, Harauchi Y, Ohta E, Nehira T, et al. HPLC profiles and spectroscopic data of cassane-type furanoditerpenoids. Data in brief. 2018; 21:1076-88. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30450403
- Jordheim M, Calcott K, Gould KS, Davies KM, Schwinn KE, et al. High concentrations of aromatic acylated anthocyanins found in cauline hairs in Plectranthus ciliatus. Phytochemistry. 2016; 128:27-34. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27165277
- Boppre M, Colegate SM. Recognition of pyrrolizidine alkaloid esters in the invasive aquatic plant Gymnocoronis spilanthoides (Asteraceae). Phytochemical Analysis. 2015; 26: 215-225.
- Du YQ, Yan ZY, Chen JJ, Wang XB, Huang XX, et al. The identification of phenylpropanoids isolated from the root bark of Ailanthus altissima (Mill.) Swingle. Nat Product Res. 2019: 1-8. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31315448
- El-Tantawy ME, Shams MM, Afifi MS. Chemical composition and biological evaluation of the volatile constituents from the aerial parts of Nephrolepis exaltata (L.) and Nephrolepis cordifolia (L.) C. Presl grown in Egypt. Natural product research. 2016; 30: 1197-201. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26211503
- Matsuda H, Nakashima S, Abdel-Halim OB, Morikawa T, Yoshikawa M. Cucurbitane-type triterpenes with anti-proliferative effects on U937 cells from an egyptian natural medicine, Bryonia cretica: structures of new triterpene glycosides, bryoniaosides A and B. Chemical and Pharmaceutical Bulletin. 2010; 58: 747-51. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20460809
- Kisielius V, Lindqvist DN, Thygesen MB, Rodamer M, Hansen HC, Rasmussen LH. Fast LC-MS quantification of ptesculentoside, caudatoside, ptaquiloside and corresponding pterosins in bracken ferns. J Chromatography B. 2020: 121966. PubMed: https://pubmed.ncbi.nlm.nih.gov/31931331
- Shulha O, Çiçek SS, Wangensteen H, Kroes J, Mäder M, et al. Lignans and sesquiterpene lactones from Hypochaeris radicata subsp. neapolitana (Asteraceae, Cichorieae). Phytochemistry. 2019; 165: 112047.
- Khan WN, Lodhi MA, Ali I, Azhar-Ul-Haq, Malik A, et al. New natural urease inhibitors from Ranunculus repens. J Enzyme Inhibition Med Chem. 2006; 21: 17-19. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/16570500
- Neag T, Olah NK, Hanganu D, Benedec D, Pripon FF, et al. The anemonin content of four different Ranunculus species. Pakistan J Pharmaceutical Sci. 2018; 31: 2027-2032. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30393208
- Mabona U, Viljoen A, Shikanga E, Marston A, Van Vuuren S. Antimicrobial activity of southern African medicinal plants with dermatological relevance: from an ethnopharmacological screening approach, to combination studies and the isolation of a bioactive compound. J Ethnopharmacol. 2013; 148: 45-55. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23545456
- You Y, Kim K, Yoon HG, Lee KW, Lee J, et al. Chronic effect of ferulic acid from Pseudosasa japonica leaves on enhancing exercise activity in mice. Phytotherapy Res. 2010; 24: 1508-1513. PubMed: https://pubs.acs.org/doi/abs/10.1021/jf010514x
- Matos P, Figueirinha A, Ferreira I, Cruz MT, Batista MT. Acanthus mollis L. leaves as source of anti-inflammatory and antioxidant phytoconstituents. Natural product research. 2019; 33: 1824-1827. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29417845
- Santos-Rebelo A, Garcia C, Eleuterio C, Bastos A, Castro Coelho S, et al. Development of Parvifloron D-Loaded Smart Nanoparticles to Target Pancreatic Cancer. Pharmaceutics. 2018; 10: 216. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6321128/
- Feng X, Wang X, Liu Y, Di X. Linarin inhibits the acetylcholinesterase activity in-vitro and ex-vivo. Iranian journal of pharmaceutical research: IJPR. 2015; 14: 949. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4518125/
- Saini NK, Singha M. Anti–inflammatory, analgesic and antipyretic activity of methanolic Tecomaria capensis leaves extract. Asian Pac J Trop Biomed. 2012; 2: 870-874. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3609241/
- Aboutabl EA, Hashem FA, Sleem AA, Maamoon AA. Flavonoids, anti-inflammatory activity and cytotoxicity of Macfadyena unguis-cati L. Afr J Tradit Complement Altern Med. 2008; 5: 18-26. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2816596/
- Park SH, Jang S, Lee SW, Park SD, Sung YY, et al. Akebia quinata Decaisne aqueous extract acts as a novel anti-fatigue agent in mice exposed to chronic restraint stress. J Ethnopharmacol. 2018; 222: 270-279. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29630998
- Guesmi F, Hmed MB, Prasad S, Tyagi AK, Landoulsi A. in vivo pathogenesis of colon carcinoma and its suppression by hydrophilic fractions of Clematis flammula via activation of TRAIL death machinery (DRs) expression. Biomedicine & Pharmacotherapy. 2019; 109: 2182-2191.
