1. INTRODUCTION
Cobalt (Co) is a crucial element for metallurgical applications (e.g., specialty steels, jet engines) and the production of rechargeable lithium-ion batteries designed to face the needs of a transition to a low-carbon economy and ambitions of space exploration (Horn et al., 2021; Petavratzi et al., 2019). Whereas 66% of the global mine production of cobalt in 2018 came as a by-product from moderate-size to giant sedimentary rock-hosted copper sulfide deposits in Central Africa (0.03–1.08% Co; Petavratzi et al., 2019), the high-grade, lens-and-vein-type Bou Azzer deposit in Morocco remains the only major orebody in the world where cobalt is exploited as the main commodity (fig. 1A, 1B, ca. 120,000 metric tons per year, 1% Co; Petavratzi et al., 2019). Yet, the origin of such a Co resource comprising arsenide minerals (e.g., safflorite – CoAs2, 21.25 weight percent [wt.%] Co; skutterudite – CoAs3, 17.95 wt.% Co) remains highly debated given controversial genetic models. Published evidence from mineralogy, mineral chemistry, and fluid inclusion microthermometry and chemistry (Ahmed et al., 2009; En-Naciri et al., 1997; Essarraj et al., 2005; Leblanc & Billaud, 1982) were reconciled in a general model for Bou Azzer-type mineralization. This model involves the dynamic and fast reduction of arsenic- and cobalt-nickel-iron-(Co-Ni-Fe)-bearing hydrothermal fluids, and controls exerted by pH variations on mineral precipitation and on the extent of Co-Ni endowment (Markl et al., 2016). However, the timing of arsenide mineralization and geodynamic setting for ore genesis remain unresolved but are paramount for a consensus on the secular distribution of Bou Azzer-type Co resources in Earth history.
The Bou Azzer Co-Ni arsenide orebodies are located in the Bou Azzer-El Graara window (‘i.e.’, “inlier”) that forms part of the Anti-Atlas Pan-African orogenic belt (fig. 1A; Gasquet et al., 2005). The inlier, which was thrust onto the Paleoproterozoic West African Craton, represents a fragment of Tonian (ca. 759 million years ago, Ma) serpentinized oceanic lithosphere and Tonian to Cryogenian (ca. 760 to 690 Ma) intra-oceanic arc lithologies intruded by Cryogenian quartz diorite (ca. 660 Ma; Hodel et al., 2020; Triantafyllou et al., 2018). Elongated lenses of Co-Ni-Fe arsenides and their carbonate ± quartz envelopes (fig. 2A) are hosted in steep fault zones between serpentinite and diorite (fig. 1C, 2B), whereas Co-Ni-Fe arsenides vein systems occur within serpentinite and diorite (fig. 2C, 2D; Tourneur et al., 2021). Previous age estimates for the timing of arsenide mineralization have ranged from late Pan-African (~ 685–580 Ma; Leblanc, 1981) to late Variscan (ca. 310 Ma) on the basis of samarium-neodymium (Sm-Nd) geochronology of bulk calcite envelope and in situ uranium-lead geochronology of brannerite (UTi2O6) inclusions in calcite (Oberthür et al., 2009). Complex mineralizing processes have been invoked to explain the presence of calcite with different initial 143Nd/144Nd values. Therefore, it cannot be excluded that the ca. 308 Ma date of calcite may signal a rejuvenation of the Sm-Nd systematics in calcite (Oberthür et al., 2009). Scanning-electron microscope imaging combined with major- and trace-element microprobe analyzes of brannerite at Bou Azzer reveals that this mineral is internally inhomogeneous (Oberthür et al., 2009). Inhomogeneous brannerite (Oberthür et al., 2009) may result from the dissolution and/or reprecipitation of an earlier phase of uraninite (Ikenne et al., 2021; Johnson & Cross, 1995; Macmillan et al., 2017; Oberthür et al., 2009). These observations call therefore for caution when interpreting the ca. 310 Ma date of brannerite associated with Co mineralization at Bou Azzer (Ikenne et al., 2021). Younger Triassic dates have also been proposed based on the argon-argon (40Ar/39Ar) thermochronometer in adularia, which is associated with sulfide and sulfosalt deposits that occur throughout the central and eastern Anti-Atlas and is interpreted as being paragenetically late with respect to arsenide ores at Bou Azzer (Levresse, 2001). However, 40Ar/39Ar ages in adularia are readily thermally reset (see Chiaradia et al., 2013). To end with, preliminary evidence from rhenium-osmium (Re-Os) dates of molybdenite at Bou Azzer imply a major ore-forming event for Co-Ni-arsenide mineralization in the Late Devonian (Stein et al., 2021). Due to this age uncertainty and the lack of direct radiometric dates on ore minerals, the geodynamic context that contributed the bulk of Co mineralization in these rich deposits has remained an open question.
To provide a genetic model for epigenetic Co-arsenide mineralization related to hydrothermal, metal-bearing fluids, there are four key components to document: (1) source(s) of metals; (2) origin of the reducing agent to fix metals as arsenides; (3) the type and chemical composition of hydrothermal, metal-bearing fluids, and (4) the geodynamic engine that put hydrothermal fluids into motion through the source towards the trap site for mineralization. Without absolute radiometric dates of the ore arsenide minerals, we lack a temporal framework to evaluate possible geodynamic triggers. In turn, without knowing the trigger, we cannot evaluate mineralization mechanisms. Therefore, the provision of radiometric ages for ore minerals is paramount for understanding the origin of the Bou Azzer Co-arsenide deposit. Here, we present new high-precision Re-Os ages for safflorite that reveal a primary introduction of bulk Co mineralization at the time of dislocation of the Anti-Atlas during tectonic subsidence controlled by normal faulting throughout the northern edge of the supercontinent Gondwana in the Late Devonian. Combining these age constraints with new sulfur stable isotope data for arsenides, and carbon and oxygen clumped isotope data on the calcite envelope surrounding the ore confirms a link between Co mineralization and oil field brines as proposed in previous petrographic and fluid inclusion microthermometry studies (En-Naciri et al., 1997; Essarraj et al., 2005). Further, these geochemical and geochronological data reveal that the Late Devonian rifting and mantle exhumation responsible for the well-known arch-and-basin geometry present from northern Africa to Arabia (Frizon de Lamotte et al., 2013), also triggered the formation of the highest-grade cobalt ore deposit known on Earth.
