ISSN: 0973-7510
E-ISSN: 2581-690X
Medicinal plants are a major source of numerous therapeutic agents, and the emergence of pathogenic bacteria has rekindled interest in traditional medicine systems as an alternative approach to overcoming resistance. The dried plant material of four medicinal plants, namely Terminalia arjuna (bark), Terminalia bellirica (fruit), Aegle marmelos (leaves), and Bacopa monnieri (leaves), was powdered, and aqueous extracts were prepared. The antimicrobial activity of the extracts was evaluated against three clinically important strains: Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli 385. As E. coli 385 was resistant to the broadest spectrum of antibiotics tested, it was classified as (MDR multi-drug resistant). E. coli, Bacillus subtilis, Mycobacterium smegmatis (MTTC), and Vibrio alginolyticus (ATCC) were also assessed using the agar well diffusion method for zones of inhibition and minimum inhibitory/bactericidal concentration (MIC/MBC). Clinically important strains were found to be sensitive to the aqueous extract of T. bellerica (19.51 ± 0.61 mm) with MICs ranging from 0.31 to 0.62 mg/ml. The MDR strain was also sensitive to Bellirica monnieri (16.10 ± 0.31 mm) aqueous extract. To determine the potential for a wide range of applications, the antioxidant activities of the extracts were evaluated using DPPH, ABTS, and FRAP assays. The T. arjuna plant extract exhibited the highest radical scavenging activity with the lowest EC50 values for DPPH (1.15 ± 0.061 mg/ml) and ABTS (1.02 ± 0.07 mg/ml). The plant extracts were characterized by UV-visible spectroscopy, Fourier-transform infrared spectroscopy, and LC-MS/MS.
Antimicrobial Activity, Antioxidant Potential, Aqueous Extract, Multi-drug Resistant Bacteria, Phytochemical Analysis
Antimicrobial resistance is a global health threat that necessitates the development of novel therapeutics. The overuse of antibiotics and their imprudent disposal into the environment have led to the emergence of antibiotic resistance in organisms.1 The antimicrobial resistance (AMR) acquired by pathogenic microbes is projected to reach 10 million by 2050.2 Based on the urgency of new antibiotics, the WHO has listed P. aeruginosa, E. coli, and S. aureus as critical antibiotic-resistant priority pathogens.3 Numerous pharmaceuticals originate from plant extracts and have been the subject of extensive pharmacological screening.1 Plants contain an array of secondary metabolites that play crucial roles in their survival and adaptation to the environment. These compounds belong to three major classes, namely, alkaloids, phenols, and terpenoids. Complex mixtures of these phytocompounds are attractive sources of bioactive compounds.2 The combination of bioactive compounds from plants and antibiotics may aid in the development of antimicrobial resistance against infectious agents. In the search for new agents, scientists have moved towards traditionally used plant sources.4 Traditional medicines have been used to treat various ailments and are recognized as a part of primary healthcare in different tribal regions of India. To cure fever, cough, skin lesions, and liver disorders, many people turn toward the well-known ayurvedic formulation Triphala, which holds the plants T. bellirica and T. arjuna as active ingredients.5 Perilously increasing multidrug resistance has accelerated the search for and identification of plant-derived antimicrobials and antioxidants that protect cells and tissues from free radical damage. These antioxidants help ameliorate the consequences of oxidative stress in various disorders, including nephrotoxicity, diabetes, Parkinson’s disease, cancer, Alzheimer’s disease, and neurodegenerative diseases.6 The Arjuna tree is widely distributed in India, especially in the Sub-Himalayan and Deccan regions.7 The bark of the arjuna tree is an important constituent of many Ayurvedic formulations and is known to have anti-cancer, anti-bacterial, and antioxidant properties.8 Different in vivo and in vitro studies have established the antitumor, anti-allergic, anti-fertility, and anti-HIV potential of T. arjuna bark.9,10 Fruits of T. bellirica (Baheda) are used to treat asthma, piles, cough, diarrhea, and throat infections.5 A. marmelos (Bael) leaves are often used in Ayurvedic and traditional medicine to treat edema, vomiting, neurological diseases, rheumatism, tuberculosis, diabetes, high blood pressure, and dysentery.11 B. monnieri (Brahmi) helps to repair injured neurons, generate new neurons, and restore synaptic activity. Traditional healers use Brahmi to treat conditions such as epilepsy and anxiety and boost brain function. They have also used it to treat UTIs as well as digestive and skin problems.12 Hence, the objective of the present work was divided into four parts: antimicrobial potential of T. arjuna, T. bellirica, A. marmelos, B. monnieri aqueous extracts against E. coli, B. subtilis, M. smegmatis, and V. alginolyticus as representative of gram-negative, gram-positive, acid fast and opportunistic pathogenic bacteria, respectively; phytochemical profiling; chemical characterization, antioxidant ability of active extracts; and antimicrobial activity against clinical and drug-resistant isolates. These results demonstrate the biological applications of these aqueous extracts against drug-resistant strains related to different types of infections and diseases.