- Chu Z, Wang H, Ni T, Tao L, Xiang L, et al. 28-Hydroxy-3-oxoolean-12-en-29-oic Acid, a Triterpene Acid from Celastrus orbiculatus Extract, Inhibits the Migration and Invasion of Human Gastric Cancer Cells in vitro. Molecules. 2019; 24: 3513. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31569766
- Habtemariam S. The therapeutic potential of Berberis darwinii stem-bark: quantification of berberine and in vitro evidence for Alzheimer's disease therapy. Nat Prod Commun. 2011; 6: 1089-1090. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/21922905
- Topolska J, Kostecka-Gugała A, Ostachowicz B, Latowski D. Selected metal content and antioxidant capacity of Sambucus nigra flowers from the urban areas versus soil parameters and traffic intensity. Environ Sci Pollut Res Int. 2020; 27: 668-677. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31808083
- Packer J, Naz T, Harrington D, Jamie JF, Vemulpad SR. Antimicrobial activity of customary medicinal plants of the Yaegl Aboriginal community of northern New South Wales, Australia: a preliminary study. BMC Res Notes. 2015; 8: 276. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26122212
- Kim HS, Jang JM, Yun SY, Zhou D, Piao Y, et al. Effect of Robinia pseudoacacia Leaf Extract on Interleukin-1β–mediated Tumor Angiogenesis. in vivo. 2019; 33: 1901-1910. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31662518
- Nawwar M, Swilam N, Hashim A, Al-Abd A, Abdel-Naim A, et al. Cytotoxic isoferulic acidamide from Myricaria germanica (Tamaricaceae). Plant Signal Behav. 2013; 8: e22642. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3745567/
- Patova OA, Smirnov VV, Golovchenko VV, Vityazev FV, Shashkov AS, et al. Structural, rheological and antioxidant properties of pectins from Equisetum arvense L. and Equisetum sylvaticum L. Carbohydrate Polymers. 2019; 209: 239-249.
- Calderon-Montano JM, Martinez-Sanchez SM, Burgos-Moron E, Guillen-Mancina E, Jimenez-Alonso JJ, et al. Screening for selective anticancer activity of plants from Grazalema Natural Park, Spain. Nat Prod Res. 2019; 33: 3454345-8. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29842791
- Pires AS, Rigueiras PO, Dohms SM, Porto WF, Franco OL. Structure‐guided identification of antimicrobial peptides in the spathe transcriptome of the non‐model plant, arum lily (Zantedeschia aethiopica). Chem Biol Drug Design. 2019; 93: 1265-1275.