2. MATERIAL AND METHODS
2.1. Petrography and preparation of monophasic sulfide mineral separates
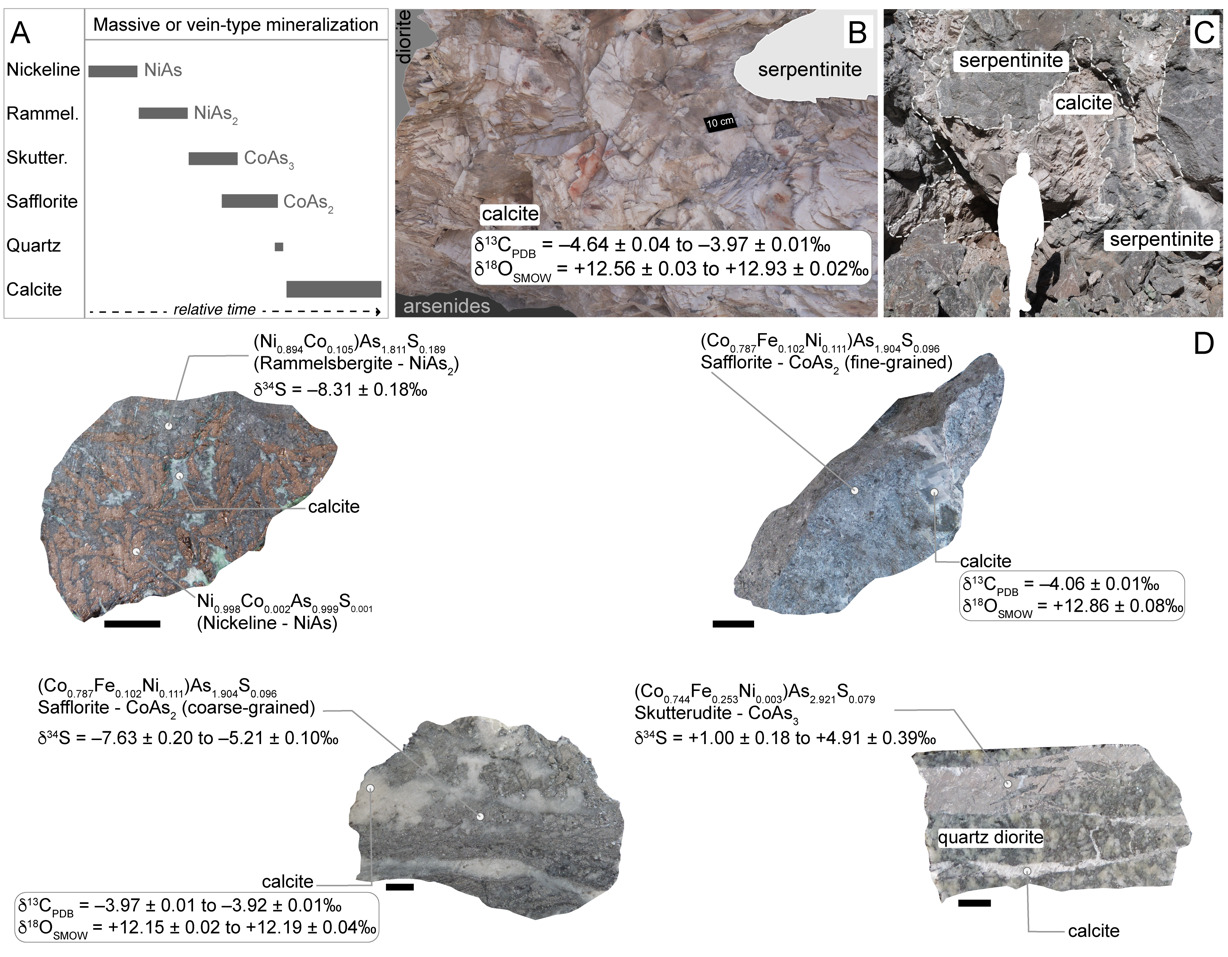
Four safflorite-mineralized samples from the Co-Ni-arsenide mineral deposit at Aït Ahmane, Bou Azzer district, Morocco were taken from the mineralized open pits F53 and F55 (fig. 1C & table S1). The paragenetic sequence between safflorite and calcite was established based on macroscopic descriptions and petrographical observations of polished thin sections using transmitted and reflected light microscopy (fig. 2A). These timing relationships constrained the workflow for optimum mineral separation of safflorite into monophase mineral separates according to the protocol presented in Saintilan et al. (2020a). This workflow is based on a first step of heavy liquid separation to isolate the bulk analyte (70–200 mesh sizes), followed by magnetic separation (I = 1.5 A) using a Frantz Isodynamic Separator to produce a safflorite final mineral separate in the non-magnetic fraction. Quality control of the mineral separates was conducted according to the method of Saintilan et al. (2020a).
2.2. Arsenide major and minor element chemistry
Quantitative microanalyses were obtained on a JEOL JXA-8230 electron microprobe at the Institute of Geochemistry and Petrology, ETH Zürich, Switzerland. This is a five-spectrometer instrument equipped with argon X-ray detectors (P-10 mixture) on spectrometers 1, 2, and 5, and xenon X-ray detectors on spectrometers 3 and 4. Analytical conditions were 15 kV and 50 nA with a 5 μm beam diameter. All analyzes were acquired using the software Probe for EPMA. Background measurements were done at interference-free spectrometer positions. Details of the element setup and standards used are listed in table S5. To improve the accuracy of some minor and trace element analyzes in arsenide, the shared background acquisition of Allaz et al. (2019) was applied. Counting times were optimized to improve detection limits while keeping analysis time reasonable at ca. 2 minutes per point. The ZAF matrix correction from Armstrong (1988) using the FFAST mass absorption coefficient table (Chantler et al., 2005) was applied throughout. Typical detection limits are on the order of 0.01 to 0.03 wt% for most elements (table S6). Major and minor element analyzes by electron microprobe were used to calculate atomic proportions and corresponding mineral formulae of arsenides.