Plants collection and authentication
Different parts of Terminalia arjuna (Roxb.) Wight & Arn (UHF-Herbarium no 13796), Terminallia bellirica (Gaertn.) Roxb. (UHF-Herbarium no 13797), Aegle marmelos (L.) Correa (UHF-Herbarium no 13799) and Bacopa monnieri (L.) Pennell (UHF-Herbarium no 13798) were collected from high altitude Himalayan region of Chamba, Himachal Pradesh, (India), based on their use in the Indian system of alternative medicine by traditional healers. The authentication of these plant materials was conducted at the Department of Forest Products, Dr. Y.S. Parmar University of Horticulture & Forestry, Nauni, Solan, Himachal Pradesh, India.
Preparation of plants extract
The dried powder of the plant material was mixed (1:10) with 200 ml of deionized water and placed in a rotatory shaker at 150 rpm for 6–7 days to ensure complete extraction, followed by filtration and centrifugation at 4000 × g for 20 min. The filtrate was collected and heat evaporated at 45-50°C.13 For further biological assays, a crude extract was prepared and stored at -20°C. Samples were designated as TA (T. arjuna), TB
(T. bellirica), AM (A. marmelos), BM (B. monnieri). The extract yield was determined using the following equation14:
Percentage (%) yield = [(Wt. of the crude plant extract obtained (g) / Wt. of plant powder used (g)] x 100
Bacterial cultures and inoculum preparation
The antibacterial potential of the extracts was initially tested against E. coli (MTCC 1678), B. subtilis (MTCC 121), M. smegmatis (MTCC 6), V. alginolyticus (ATCC 17749), P. aeruginosa (MTCC 2297), S. aureus (Lab isolate), and drug-resistant E. coli 385 (Lab isolate), a kind gift from the Special Center for Molecular Medicine (JNU), New Delhi. The test organisms were cultured overnight at 37°C in Muller Hilton broth (MHB), and the turbidity was adjusted to 108 CFU/ml using the 0.5 McFarland standard.
Preparation of stock and working solution
A 200 mg/ml stock solution was prepared by mixing 2 g of the dried crude extract with 10 ml of ddH2O. From this stock solution 20 mg/ml working solution were prepared by reconstituting 100 µl of stock solution in 900 µl of ddH2O. Prior to application, plant extracts and commercially available antibiotics underwent filtration using a 0.22 µm membrane filter.
Antimicrobial activity of extracts
The antimicrobial activity of the aqueous extracts was evaluated using the agar well diffusion method, following the guidelines of the CLSI (Clinical and Laboratory Standards Institute).15 Sterile Muller-Hilton agar (MHA) plates were cultured in a standardized microbial culture broth using sterile cotton swabs. Three wells (6 mm diameter each) were punched into the MHA using a sterile borer. 100 µl of plant extract, standard antibiotic as a positive control, and demineralized water as a negative control were added to the respective wells. For diffusion of extracts, plates were placed in the refrigerator for 30 minutes, followed by incubation in an upright position at 37°C for 14–16 hours. Antimicrobial activity was calculated by measuring the inhibition zones (mm) around the wells, with three replicates for each treatment. Considering their high prescription frequency to combat the microbial agents, antibiotics Chloramphenicol (15 mcg) and Tetracycline (15 mcg) were used as positive controls.