- Radulovic N, Denic M, Stojanovic-Radic Z. Antimicrobial phenolic abietane diterpene from Lycopus europaeus L.(Lamiaceae). Bioorg Med Chem Lett. 2010; 20: 4988-4891. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20674349
- Chen X, Li T, Qing D, Chen J, Zhang Q, et al. Structural characterization and osteogenic bioactivities of a novel Humulus lupulus polysaccharide. Food Function. 2020; 11: 1165-1175. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31872841
- Bournine L, Bensalem S, Wauters JN, Iguer-Ouada M, Maiza-Benabdesselam F, et al.. Identification and quantification of the main active anticancer alkaloids from the root of Glaucium flavum. Int J Mol Sci. 2013; 14: 23533-23544. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3876061/
- Basic M, Elgner F, Bender D, Sabino C, Herrlein ML, et al. A synthetic derivative of houttuynoid B prevents cell entry of Zika virus. Antiviral Res. 2019; 172: 104644. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31697958
- Akhtar M, Shaukat A, Zahoor A, Chen Y, Wang Y, et al.. Anti-inflammatory effects of Hederacoside-C on Staphylococcus aureus induced inflammation via TLRs and their downstream signal pathway in vivo and in vitro. Microbial Pathogenesis. 2019; 137: 103767. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31580956
- García ME, Borioni JL, Cavallaro V, Puiatti M, Pierini AB, et al. Solanocapsine derivatives as potential inhibitors of acetylcholinesterase: Synthesis, molecular docking and biological studies. Steroids. 2015; 104: 95-110. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26362598
- Dougnon G, Ito M. Sedative effects of the essential oil from the leaves of Lantana camara occurring in the Republic of Benin via inhalation in mice. J Nat Med. 2020; 74: 159-169. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31446559
- Hsu CL, Fang SC, Yen GC. Anti-inflammatory effects of phenolic compounds isolated from the flowers of Persicaria hydropiperPersicaria hydropiper Zucc. Food & function. 2013; 4: 1216-1222. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23727892
- Ticona LA, SAnchez AR, GonzAles OO, Domenech MO. Antimicrobial compounds isolated from Tropaeolum tuberosum. Natural Product Research. 2020: 1-5. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31913056
- Luis A, Breitenfeld L, Ferreira S, Duarte AP, Domingues F. Antimicrobial, antibiofilm and cytotoxic activities of Hakea sericea Schrader extracts. Pharmacognosy magazine. 2014; 10: S6. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24914310
- Yesilada E, Kupeli E. Clematis vitalba L. aerial part exhibits potent anti-inflammatory, antinociceptive and antipyretic effects. J Ethnopharmacol. 2007; 110: 504-515.
- Amabeoku GJ, Green I, Kabatende J. Anticonvulsant activity of Cotyledon orbiculata L.(Crassulaceae) leaf extract in mice. J Ethnopharmacol. 2007; 112: 101-107. PubMed: https://pubmed.ncbi.nlm.nih.gov/17398051
- Neuman MG, Jia AY, Steenkamp V. Senecio latifolius induces in vitro hepatocytotoxicity in a human cell line. Canadian journal of physiology and pharmacology. 2007; 85: 1063-1075.
- dos Santos Alves CF, Bonez PC, de Souza MD, da Cruz RC, Boligon AA, et al. Antimicrobial, antitrypanosomal and antibiofilm activity of Equisetum hyemale. Microbial pathogenesis. 2016; 101: 119-125. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27856271
- Schmidt M, Skaf J, Gavril G, Polednik C, Roller J, et al. The influence of Osmunda regalis root extract on head and neck cancer cell proliferation, invasion and gene expression. BMC Complement Altern Med. 2017; 17: 518. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5716017/
- Tian G, Chen J, Luo Y, Yang J, Gao T, Shi J. Ethanol extract of Ligustrum lucidum Ait. leaves suppressed hepatocellular carcinoma in vitro and in vivo. Cancer Cell Int. 2019; 19: 246. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31572063
- Saddiqe Z, Maimoona A, Abbas G, Naeem I, Shahzad M. Pharmacological screening of Hypericum androsaemum extracts for antioxidant, anti-lipid peroxidation, antiglycation and cytotoxicity activity. Pakistan J Pharmaceut Sci. 2016; 29.
- Tiana C, Yang C, Zhang D, Han L, Liu Y, et al. Antibacterial and antioxidant properties of various solvents extracts of Abutilon theophrasti Medic. leaves. Pak J Pharmaceut Sci. 2017; 30. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/28653920
- Ling B, Xiao S, Yang J, Wei Y, Sakharkar MK, et al. Probing the Antitumor Mechanism of Solanum nigrum L. Aqueous Extract against Human Breast Cancer MCF7 Cells. Bioengineering. 2019; 6: 112. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31835887
- Aghajanyan A, Nikoyan A, Trchounian A. Biochemical activity and hypoglycemic effects of Rumex obtusifolius L. seeds used in Armenian traditional medicine. BioMed research international. 2018; 2018.