2.3. Rhenium-osmium radiogenic isotope geochemistry of safflorite
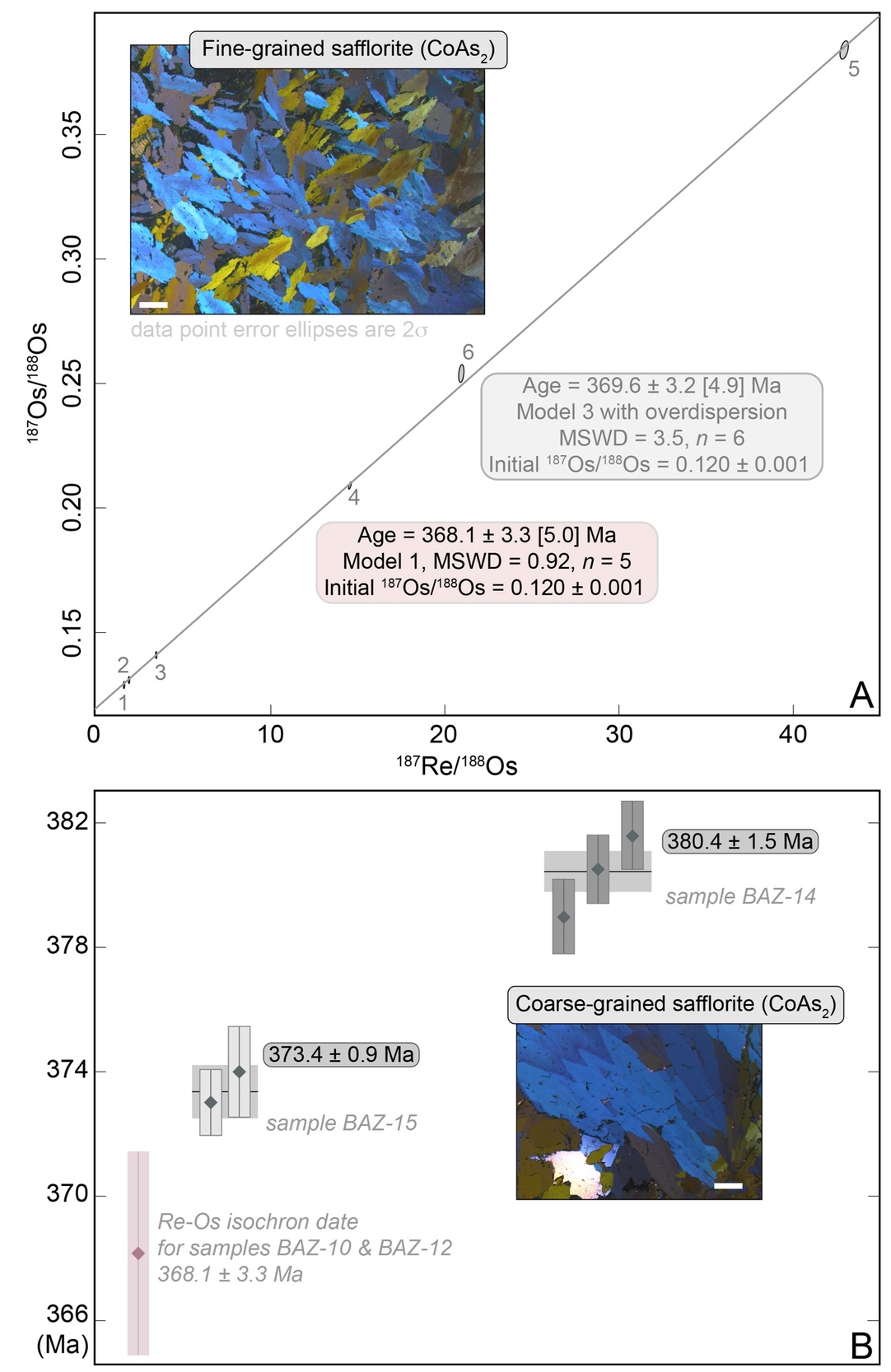
For each analysis, between 269 and 440 mg of safflorite mineral separates were weighed and transferred into thick-walled borosilicate Carius tubes (Shirey & Walker, 1995). Each sulfide aliquot was dissolved in inverse Aqua Regia (~3 mL of 11N HCl and ~ 6 mL 16N HNO3) with a known amount of “185Re+190Os spike” solution at 210ºC for 24 h (Laboratory of Rhenium-Osmium Isotope Geochemistry and Geochronology, Isotope Geochemistry and Cosmochemistry Group, Institute of Geochemistry and Petrology, ETH Zürich). The laboratory protocol used in the present work is described in full details in Selby and Creaser (2001), Selby et al. (2009), Hnatyshin et al. (2016), and Li et al. (2017). In brief, Os was isolated and purified from the inverse Aqua Regia solution by chloroform (CHCl3)-hydrobromic acid (HBr) solvent extraction at room temperature, and, by microdistillation (Birck et al., 1997; Cohen & Waters, 1996; Roy-Barman & Allègre, 1995; Selby & Creaser, 2001; Shen et al., 1996). The Re was isolated using an acetone-sodium hydroxide (Acetone-NaOH) step (Bozhkov et al., 1985; C. Li et al., 2009; Matthews & Riley, 1970), followed by HCl–HNO3-based anion chromatography (Cumming et al., 2013; Morgan et al., 1991). The Re and Os isotopic compositions were determined by negative thermal ionization mass spectrometry (N-TIMS) using a Thermo Scientific Triton mass spectrometer at the Institute of Geochemistry and Petrology, ETH Zürich, Switzerland. Rhenium and Os were loaded onto outgassed Ni and Pt filaments, respectively. Rhenium was measured as ReO4– in static mode on Faraday collectors, whereas Os was measured as OsO3– in peak-hopping mode on a single electron multiplier (Creaser et al., 1991; Völkening et al., 1991). Measurement quality was monitored by repeated measurements of in-house standard solution for Re (125 pg aliquot – 185Re/187Re = 0.5986 ± 0.0005, 2σ, n = 22) and Os (Durham Romil Os Standard, DROsS; Nowell et al., 2008; 50 pg aliquot – 187Os/188Os = 0.16092 ± 0.00069, 2σ, n = 12). Rhenium analyses are (i) corrected for isobaric oxide interferences using our long-term measurements of our in-house standard Re solution and (ii) normalized to an accepted value of the natural 185Re/187Re ratio (0.5974; Gramlich et al., 1973). Raw OsO3– ratios are corrected using an exponential law for isobaric oxide interferences, spike contribution and mass fractionation using an accepted value of the 192Os/188Os ratio of 3.08261 (Creaser et al., 1991; Hnatyshin et al., 2016). Total procedural blank using inverse Aqua Regia was: (i) for batch RO-006, 1.55 ± 0.02 pg Re and 28 ± 2 fg Os with a blank 187Os/188Os isotope composition (IC) of 0.43 ± 0.08 (2σ, n = 2); (ii) for batches RO-008, RO-010 & RO-011, 3.27 ± 0.02 pg Re and 44 ± 2 fg Os with a blank 187Os/188Os IC of 0.43 ± 0.09 (2σ, n = 3); (iii) for batch RO-009, 2.23 ± 0.01 pg Re and 113 ± 11 fg Os with a blank 187Os/188Os IC of 2.96 ± 0.92 (2σ, n = 1). The analytical uncertainties result from full error propagation of weighing errors, standard measurements, mass spectrometry analyzes and blanks. The Re-Os data for safflorite are plotted in the 187Os/188Os vs. 187Re/188Os space with the error correlation value (rho; Ludwig, 1980) and the 2σ calculated uncertainties for the 187Os/188Os and 187Re/188Os values (fig. 3A). An isochron date is calculated using IsoplotR (Vermeesch, 2018). Total uncertainty on the Re-Os dates is presented with and without including the decay constant λ of 187Re as determined by Smoliar et al. (1996; λ = 1.666e–11 ± 5.165e–14 a–1, 2σ).