Minimum inhibitory (MIC) and bactericidal (MBC) concentration
Macro broth dilution method
The Minimum Inhibitory Concentration (MIC) of each extract was determined using the broth dilution method. Plant extract concentrations of the plant extracts (20, 10, 5, 2.5, 1.25, 0.625, and 0.312 mg/ml) were prepared. Mueller-Hinton broth (MHB; 10 mL) was dispensed into test tubes. Successive two-fold serial dilutions of the extract concentrations (100 µl) were mixed with the MHB. Next, a standardized inoculum (50 µl) of the test organisms was added to each test tube containing the MHB-extract suspension. Subsequently, all test tubes were incubated at 37°C for 14–16 hours. The MICs was determined as the lowest concentration that completely inhibited visible growth (indicated by the absence of turbidity) of the test organisms.
Micro broth dilution assay (96 well plates)
Plant extracts with a positive MIC result in the macrodilution assay were used to determine the MIC using the microbroth dilution method. The assay was performed using MHB as recommended by the NCCLS (National Committee for Clinical Laboratory Standards). Briefly, serial dilutions of plant extracts were prepared in sterile 96 well plates. The growth controls were prepared by replacing the extracts with equal volumes of ddH2O. Sterility controls were prepared using MHB alone. The plates were incubated at 37°C for 14-16 hours. Turbidity indicates bacterial growth. Antimicrobial activity was assessed by measuring the absorbance at 630 nm. The MIC was defined as the lowest concentration that inhibited growth. Broth (20 µl) were taken from each well of micro titer plate and streaked over MH agar plates to determine the MBC.
In Vitro Radical Scavenging potential
The free radical-scavenging activity and reducing power of each extract were determined using DPPH, ABTS, and FRAP assays. To achieve different concentrations, plant extract stocks (20 mg/ml) were serially diluted two-fold.16
Freshly prepared DPPH (2,2-Diphenyl-1-picrylhydrazyl) free radical (0.0045 g dissolved in few ml methanol) was diluted to achieve 0.98-1.00 absorbance at 517 nm. 150 µl of DPPH working solution and 50 µl of each different sample concentration and the standard were mixed in a 96-well microtiter plate followed by dark incubation at RT for half an hour. The control well was prepared by adding 150 µl of DPPH and 50 µl of methanol. The absorbance was recorded at 517 nm using a UV-visible spectrophotometer.17
Scavenging activity (%) of the crude extracts was calculated:
DPPH scavenging activity (%) = [Absorbance (control) – Absorbance (sample) / Absorbance (control)] x 100
where is the control (DPPH + methanol) and is the sample (DPPH + sample concentration).