- Boniface PK, Verma S, Shukla A, Cheema HS, Srivastava SK, et al. Bioactivity-guided isolation of antiplasmodial constituents from Conyza sumatrensis (Retz.) EH Walker. Parasitol Int. 2015; 64: 118-123. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25449289
- Genc Y, Dereli FT, Saracoglu I, Akkol EK. The inhibitory effects of isolated constituents from Plantago major subsp. major L. on collagenase, elastase and hyaluronidase enzymes: Potential wound healer. Saudi Pharmaceut J. 2020; 28: 101-106. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31920436
- Rogozhin EA, Slezina MP, Slavokhotova AA, Istomina EA, Korostyleva TV, et al. A novel antifungal peptide from leaves of the weed Stellaria media L. Biochimie. 2015; 116: 125-132. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/26196691
- Ilina T, Kashpur N, Granica S, Bazylko A, Shinkovenko I, et al. Phytochemical Profiles and in vitro Immunomodulatory Activity of Ethanolic Extracts from Galium aparine L. Plants. 2019; 8: 541. PubMed: https://pubmed.ncbi.nlm.nih.gov/31775336
- Aremu OO, Oyedeji AO, Oyedeji OO, Nkeh-Chungag BN, Rusike CR. in vitro and in vivo Antioxidant Properties of Taraxacum officinale in Nω-Nitro-l-Arginine Methyl Ester (L-NAME)-Induced Hypertensive Rats. Antioxidants. 2019; 8: 309. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31443195
- Jahan S, Azad T, Ayub A, Ullah A, Afsar T, et al. Ameliorating potency of Chenopodium album Linn. and vitamin C against mercuric chloride-induced oxidative stress in testes of Sprague Dawley rats. Environ Health Prevent Med. 2019; 24: 62.
- Parzonko A, Kiss AK. Caffeic acid derivatives isolated from Galinsoga parviflora herb protected human dermal fibroblasts from UVA-radiation. Phytomedicine. 2019; 57: 215-22. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30785017
- Di Sotto A, Di Giacomo S, Toniolo C, Nicoletti M, Mazzanti G. Sisymbrium Officinale (L.) Scop. and its polyphenolic fractions inhibit the mutagenicity of Tert‐butylhydroperoxide in Escherichia Coli WP2uvrAR strain. Phytotherapy Res. 2016; 30: 829-834.
- Di Napoli M, Varcamonti M, Basile A, Bruno M, Maggi F, et al. Anti-Pseudomonas aeruginosa activity of hemlock (Conium maculatum, Apiaceae) essential oil. Nat Prod Res. 2019; 33: 3436-3440.
- Song SY, Hyun JE, Kang JH, Hwang CY. in vitro antibacterial activity of the manuka essential oil from Leptospermum scoparium combined with Tris‐EDTA against Gram‐negative bacterial isolates from dogs with otitis externa. Vet Dermatol. 2019.
- Doukkali Z, Taghzouti K, Bouidida EH, Nadjmouddine M, Cherrah Y, et al. Evaluation of anxiolytic activity of methanolic extract of Urtica urens in a mice model. Behav Brain Funct. 2015; 11: 19. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4423131/
- Farid O, Zeggwagh NA, Ouadi FE, Eddouks M. Mentha pulegium Aqueous Extract Exhibits Antidiabetic and Hepatoprotective Effects in Streptozotocin-induced Diabetic Rats. Endocrine, Metabolic & Immune Disorders-Drug Targets (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders). 2019; 19: 292-301. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/30289084
- Bubueanu C, Iuksel R, Panteli M. Haemostatic activity of butanolic extracts of Lamium album and Lamium purpureum aerial parts. Acta Pharmaceutica. 2019; 69: 443-449. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31259737
- Ibrahim AM, Ghoname SI. Molluscicidal impacts of Anagallis arvensis aqueous extract on biological, hormonal, histological and molecular aspects of Biomphalaria alexandrina snails. Experimental Parasitology. 2018; 192: 36-41.