For samples BAZ-14 & BAZ-15, preliminary analyzes using dissolution with a known amount of “185Re+190Os spike” solution showed an Os budget dominated by radiogenic 187Os* and lacking common Os. Therefore, new analyzes of each sulfide aliquot were performed by using the same protocol as above but with a known amount of “185Re+188Os+190Os spike” solution at 220ºC for 48 h. The Re and Os isotopic compositions were determined by N-TIMS using a Thermo Scientific Triton Plus mass spectrometer at the Canadian Centre for Micro-Analysis, Department of Earth and Atmospheric Sciences, University of Alberta, Canada. Measurement quality was monitored by measurements of in-house Re and Os standards over the analysis period (185Re/187Re = 0.59775 ± 0.00042, n = 8; AB-2 Os Standard, 187Os/188Os = 0.10689 ± 0.00061 1SD, n = 14). An average total procedural blank of 3.0 ± 0.3 pg Re and 44 ± 33 fg Os with a blank 187Os/188Os ratio of 0.30 ± 0.06 (n = 3) was measured over the duration of the safflorite analyzes. Reference Material 8599 Henderson molybdenite (Wise & Watters, 2011) was routinely analyzed as an age standard with the “185Re+188Os+190Os spike”. During the past 8 years, this reference sample has returned an average Re-Os date of 27.68 ± 0.07 Ma (n = 37), indistinguishable from the reported reference age of 27.66 ± 0.02 Ma (Markey et al., 2007). Individual model ages t for each safflorite aliquot are calculated using equation (1):
t=(1λ)⋅ln(187Os∗187Re+1)
2.4. Sulfur stable isotope geochemistry
Sulfur isotope geochemistry was carried out at the Institute of Earth Surface Dynamics, University of Lausanne, Switzerland. Based on microprobe analysis, only rammelsbergite, safflorite and skutterudite have sulfur contents greater than 0.03 wt.%. In addition to the safflorite mineral separates used for Re-Os isotope geochemistry, mineral separates of rammelsbergite and skutterudite were produced by following and modifying where necessary the protocol by Saintilan et al. (2020a). About 15 to 40 mg of pure nickeline, safflorite and skutterudite mineral separates were powdered in a hand-held agate mortar and pestle. Arsenide aliquots were analyzed by the new protocol of Spangenberg et al. (2022) for sulfur (S) concentration and sulfur stable isotope composition. Standards distributed by the International Atomic Energy Agency (IAEA-S1, -S2, and -S3) were analyzed for calibration. Repeated measurements of these standards gave reproducibility better than 0.14‰. Data are reported in δ34S notation as per mil (‰) variations from the Vienna Cañon Diablo Troilite (V-CDT) standard.
2.5. Carbon, oxygen, and clumped isotope geochemistry
A meter-size calcite block was collected in situ from the envelope of calcite at the contact between serpentinite and diorite in the vicinity of Ni ± Co-arsenide concentrations (fig. 2B) in open pit F51 (Aït Ahmane deposit, Bou Azzer district, fig. 1C). This block was cut into four sub-samples (samples BAZ-01a, b, c, d). In addition, pluricentimetric calcite crystals were extracted with tweezers from hand samples of fine-grained (BAZ-10, open pit F55) or coarse-grained (BAZ-15, open pit F53) safflorite-bearing samples (fig. 2D) prior to production of arsenide mineral separates from those samples. An additional sample of calcite associated with coarse-grained safflorite was collected (BAZ-17, open pit F53). Individual calcite samples were powdered in a hand-held agate mortar and pestle to obtain at least 1g of final powdered product.
The carbon, oxygen, and clumped isotope compositions of carbonates were determined at ETH Zürich using a Thermo Fisher Scientific 253Plus mass spectrometer coupled to a Kiel IV carbonate preparation device, following the methods described in Schmid and Bernasconi (2010), Meckler et al. (2014), and Müller et al. (2017). The Kiel IV device includes a custom built PoraPakQ trap held a –40 °C to eliminate potential organic contaminants. Prior to each sample run, the pressure-dependent backgrounds were determined on all beams to correct for non-linearity effects in the mass spectrometer. During each run, 22 replicates of 90–110 µg of different samples and five replicates of each carbonate standards, ETH-1, ETH-2, and 10 replicates of ETH-3, were analyzed for data normalization. Two replicates of the international standard, IAEA C2, were analyzed to monitor the long-term reproducibility of the method. All instrumental and data corrections are carried out with the software Easotope (John & Bowen, 2016) using the revised IUPAC parameters for 17O correction (Daëron et al., 2016). Results are presented in the I-CDES (Intercarb Carbon Dioxide Equilibration Scale) according to Bernasconi et al. (2021). Temperatures are calculated using the N. T. Anderson et al. (2021) universal calibration.
3. RESULTS
3.1. Mineralogy, mineral chemistry, and sulfur stable isotope composition of arsenides
Arsenide deposits with meter-scale envelopes of calcite are mostly located at the contact between serpentinite and intrusive Pan-African diorite (figs. 1C, 2B). The arsenide mineralization occurs also as localized and mineral-specific replacements (e.g., magnetite; Tourneur et al., 2021) in brecciated blocks of serpentinite cemented by calcite (fig. 2C). In the studied samples, skutterudite (Co tri-arsenide) is only found in anastomosing veins with accessory calcite within brittle structures in diorite (fig. 2D). Nickeline, which initiates the paragenetic sequence (fig. 2A, 2D), is bereft of sulfur and has a stoichiometric composition (fig. 2D). In contrast, the Ni di-arsenide rammelsbergite (fig. 2D) forms a solid solution with the Co di-arsenide safflorite with an average of 10.5% Co substituting for Ni (table S2). Rammelsbergite contains 1.63 ± 0.30 wt.% S (2 standard errors – SE) and has the lowest δ34S values among the analyzed arsenides (–8.31 ± 0.18‰, 2SE; table S3). The sulfur contents in safflorite lie between 1.18 ± 0.47 and 2.25 ± 0.49 wt.% S with similarly low δ34S values of –7.63 ± 0.20 to –5.21 ± 0.10‰. Safflorite (fig. 2D), which contains an average of 10.2% Fe and 11.1% Ni substituting for Co, forms a solid solution with the iron (Fe) di-arsenide löllingite, in addition to rammelsbergite. Skutterudite, with an average of 25% Fe substituting for Co, stands out as the arsenide species least enriched in sulfur (0.31 ± 0.13 to 0.65 ± 0.54 wt.% S) and with distinct positive δ34S values (+1.00 ± 0.18 to +4.91 ± 0.39‰).