ABTS 2, 2′-azino-bis (3-ethylbenzthiazoline -6-sulphonic acid) stock solution (7 mM) was mixed with K2S2O8 (2.45 mM) in a ratio of 1:1, followed by incubation in dark for 12 hours at RT. The blue-green radicals (ABTS+) generated by this reaction were diluted with methanol to obtain an OD of 0.7 at 734 nm. ABTS+ was reduced in the presence of antioxidant compounds in the extracts. The sample concentration (50 µl) was mixed with 150 µl of ABTS+ working solution, and the control was prepared by adding 150 µl of ABTS+ working solution with 50 µl of methanol. Absorbance was recorded at 734 nm.18
Scavenging activity (%) of the crude extracts was calculated:
ABTS scavenging activity (%) [Absorbance (control) – Absorbance (sample) / Absorbance (control)]= x 100
The antioxidant activities of the crude aqueous extracts were evaluated using a reducing power assay. The FRAP (Ferric reducing antioxidant power) assay relies on the reduction of Fe3+ to Fe2+ using a reductant. The binding of Fe2+ to the ligand creates a blue complex. Absorbance was recorded at 593 nm. The presence of antioxidants in the sample increased the optical density, which may be related to the reduction of iron.19
Phytochemical analysis of extracts
To identify the different classes of active chemical constituents, the extracts were subjected to preliminary phytochemical screening with minor modifications.20-22
Estimation of Total Phenolic and Tannin Content
TPC was determined following a procedure based on Gonzalez-Palma et al. with slight modifications. In a 96-well microtiter plate, 10 µl of the sample was combined with 80 µl of dH2O and mixed with 10 µl of the FC reagent (10%) and 100 µl of Na2CO3 (7%). The plates were then incubated in the dark for 60 min at room temperature (RT). The absorbance was recorded using a UV spectrophotometer at 725 nm. Gallic acid in concentrations ranging from 7.81 to 1000 µg/ml was used as a standard for calibration.
Quantitative analysis of tannins was conducted using the Folin-Ciocalteu method, as described by Haile and Kang, with slight modifications. In this method, 10 µl of the sample was added to a 96-well microtiter plate containing 40 µl of distilled water, 50 µl of FC phenol reagent, and 100 µl of a 35% Na2CO3 solution. The plates were shaken and incubated at RT for 30 min. Absorption readings were recorded at 760 nm, both for tannic acid standard solutions ranging from 7.81 to 1000 µg/ml and for the sample. The amount of total tannic acid was quantified in milligrams of tannic acid equivalents using the calibration curve method.23
Chemical characterization
To prepare the extracts for analysis, they were first centrifuged at 3,000 rpm to remove any particulate matter, followed by filtration through a 0.4 µm filter. The resulting filtrate was diluted 1:10 using the same solvent. A UV-visible spectrophotometer (slit width of 2 mm, 10 mm cell at RT) was used for the measurements. The spectrophotometer covered a wavelength range of 200–1,100 nm, allowing the assessment of absorbance and peak values in the visible and UV light spectra.24
Fourier transform infrared (FTIR) analysis was used to identify the characteristic functional groups present in the aqueous extracts. For this analysis, 1 mg of the dried extract powder was mixed with 10 mg of dry KBr pellets to create translucent sample discs. The discs were then placed in the sample cups within a diffuse reflectance accessory. The peak values of the IR spectrum were obtained using an FTIR spectroscope (scan range from 400 to 4,000 cm-1 with a resolution of 4 cm-1 Shimadzu, Japan).24
LC-MS/MS analysis
The aq. Extracts from the four selected plants were subjected to LC-MS analysis. The analysis was conducted using a UHPLC system coupled with a Thermo Fisher-QExactive plus mass spectrometer in positive ionization mode. The mass spectrometer was equipped with a heated electrospray ionization (ESI) source in a Z-spray configuration. The analytical column used was a reverse phase syncronis C-18 column with dimensions of 4.6 x 250 mm and maintained at a temperature of 30°C. A 10 µl sample was injected onto the column, and the flow rate was set at 200 µl/min. A mobile phase consisting of 0.1% formic acid in acetonitrile and 0.1% formic acid in water in a ratio of 75:25 was used. Data acquisition and processing were conducted using Compound Discoverer software, which facilitated the identification and quantification of the compounds present in the extracts.
Yield percentage of extracts
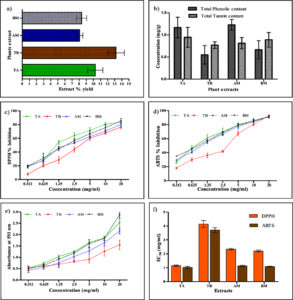
The percentage yields of the extracts obtained from various plant parts are shown in Figure 1a. Our study found that The crude extraction yields were as follows: TB, 14.5%; TA, 10.75%; AM, 8.25%; and BM, 7.5% (w/w). Previous studies have consistently reported that aqueous extracts from different plant parts tend to yield higher amounts than solvent-based extracts from the same plant.25 This phenomenon can be attributed to the prolonged incubation time and the high polarity of water as the extraction solvent.