- Fernandez-Martinez E, Jimenez-Santana M, Centeno-Alvarez M, Torres-Valencia JM, Shibayama M, et al. Hepatoprotective effects of nonpolar extracts from inflorescences of thistles Cirsium vulgare and Cirsium ehrenbergii on acute liver damage in rat. Pharmacognosy magazine. 2017; 13: S860. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5822512/
- Sharifi-Rad J, Iriti M, Setzer WN, Sharifi-Rad M, Roointan A, et al. Antiviral activity of Veronica persica Poir. on herpes virus infection. Cell Mol Biol. 2018; 64: 11-17. PubMed: https://pubmed.ncbi.nlm.nih.gov/29981678
- Li BY, Hu Y, Li J, Shi K, Shen YF, et al. Ursolic acid from Prunella vulgaris L. efficiently inhibits IHNV infection in vitro and in vivo. Virus Res. 2019; 273: 197741.
- Torres-GonzAlez L, Cienfuegos-Pecina E, Perales-Quintana MM, Alarcon-Galvan G, Munoz-Espinosa LE, et al. Nephroprotective effect of Sonchus oleraceus extract against kidney injury induced by ischemia-reperfusion in wistar rats. Oxidative medicine and cellular longevity. 2018; 2018: 9572803. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29643981
- Ayaz M, Ahmad I, Sadiq A, Ullah F, Ovais M, Khalil AT, Devkota HP. Persicaria hydropiper (L.) Delarbre: A review on traditional uses, bioactive chemical constituents and pharmacological and toxicological activities. Journal of Ethnopharmacology. 2019: 112516. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31884037
- Guz L, Adaszek L, Wawrzykowski J, Zietek J, Winiarczyk S. in vitro antioxidant and antibabesial activities of the extracts of Achillea millefolium. Polish journal of veterinary sciences. 2019: 369-376. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31269341
- Mahdavi S, Amiradalat M, Babashpour M, Sheikhlooei H, Miransari M. The antioxidant, anticarcinogenic and antimicrobial properties of Verbascum thapsus L. Medicinal chemistry (Shariqah (United Arab Emirates)). 2019. PubMed: https://pubmed.ncbi.nlm.nih.gov/31456524
- Oszmianski J, Wojdyło A, Juszczyk P, Nowicka P. Roots and Leaf Extracts of Dipsacus fullonum L. and Their Biological Activities. Plants. 2020; 9: 78. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31936189
- Kim J, Jung KH, Ryu HW, Kim DY, Oh SR, et al. Apoptotic Effects of Xanthium strumarium via PI3K/AKT/mTOR Pathway in Hepatocellular Carcinoma. Evidence-Based Complementary and Alternative Medicine. 2019; 2019.
- Shiomi N, editor. Advances in Bioremediation and Phytoremediation. BoD–Books on Demand; 2018.
- Macci C, Peruzzi E, Doni S, Iannelli R, Masciandaro G. Ornamental plants for micropollutant removal in wetland systems. Environ Sci Pollut Res. 2015; 22: 2406-2415. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/24798922
- Augustynowicz J, Lukowicz K, Tokarz K, Płachno BJ. Potential for chromium (VI) bioremediation by the aquatic carnivorous plant Utricularia gibba L. (Lentibulariaceae). Environ Sci Pollut Res. 2015; 22: 9742-9748.
- Cui X, Fang S, Yao Y, Li T, Ni Q, et al. Potential mechanisms of cadmium removal from aqueous solution by Canna indica derived biochar. Sci Total Environ. 2016; 562: 517-525. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/27107650
- Wechtler L, Laval-Gilly P, Bianconi O, Walderdorff L, Bonnefoy A, et al. Trace metal uptake by native plants growing on a brownfield in France: zinc accumulation by Tussilago farfara L. Environ Sci Pollut Res . 2019; 26: 36055-36062
- Maleva M, Garmash E, Chukina N, Malec P, Waloszek A, et al. Effect of the exogenous anthocyanin extract on key metabolic pathways and antioxidant status of Brazilian elodea (Egeria densa (Planch.) Casp.) exposed to cadmium and manganese. Ecotoxicol Environ Safety. 2018; 160: 197-206.