3.2. Mineral-specific rhenium-osmium geochronological constraints on mineralization
The Re-Os data for fine-grained safflorite yield a Model 3 date of 369.6 ± 3.2 [4.9] Ma [bracketed uncertainty includes the 0.31% uncertainty in the decay constant of 187Re; n = 6; mean square of weighted deviates (MSWD) = 3.5; 2σ; initial 187Os/188Os ratio – Osi, 0.120 ± 0.001; fig. 3A and table S4]. A Model 3 isochron regression includes a measure of overdispersion attributed to geological uncertainty in the value for Osi, ‘i.e.’, all aliquots may not have the same Osi within the uncertainty of a Model 1 regression (Vermeesch, 2018). A Model 1 inverse isochron regression (not shown; Y. Li & Vermeesch, 2021) shows that analytical point 6 in figure 3A is a statistical outlier. Thus, a Model 1 isochron regression on the other 5 data points (MSWD = 0.92) yields a date of 368.1 ± 3.3 [5.0] Ma with an Osi at 0.120 ± 0.001. The Re-Os isochron age results of 368.1 ± 3.3 Ma (Model 1, n = 5) vs. 369.6 ± 3.2 Ma (Model 3, n = 6) does not change the interpretations and conclusions that mineralization is Late Devonian in age. In contrast, coarse-grained safflorite (sample BAZ-15), which is bereft of common Os (≤0.1 pg common Os), yields model ages that replicate with a weighted mean average of 373.4 ± 0.9 [1.2] (n = 2, fig. 3B; table S4). Coarse-grained safflorite (sample BAZ-14), which has low amounts of common Os (6.8–16.1 pg common Os) in comparison to radiogenic 187Os contents (2.13–2.24 ppb 187Os), yields model ages of ca. 380 Ma with a weighted average of 380.4 ± 1.5 [2.9] (n = 3, fig. 3B; table S4).
The three distinct Re-Os date groupings indicate at least three episodes of safflorite mineralization spanning ca. 12.8 ± 3.4 million years. We suggest that these dates reflect discrete episodes of mineralization, rather than resetting or disturbance of the Re-Os geochronometer in safflorite. We base this reasoning on independent and empirical evidence for the Re-Os system in sulfarsenides (arsenopyrite FeAsS and its solid solution with cobaltite – CoAsS – and gersdorffite – NiAsS; Davies et al., 2010; Majzlan et al., 2022; Morelli et al., 2010; Saintilan, Creaser, & Bookstrom, 2017; Saintilan, Creaser, Spry, et al., 2017; Saintilan et al., 2020b) and one arsenide (löllingite FeAs2; Saintilan, Creaser, Spry, et al., 2017). The robustness of the mature Re-Os arsenopyrite geochronometer was demonstrated in case studies against independent U-Pb age constraints on zircon or monazite (e.g., Davies et al., 2010; Morelli et al., 2010; Saintilan, Creaser, Spry, et al., 2017; Saintilan et al., 2020b; Scherstén et al., 2012). For arsenopyrite, closure temperature of the Re-Os system was constrained at ca. 600 °C (e.g., Davies et al., 2010; Morelli et al., 2010; Rooney et al., in press; Saintilan, Creaser, Spry, et al., 2017; Saintilan et al., 2020b). Further, Saintilan, Creaser, Spry and Hnatyshin (2017) in their study of the Re-Os chronometer in arsenopyrite weighed against independent monazite U-Pb age constraints concluded on the robustness of the löllingite Re-Os geochronometer with closure temperature between 550 and 600 °C. Given that löllingite (FeAs2) forms a solid solution with safflorite (CoAs2), we targeted safflorite at Bou Azzer for Re-Os isotope geochemistry and geochronology, assuming similar closure temperatures as for löllingite (i.e., 550 to 600 °C).
The aspects of diffusion of Os have only been studied in molybdenite (Selby & Creaser, 2004; Stein et al., 2003) and in pyrite and pyrrhotite (Brenan et al., 2000). For sulfarsenides and arsenides, such knowledge is not yet available and as such, direct calculation of a closure temperature for safflorite is not possible. The most robust mineral for Re-Os dating is molybdenite (Selby & Creaser, 2001; Stein et al., 2001; Takahashi et al., 2007). At Bou Azzer, a range of recently reported molybdenite dates indicates a major ore-forming event in the Late Devonian (Stein et al., 2021) closely matching our arsenide ages. Thus, we suggest that the safflorite Re-Os dates in the present work are not recording resetting by Os diffusion or recrystallization effects.
3.3. Clumped and carbon-oxygen stable isotope composition of ore-stage calcite
To complement previous evidence that mineralizing fluids were derived from heated seawater-derived brines (En-Naciri et al., 1997; Essarraj et al., 2005) and place new geochemical constraints on the redox conditions for mineralization, powdered calcite aliquots (n = 66) from eight samples were analyzed for carbon and oxygen isotope composition (δ13C in ‰ VPDB and δ18O in ‰ VSMOW; fig. 4) and clumped isotopes (I-CDES, table S5). The δ18Ofluid values of the hydrothermal fluid in equilibrium with calcite are calculated according to the calibration of O’Neil et al. (1969). Calcite at the contact between serpentinite and diorite has low δ13C values (–4.64 ± 0.04 to –3.97 ± 0.02‰, 1 standard deviation – SD). Its clumped isotope data (∆47 = 0.350 ± 0.006 to 0.375 ± 0.006‰, 1 standard error – SE) and δ18O values (+12.57 ± 0.03 to 12.93 ± 0.05‰) signal a minimum average temperature of the hydrothermal fluid (Tmin-fluid) of 146 to 172 °C and δ18Ofluid at –0.34 to +1.90‰. Calcite intergrown with safflorite has similarly low δ13C values (–4.06 ± 0.02 to –3.92 ± 0.01‰). The hydrothermal fluid at the root of this calcite associated with Co-arsenides has overall slightly higher Tmin-fluid (172–219 °C for ∆47 = 0.314 ± 0.021 to 0.349 ± 0.028‰) and slightly higher δ18Ofluid of +1.26 to +3.72‰ (for δ18Ocalcite = +12.15 ± 0.02 to 12.86 ± 0.08‰).
4. DISCUSSION AND CONCLUSIONS
4.1. Geochronological implications and regional geodynamic context
Our new Re-Os ages constrain the timing of cobalt arsenide mineralization at Bou Azzer in the Late Devonian starting in the Frasnian at ca. 380.4 Ma until shortly after the Frasnian–Famennian boundary at ca. 373.4 to 368.1 Ma (figs. 3 & 5). These time stamps signal the primary introduction of bulk Co mineralization during dislocation of the Anti-Atlas in the Middle to Late Devonian, in connection with subsidence controlled by normal faulting that occurred throughout the northern edge of the Gondwana supercontinent (Wendt, 1985). This tectonic episode is part of a global event of rifting, mantle exhumation and thermal uplift that is responsible for the well-known arch-and-basin geometry that is recognized from present-day northern Africa to Arabia and is inherited from the breakup of northern Gondwana (Frizon de Lamotte et al., 2013).