Antibacterial potential of extracts
The antimicrobial activities of the extracts against the test organisms displayed varying degrees of inhibition, as summarized in Table 1. All four extracts were effective against the tested organisms, and the zones of inhibition were quantified, excluding the diameter of the well. All extracts demonstrated substantial inhibitory effects at 20 mg/mL. TA extract showed maximum inhibition against E. coli (13.43 ± 0.74 mm) and was less effective against V. alginolyticus (8.40 ± 0.52 mm). TA extract exhibited the highest level of inhibition against E. coli (13.43 ± 0.74 mm) but showed relatively lower effectiveness against V. alginolyticus (8.40 ± 0.52 mm). Similarly, it demonstrated equal inhibitory activity against M. smegmatis and three clinically significant bacterial isolates, S. aureus, P. aeruginosa, and E. coli 385. Previous studies have reported the antibacterial activity of TA (bark) extracts against various clinical isolates, including ear infections, with inhibition zones reported for E. coli (15 mm), S. aureus (17 mm), P. aeruginosa (16 mm), and B. subtilis (11 mm).26
Table (1):
Antimicrobial activity of aqueous extracts (20 mg/ml) of selected plants against bacterial strains and lab isolates
| Test organisms | Zone of inhibition (mm) | |||
|---|---|---|---|---|
| T. arjuna | T. bellirica | A. marmelos | B. monnieri | |
| E. coli | 13.43 ±.74 | 13.91±.35 | 16.85±.57 | 11.42±.35 |
| B. subtilis | 11.37±.62 | 14.51±.64 | 16.09±.60 | 9.11±.35 |
| M. smegmatis | 9.65±.49 | 12.95±.55 | 17.60±.31 | 11.92±.56 |
| V. alginolyticus | 8.40±.52 | 16.20±.31 | 15.63±.55 | 8.81±.33 |
| P. aeruginosa | 8.95±.56 | 16.31±.46 | 13.64±.56 | 10.90±.32 |
| S. aureus | 9.37±.55 | 17.19±.58 | 15.67±.58 | 20.40±.31 |
| E. coli 385 | 9.42±.50 | 19.51±.61 | 10.52±.45 | 16.10±.31 |
Another study found that TA bark extract (both cold and hot) was effective against S. aureus but lacked inhibitory potential against P. aeruginosa and E. coli.27 Kumar et al. reported similar inhibitory potential of TA bark extract against B. subtilis, E. coli, S. aureus, and P. aeruginosai.6 These findings align with the observations of Gupta and Kumar, who investigated the antimicrobial effects of TA against E. coli, P. aeruginosa, and S. aureus, all of which exhibited high sensitivity to the chloroform-methanol extract.28 Furthermore, this antimicrobial effect of TA is consistent with a study conducted by Jaiswal and Kumar, who assessed the antimicrobial effect of the ethyl acetate extract of TA against six pathogenic microbes.29 Numerous other studies support the results of the current study.27,30,31
The strains of S. aureus, P. aeruginosa, and E. coli 385 used in this study were susceptible to TB. The aqueous extract of TB exhibited significant inhibition of E. coli 385 (19.51 ± 0.61 mm), followed closely by BM (16.10 ± 0.31 mm), while TA extract was less effective against E. coli 385. These findings are consistent with the results of a study conducted by Aqil et al, who investigated the antimicrobial effect of a TB ethanolic extract against methicillin-sensitive and methicillin-resistant S. aureus.32 Devi et al assessed the antimicrobial potential of an aqueous extract of TB in restricting the growth of various microbes, including E. coli and P. aeruginosa, as well as some fungal isolates.33 Several other researchers have examined the antimicrobial properties of TB, and their findings are consistent with those of the current study.34,35
As indicated in Table 1, the TB extract displayed the most potent inhibitory effect against all the selected bacterial strains compared to the aqueous extracts of the other plants. Notably, this study used water for phytochemical extraction and the aqueous extract exhibited strong inhibitory effects against the clinical isolates used in this study, which are known to exhibit high levels of drug resistance.