- Marchand L, Lamy P, Bert V, Quintela-Sabaris C, Mench M. Potential of Ranunculus acris L. for biomonitoring trace element contamination of riverbank soils: photosystem II activity and phenotypic responses for two soil series. Environ Sci Pollut Res . 2016; 23: 3104-3119. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25956517
- Pardo-Muras M, G Puig C, Pedrol N. Cytisus scoparius and Ulex europaeus Produce Volatile Organic Compounds with Powerful Synergistic Herbicidal Effects. Molecules. 2019; 24: 4539. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6943486/
- Salinitro M, Tassoni A, Casolari S, de Laurentiis F, Zappi A, et al. Heavy Metals Bioindication Potential of the Common Weeds Senecio vulgaris L., Polygonum aviculare L. and Poa annua L. Molecules. 2019; 24: 2813. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31374997
- Guo X, Wang P, Li Y, Zhong H, Li P, et al. Effect of copper on the removal of tetracycline from water by Myriophyllum aquaticum: Performance and mechanisms. Bioresource Technol. 2019; 291: 121916. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31377514
- Noori A, Zare Maivan H, Alaie E, Newman LA. Leucanthemum vulgare Lam. crude oil phytoremediation. Int J Phytoremediation. 2018; 20: 1292-1299. PubMed: https://pubmed.ncbi.nlm.nih.gov/26121329
- Yu X, Brown JM, Graham SE, Carbajal EM, Zuleta MC, Milla-Lewis SR. Detection of quantitative trait loci associated with drought tolerance in St. Augustinegrass. PloS one. 2019; 14. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/31671135
- Alur HH, Pather SI, Mitra AK, Johnston TP. Evaluation of the gum from Hakea gibbosa as a sustained-release and mucoadhesive component in buccal tablets. Pharmaceutical development and technology. 1999; 4: 347-358. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/10434280
- Keshari AK, Srivastava R, Singh P, Yadav VB, Nath G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J Ayurveda Integrative Med. 2018.
- Hussain N, Abbasi T, Abbasi SA. Generation of highly potent organic fertilizer from pernicious aquatic weed Salvinia molesta. Environ Sci Pollut Res Int. 2018; 25: 4989-5002. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/29209963
- Balsamo RA, Willigen CV, Bauer AM, Farrant J. Drought tolerance of selected Eragrostis species correlates with leaf tensile properties. Ann Botany. 2006; 97: 985-991.
- Zhou Y, Lambrides CJ, Kearns R, Ye C, Fukai S. Water use, water use efficiency and drought resistance among warm-season turfgrasses in shallow soil profiles. Functional plant biology. 2012; 39: 116-125. PubMed: https://www.publish.csiro.au/fp/FP11244
- Baracho NC, Vicente BB, Arruda GD, Sanches BC, Brito JD. Study of acute hepatotoxicity of Equisetum arvense L. in rats. Acta Cirurgica Brasileira. 2009; 24: 449-453. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/20011829/
- Slaughter RJ, Beasley DM, Lambie BS, Wilkins GT, Schep LJ. Poisonous plants in New Zealand: a review of those that are most commonly enquired about to the National Poisons Centre. N Z Med J. 2012; 125: 87-118. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/23321887
- Bregnbak D, Menné T, Johansen JD. Airborne contact dermatitis caused by common ivy (Hedera helix L. ssp. helix). Contact Dermatitis. 2015; 72: 243-244. PubMed: https://www.ncbi.nlm.nih.gov/pubmed/25630853
- Pour BM, Sasidharan S. in vivo toxicity study of Lantana camara. Asian Pac J Trop Biomed. 2011; 1: 230-232. PubMed: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3609184/
- Botha CJ, Lessing D, Rösemann M, Van Wilpe E, Williams JH. Analytical confirmation of Xanthium strumarium poisoning in cattle. J Vet Diagn Invest. 2014; 26: 640-645. PubMed: https://pubmed.ncbi.nlm.nih.gov/25012081