In the Late Devonian, the central Anti-Atlas, including the Bou Azzer area, was a continental platform to major sedimentary basins located in the present-day eastern Anti-Atlas (Mader and Tafilalt Basins; fig. 1A; fig. 6). Hereafter, we refer to the carbonate-shale platform at Bou Azzer on the eastern edge of the Anti-Atlas as the “Bou Azzer platform”. During the Middle to Late Devonian, the eastern edge of the Anti-Atlas (in particular, the Tafilalt basin & platform) underwent extensional tectonic activity. This tectonic activity is attested by sharp variations in sediment thickness and syn-sedimentary faulting (Pouclet et al., 2017; Wendt, 2021) which caused major transgressions during the Late Devonian with an increased frequency of syn-sedimentary fault movements (Wendt, 2021). This tectonic context is also contemporaneous with significant calc-alkaline magmatic activity (Franchi et al., 2015; Pouclet et al., 2017). Protracted sodic alkaline magmatism is attested in the Tafilalt Basin from the late Devonian (Famennian) to the end of the Lower Carboniferous (Tournaisian) (Pouclet et al., 2017). This geodynamic configuration has been interpreted as being related to a thermal anomaly and uplift associated with a mantle anomaly (Frizon de Lamotte et al., 2013). In the Tafilalt platform, this magmatic activity fueled the circulation of warm hydrothermal fluids within the overlying carbonate units (Franchi et al., 2015). Further, in the context of sustained syn-sedimentary faulting, the eastern edge of the Anti-Atlas and the Bou Azzer platform could have focused large quantities of hydrothermal fluid flow resulting in hydrothermal mineralization triggered by episodic and seismically induced fluid pulses (see Sibson et al., 1975).
Further to this, the timing of the mineralizing processes leading to the highest-grade Co ore deposit on Earth coincides with the two events comprising the Kellwasser episode (fig. 5) as illustrated by specific hydrocarbon source rocks in the sedimentary record in the eastern and central Anti-Atlas in the Mader-Tafilalt Basins and adjacent carbonate platforms (Wendt & Belka, 1991). The Bou Azzer pelagic platform comprised black bituminous limestone and shale (Wendt & Belka, 1991) above a basement that included the future loci of the Bou Azzer deposits at the tectonic contacts between Neoproterozoic serpentinite and Pan-African granodiorite (fig. 6). The hydrocarbon source rocks in the Bou Azzer platform may have been deposited in the context of a major biotic crisis, ‘i.e.’, the Kellwasser event (fig. 5; Carmichael et al., 2019; Hollard, 1974; Schindler, 1990; Wendt & Belka, 1991). The Late Devonian Kellwasser event was a series of modification of marine ecosystems of global scope with variable lithological and geochemical expressions depending on paleogeography and paleoenvironment (Carmichael et al., 2019). At its type locality (Steinbruch Schmidt outcrops, Germany; Devleeschouwer et al., 2002), the Kellwasser event is calibrated immediately prior the Frasnian-Famennian boundary at 371.86 ± 0.08 Ma and comprises two horizons above and below a bentonite layer (uranium-lead isotope dilution thermal ionization mass spectrometry zircon age of 372.36 ± 0.05 Ma; Percival et al., 2018; fig. 5) assigned to the Upper rhenana conodont Zone (Ziegler & Sandberg, 1990). In Morocco, the sedimentary record of potential Kellwasser anoxic horizons is not calibrated by absolute radiometric ages nor astrochronological correlations (fig. 5) as done for other Kellwasser sequences at the global scale (De Vleeschouwer et al., 2017; Percival et al., 2018). Current interpretations suggest that a first and short Lower Kellwasser episode at ca. 382 to 381 Ma (lowermost Frasnian, fig. 5; Wendt & Belka, 1991) preceded a longer Upper Kellwasser episode starting possibly at ca. 373 to 371 Ma across the Frasnian-Fammenian boundary. The Upper Kellwasser overlapped with the peak of extensional tectonics in the eastern Anti-Atlas during the late Frasnian to early Famennian (Wendt & Belka, 1991).
The two types of hydrocarbon source rocks in the Bou Azzer platform are known to be able to generate hydrocarbons involved in epigenetic sulfide mineralization related to hydrothermal, metal-bearing fluids derived from basinal brines (e.g., Laisvall sandstone-hosted sulfide deposit related to exogenous hydrocarbons generated from buried black shale, Saintilan et al., 2019; Saintilan, Spangenberg, Samankassou et al., 2016; Ruby Creek sulfide deposit in dolomitized carbonaceous limestone; Saintilan et al., 2023). In addition, carbonate-shale platforms are ideal loci for evaporative, seawater-derived brine factories adjacent to sedimentary basins (Leach et al., 2010; fig. 6). In the context of an increased geothermal gradient (Frizon de Lamotte et al., 2013; Pouclet et al., 2017), such brines were involved in the form of heated hydrothermal fluids (En-Naciri et al., 1997; Essarraj et al., 2005) in the episodic mineralizing processes at Bou Azzer as evidenced by our new safflorite Re-Os ages. These hydrothermal fluids had the appropriate salinity and temperatures (as shown by evidence from fluid inclusions microthermometry studies; En-Naciri et al., 1997; Essarraj et al., 2005) to leach cobalt and, to a lesser extent, nickel from serpentinite (see details of the nature of the mineralizing fluids and mineralizing mechanisms in the next section 4.2.). In addition, such brines sinking into and refluxing within the Mader and Tafilalt sedimentary basins adjacent to the Bou Azzer platform (fig. 1A) would have resurged episodically via seismically induced fluid pulses in the context of intense extensional activity in the Middle to Late Devonian (Pouclet et al., 2017; Wendt, 2021). Then, according to the general model of Markl et al. (2016) for Co-Ni-arsenide mineralization, we speculate these warm brines triggered hydrothermal alteration of hydrocarbon source rocks of the Kellwasser episode (fig. 6 and its inset) that were either deposited previously (ca. 382–381 Ma Lower Kellwasser event of Morocco, fig. 5) or were being deposited at the time of Co-arsenide mineralization (Upper Kellwasser event of Morocco, fig. 5).This hydrothermal alteration of hydrocarbon source rocks would have produced methane for episodic Co-arsenide mineralization at Bou Azzer (see details of mineralization mechanisms in the next section).