BM also effectively inhibited the growth of the microbes examined in this study. BM exhibited the largest zone of inhibition against S. aureus (20.40 ± 0.31 mm). Previous studies, such as that by TA et al., have assessed the antimicrobial potential of BM against clinical isolates of pathogenic strains, with the methanolic extract displaying a more potent inhibitory effect against K. pneumoniae and S. aureus.36 Haque et al. have described the inhibitory potential of BM against multidrug-resistant urinary tract infections (UTI) and respiratory tract infections (RTI). The diverse array of phytochemicals found in these extracts underscores their pharmaceutical significance.37 Furthermore, Kothari et al., Abhirami et al. and Ganapathy et al. investigated the antimicrobial effects of AM against five bacterial and three fungal strains using various solvents, such as chloroform, petroleum ether, and ethyl acetate.38-40 The current findings also confirmed the effectiveness of AM against multidrug-resistant E. coli 385.
Minimum inhibitory (MIC) and bactericidal (MBC) concentration
The results of the current study demonstrated that the water extracts efficiently inhibited the growth of both the test strains and laboratory isolates to varying degrees within the wells. The antimicrobial potential of the extracts directly correlated with an increase in extract concentration, as supported by previous studies.41 All microorganisms used in this study were susceptible to the Minimum Inhibitory Concentration (MIC) of each plant extract, as presented in Table 2. The lowest MIC (0.312 mg/l) and MBC (0.625 mg/ml) values were observed for TB against S. aureus, with an MIC of (0.625 mg/ml) against B. subtilis, V. alginolyticus, P. aeruginosa, and E. coli 385. TB fruit extract has previously been reported to be effective against drug-resistant P. aeruginosa and MRSA at very low concentrations, with MIC values ranging from 0.25 to 0.5 mg/ml.35
Table (2):
Minimum inhibitory concentration (MIC) and MBC of aqueous extracts of plant parts
| Test organisms | Plant extracts (mg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|
| T. arjuna | T. bellirica | A. marmelos | B. monnieri | |||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| E. coli | 2.5 | 5 | 1.25 | 2.5 | 1.25 | 2.5 | 2.5 | 5 |
| B. subtilis | 2.5 | 5 | 0.625 | 0.125 | 1.25 | 2.5 | 2.5 | 5 |
| M. smegmatis | 2.5 | 5 | 2.5 | 5 | 1.25 | 2.5 | 2.5 | 5 |
| V. alginolyticus | 2.5 | 5 | 0.625 | 1.25 | 1.25 | 2.5 | 2.5 | 5 |
| P. aeruginosa | 2.5 | 5 | 0.625 | 1.25 | 1.25 | 2.5 | 2.5 | 5 |
| S. aureus | 5 | 10 | 0.312 | 0.625 | 1.25 | 2.5 | 1.25 | 2.5 |
| E. coli 385 | 2.5 | 5 | 0.625 | 1.25 | 1.25 | 2.5 | 2.5 | 5 |
The TA and BM extracts exhibited MIC values of (2.5 mg/ml) and MBC values of (5.0 mg/ml) against all tested strains, except S. aureus. In a previous study, the MIC value of TA bark aqueous extract was reported to be 25 mg/ml against S. aureus, 12.5 mg/ml against E. coli and B. subtilis, and 6.3 mg/ml against P. aeruginosa.6 TA displayed activity at an MIC of (5.0 mg/ml) and an MBC of (10 mg/ml), while BM exhibited an MIC and MBC of 1.25 mg/ml and 2.5 mg/ml, respectively, against S. aureus.