4.2. Origin of fluids and dynamics of fluid mixing
The wider geodynamic environment of extensional tectonics in the Late Devonian on the northern edge of Gondwana is compatible with deposit-scale fault patterns and petrographic observations documenting mineralization at Bou Azzer as open-space filling, including within brecciated zones. Specifically, in this extensional context, low-angle detachment faulting led to the formation of open spaces between fragments of brecciated country rocks (e.g., serpentinite; Tourneur et al., 2021; figs. 2B, 2C). Consequently, euhedral to subhedral minerals (e.g., arsenides and calcite; figs. 2B-D) could fill large-scale pockets in open spaces within an extensional environment (Tourneur et al., 2021). This tectonic setting would favor hydrothermal fluid flow and mineralization resulting from the involvement of two originally immiscible fluids (inset in fig. 6; En-Naciri et al., 1997; Essarraj et al., 2005). Previous work on fluid inclusion data concluded that the first fluid was sodium-calcium chloride (NaCl-CaCl2), seawater-derived brines (En-Naciri et al., 1997; Essarraj et al., 2005). These brines, which were saturated with respect to halite, had salinities (5.5 to 22.0 weight percent equivalent [wt.% eq.] NaCl and 13.5 to 18.5 wt.% eq. CaCl2) two to six times the average Cambrian–Devonian seawater salinity (Demicco et al., 2005; En-Naciri et al., 1997; Essarraj et al., 2005). Fluid inclusion halogen and cation ratios are typical of deep basinal brines, in particular oil-field brines, which were derived from surface evaporation of seawater (Essarraj et al., 2005). Given the timing of Co-arsenide mineralization in the Upper Devonian, the δ18Oseawater was likely at –0.10 ± 1.30‰ (Galili et al., 2019). Our new δ18Ofluid and δ34Sskutterudite data support previous observations and conclusions that primary seawater-derived brines in the Bou Azzer region were modified by fluid-rock interaction during burial and migration through basement rocks: (1) The calculated δ18Ofluid for Bou Azzer is a testament of limited fluid-rock interaction during burial and migration of the evaporative primary brines that evolved to the chemistry of deep basinal oil-field brines (En-Naciri et al., 1997; Essarraj et al., 2005), until eventual calcite precipitation in open spaces at the contact between serpentinite and diorite (δ18Ofluid of –0.34 to +1.90‰) and calcite associated with Co-arsenides (δ18Ofluid of +1.26 to +3.72‰). Assuming that the temperatures of calcite precipitation determined from clumped isotope data were not reset after the original arsenide-calcite mineralization, and considering that calcite follows arsenides in the paragenetic sequence (fig. 2A), the minimum temperatures of calcite precipitation (146–219 °C) are compatible with previous temperature estimates of Bou Azzer Co-arsenide mineralization at ca. 200 to 225 °C (En-Naciri et al., 1997; Essarraj et al., 2005); (2) Low Na/Ca ratios in fluid inclusions are diagnostic of the interaction of Na-rich brines with Ca-rich rocks because brines that evaporate beyond the point of halite saturation should have high Na/Ca ratios (Essarraj et al., 2005). At Bou Azzer, a gain in Ca is related to feldspar alteration in diorite (Ca-Na exchange during albitization of plagioclase; Essarraj et al., 2005; Leblanc & Lbouabi, 1988; fig. 2D) during brine migration at the interface with serpentinite (fig. 6). The sulfur isotope composition of skutterudite (δ34Sskutterudite = +1.00 ± 0.18 to +4.91 ± 0.39‰) is compatible with derivation of hydrogen sulfide from trace magmatic sulfides in diorite during this hydrothermal alteration.
The seawater-derived brines coexisted with another fluid comprising methane ± molecular nitrogen (CH4 ± N2) gas (En-Naciri et al., 1997; Essarraj et al., 2005). The ore-stage calcite δ13C and δ18O isotope data overlap with the δ13C and δ18O values of calcite in sediment-hosted petroleum reservoirs worldwide (e.g., Alberta, Canada; fig. 4A), and with replacement and pore-filling calcite veins associated with bitumen in basalt-hosted petroleum reservoirs in Greenland (Rogers et al., 2006). In such geological settings, δ13C of calcite cements is diagnostic of the processes altering hydrocarbons in reservoirs (fig. 4B; Macaulay et al., 2000). Thus, the δ13C composition of calcite may commonly reflect a mixing of marine bicarbonate (δ13C of ca. 0‰) with dissolved inorganic carbon with light δ13C values (<–25‰) derived from altered organic matter (Peckmann et al., 1999). Here, we propose that calcite at Bou Azzer results from the interaction of basinal brines with CH4–N2 gaseous fluids, involving the abiotic degradation of hydrocarbons at the site of arsenide mineralization. The resulting pool of dissolved inorganic carbon available to form calcite with δ13C values of –4.64 ± 0.04 to –3.92 ± 0.01‰ would be dominated by marine bicarbonate with only a minor contribution from methane decomposition (fig. 4B).
This methane gas was probably sour gas, ‘i.e.’, hydrocarbon gases bearing hydrogen sulfide (H2S) with a light sulfur isotope composition (G. M. Anderson, 2008). This interpretation is supported by the isotopically light δ34S values of NiAs2 (–8.31 ± 0.18‰) and CoAs2 (–7.63 ± 0.20 to –5.21 ± 0.10‰) at Bou Azzer (fig. 2D). Thermal decarboxylation of hydrocarbons (Macaulay et al., 2000) is a process of hydrocarbon degradation that is compatible with the δ13C signatures of Bou Azzer calcite (fig. 4B; Irwin et al., 1977; Macaulay et al., 2000). This degradation of hydrocarbons is probably the pre-requisite for mixing of the two above-mentioned fluids that were initially immiscible. We conceptualize that the oxidation of organic molecules mainly controlled the reduction of As for arsenide mineralization (see details in the next section).
Considering the present new findings and consequent genetic model for Co-arsenide mineralization at Bou Azzer (fig. 6), the identification of the exact hydrocarbon source rocks should be the aim of future studies (see section 5 and the detailed explanations of limitation #2 about the present work). Yet, there are several working hypotheses regarding the source(s) of hydrocarbons on which we speculate here. In the context of extensional tectonics in the central and eastern Anti-Atlas in the Devonian, several sources of hydrocarbon accumulations (especially methane) are possible for explaining the sudden influx and availability of methane gas for arsenide mineralization (according to the model by Markl et al., 2016) in the Bou Azzer area. On the one hand, carbonate mounds related to methane ± H2S venting in sedimentary basins in the Emsian and Eifelian–Givetian are known as the “Kess Kess” and “Hollard” Mounds in the eastern Anti-Atlas, respectively (fig. 1A; Belka et al., 2018; Peckmann et al., 1999). On the other hand, deposition of black bituminous limestone and shale took place in shallow basins (eastern Anti-Atlas) and adjacent pelagic platforms (including the Bou Azzer area in central Anti-Atlas; fig. 1A) during the Kellwasser event as summarized in the previous section (Wendt & Belka, 1991). The timing of this “Moroccan” Upper Kellwasser event (ca. 373 to 371 Ma) overlaps with or slightly precedes the time of extensive fine-grained Co-arsenide mineralization at Bou Azzer determined here (ca. 373.4 to 368.1 Ma), whereas the “Moroccan” Lower Kellwasser episode at ca. 382–381 Ma shortly precedes the timing of a first stage of coarse-grained safflorite mineralization at ca 380.4 Ma (fig. 5). Therefore, our geochronological and lithological evidence would collectively favor the hypothesis that supply and migration of hydrocarbons (including methane) from hydrocarbon source rocks in the Bou Azzer platform (fig. 6) probably took place following warm hydrothermal alteration of Upper Devonian Kellwasser hydrocarbon source rocks (‘i.e.’, either carbonaceous limestone or organic-rich shale) by heated, seawater-derived basinal brines. We cannot exclude that hydrocarbons (1) were also produced by warm hydrothermal alteration of Upper Devonian Kellwasser hydrocarbon source rocks deposited in the basins adjacent to the Bou Azzer platform, and (2) migrated from basin to platform setting. In fact, hydrocarbon migration from basin to carbonate platform is known in the petroleum systems related to Jurassic hydrocarbon sources rocks in the Gotnia and Arabian basins in south-western Iraq and Central Saudi Arabia. In such systems, hydrocarbon migration took place up dip and laterally in permeable conduits (Fox & Ahlbrandt, 2002).
4.3. Source of metals and reduction pathways for Co-arsenide mineralization
Osmium isotope data and microscopic observations reported here provide new insights into the source of metals and the chemical and mineralogical controls on the mechanisms of reduction leading to Co-arsenide mineralization at Bou Azzer. Serpentinites derived from Re-depleted, harzburgitic, oceanic lithospheric mantle possess unradiogenic 187Os/188Os ratios (Agranier et al., 2007; Meisel et al., 1996; Reisberg et al., 1991; Smith et al., 2021) that fall within the range of 187Os/188Os ratios reported for fresh lithospheric mantle harzburgite peridotite (0.118–0.128; Snow & Reisberg, 1995). Such Os isotopic signatures are explained by serpentinization at low fugacity of hydrogen sulfide that results in newly formed Os-rich alloys retaining the unradiogenic 187Os/188Os composition of primary mantle sulfides in the original unaltered peridotite (Foustoukos et al., 2015). The Osi of safflorite at 0.120 ± 0.001 points to a derivation of Os from the pre-existing serpentinized oceanic lithosphere including harzburgite at Bou Azzer (Hodel et al., 2020) that was leached by highly saline basinal brines in the Late Devonian. By corollary, using Os a tracer for the source of other metals (Saintilan et al., 2021), serpentinite logically stands as a major source of cobalt and nickel (Bouabdellah et al., 2016; Leblanc & Billaud, 1982) that could be leached effectively by NaCl-CaCl2-bearing basinal brines (Liu et al., 2011, 2012; Williams-Jones & Vasyukova, 2022). Such hydrothermal fluids have the chemistry to effectively leach and transport cobalt (Liu et al., 2012).
The hydrothermal alteration of pre-existing Neoproterozoic serpentinite by acidic, Cl-rich fluids in the Late Devonian led to the formation of distinctive massive magnetite veins within serpentinite at Bou Azzer (Hodel et al., 2017). This process resulted in an increase in modal magnetite content with veins of secondary magnetite augmenting the primary disseminated magnetite that formed during serpentinization in the Neoproterozoic (Hodel et al., 2017). These magnetite veins signal the availability and mobility of iron from serpentinite, probably in the form of aqueous Fe(II)-Cl2 species (Debret et al., 2016). Therefore, by piecing together evidence for the involvement of an oil-field brine system with the availability and mobility of reduced Fe(II), we conceptualize two pathways of reduction of arsenic leading to the production of reduced species of arsenic available for Co-Ni-arsenide mineralization:
-
an abiotic process of reduction of As(V) by Fe(II) as previously evidenced in sub-surface settings in studies dealing with environmental monitoring of groundwater quality (Bose & Sharma, 2002). Such a process taking place in deeper crustal settings at Bou Azzer would equally explain petrographic observations whereby magnetite in serpentinite is replaced by Ni-arsenides in particular (Tourneur et al., 2021);
and
-
an additional pathway involving the abiotic thermal decarboxylation of hydrocarbon mentioned above. The oxidation of hydrocarbons would be accompanied by reduction of arsenic species and a decrease in solubility of aqueous Co-chloride and Ni-chloride species that would dissociate to form cobalt and nickel arsenides (see Markl et al., 2016). The newly produced dissolved inorganic carbon derived from the oxidation of hydrocarbon would be fixed in calcite in breccia zone within serpentinite, and at the rheological contact between serpentinite and diorite (figs. 2B, 2C).
5. LIMITATIONS AND OPEN QUESTIONS
Limitation #1. Although serpentinites are known to be sponges for As (Deschamps et al., 2010; Hattori et al., 2005; Ryan et al., 2011), a demonstration that the Bou Azzer serpentinite is not only the source of Co and Ni but also a volumetrically sufficient local source of As remains necessary (‘i.e.’, through replacement of As-bearing magnetite by arsenides and leaching of As at a specific oxidation state from the lattice of serpentine minerals; Hattori et al., 2005; Wu et al., 2021). Such a study would probably signal that a pre-requisite for the formation of giant Co-Ni-arsenide ore deposits versus the numerous Co-Ni arsenide mineral occurrences in basement rocks of supercontinents (see Burisch et al., 2022) is the presence of serpentinite that acts as a source of both metals and metalloid (As).
Limitation #2. Our present study calls for follow-up work that could yield direct geochemical evidence in support of our preferred hypothesis that hydrocarbons involved in mineralization at Bou Azzer were sourced from shale hydrocarbon source rocks and/or dark bituminous fetid limestone deposited at the time of the Kellwasser biotic crisis. We suggest that compound-specific carbon isotope data combined with organic geochemical studies of a suite of samples comprising arsenides, calcite, serpentinite, and Kellwasser source rocks may prove fruitful.
Acknowledgments
N.J.S., M.I. and M.S. are indebted to the administration and employees of the CTT-Managem Group at Bou Azzer for their kind hospitality and logistical support during fieldwork. N.J.S. acknowledges support from the Swiss National Science Foundation through an Ambizione Fellowship (Grant PZ00P2_180133). M.I. acknowledges financial support from Ibn Zohr University, Agadir, Morocco. R.A.C. acknowledges an NSERC Discovery Grant. Dr. J. Slack (Emeritus USGS) is thanked for his constructive comments on an earlier version of this manuscript. We thank Associate Editor Ethan Baxter and reviewer Alan D. Rooney for stimulating, constructive, and pertinent comments and thoughtful advice to clarify several points in our manuscript. The additional comments by an anonymous reviewer are acknowledged. The overall editorial handling by Editor-in-chief Page Chamberlain is greatly appreciated as well as additional constructive comments throughout the review process.
Author contributions
N.J.S. and M.I. designed the study. N.J.S., M.I., M.S. performed fieldwork and sample collection. A.K. and L.M. organized fieldwork and contributed to sample collection. N.J.S. carried out petrographic investigations, mineral separation, arsenide Re-Os isotope geochemistry procedures and N-TIMS mass spectrometry analyzes at ETH Zürich. J.T. and R.A.C. conducted arsenide Re-Os isotope geochemistry procedures and N-TIMS mass spectrometry analyzes at the University of Alberta on aliquots provided by N.J.S. S.M.B. performed carbon, oxygen and clumped isotope analyzes of calcite using samples prepared by N.J.S. J.E.S. performed sulfur stable isotope analyzes of arsenides using samples prepared by N.J.S. N.J.S. and J.M.A. performed mineral chemistry analyzes. N.J.S. wrote the paper. All co-authors contributed comments and improvements to both the original draft and the revised manuscript.
Data and materials availability
All data are available in the main text or the supplementary materials.
Supplementary Data
https://earth.eps.yale.edu/~ajs/SupplementaryData/2023/Saintilan/"
Editor: C. Page Chamberlain, Associate Editor: Ethan Baxter