Determination of phytochemical components
Preliminary analysis of the extracts revealed the presence of phenols, sterols, coumarins, tannins, and saponins in the selected plants. Phytochemicals play important roles in the medicinal properties of plants.
Phenols, which were found in all extracts, are well-known secondary metabolites with significant antioxidant potential. They reduce the risk of cancer, inflammation, and cardiovascular diseases.42 Coumarins, also identified in the extracts, exert anti-inflammatory and antioxidant effects by blocking the generation of ROS (reactive oxygen species) and downregulating key pathways like NF-κB, TNF-α, and MAPKs, which are associated with inflammation and oxidative stress.43 Tannins, another group of phytochemicals present in plant extracts, are polyphenolic compounds with known antioxidant properties. Traditionally, they have been used to treat a range of health issues, including gastrointestinal, respiratory, and skin problems.44 Saponins have various health benefits, including hypocholesterolemic, immune modulation, and anti-inflammatory properties, which have led to their use in traditional pharmaceutical applications.
Quantitative analysis of phytochemicals
Using the FC reagent, the total phenolic content (TPC) of the extracts (TA, TB, AM, and BM) was calculated (Figure 1b) and expressed as gallic acid equivalent (GAE). The results indicated that the extract of AM had the highest TPC with a value of 1.22 ± 0.12 mg/g, followed by TA (1.17 ± 0.23 mg/g), TB (0.66 ± 0.20 mg/g), and BM (0.55 ± 0.21 mg/g). Previous studies have reported much higher concentrations of TPC when different solvents were used for the extraction of phytochemicals, as opposed to water. For example, in the case of TA, TPC values of 96.42, 184.59, and 137.98 mg/g GAE were determined in the methanolic extracts of the leaves, bark, and fruits, respectively.45 Meena et al. reported TPC values ranging from 128 to 1228 µg/mL GAE in the distilled water extract of TA bark, which are quite similar to the present study. Kumar et al. found a TPC of 131.9 mg/g GAE in the aqueous fraction of TA bark extract, whereas Kumar et al. reported TPC values of 233.64 mg/g GAE in TA bark and nearly 200 mg/g in the fruit methanolic
extract of TA.6, 46,47
It is well-established that the phenolic content in different solvent extracts is generally higher than that in aqueous extracts.48 The high concentration of phenolic compounds in the plant extracts indicated their strong radical-scavenging potential.49-52 For example, a 2019 study reported nearly 60 mg/g GAE in the aqueous extract of TB fruit.53 The BM contained 67-79 mg/g GAE in the aqueous extract, whereas the methanolic extract contained 37-55 mg/g GAE.54 The AM extract to have 31.6 mg/g GAE.55 The presence of phenols in AM indicates the antidiabetic potential of plants.
The total tannic acid contents of the extracts (TA, TB, AM, and BM) ranged from 0.775 to 0.952 mg TAE/g (Figure 1b). TA extract contained the highest amount of tannins (0.95 ± 0.22 mg/g), followed by TB (0.89 ± 0.16 mg/g), AM (0.87 ± 0.15 mg/g), and BM (0.77 ± 0.07 mg/g). The tannin contents reported in previous studies were often higher than those observed in the present study. For instance, the fruits of TB are generally reported to be enriched with 23.60–37.36% tannin content.56 However, the quantity of phytochemicals in the same plant can vary owing to environmental factors that vary by location.57
Radical scavenging and reducing power of plants extracts
The antioxidant potential of the extracts was evaluated using various assays, including DPPH and ABTS radical scavenging assays as well as a reducing power assay. Antioxidants play a crucial role in protecting the body from the damage caused by free radicals by donating hydrogen atoms or electrons to neutralize these harmful molecules. In the DPPH assay, antioxidants reduce DPPH radicals, resulting in a color change from purple to yellow. The effectiveness of antioxidants in scavenging DPPH radicals was indicated by their ability to reduce the absorbance of the radical solution. The lower the EC50 value, the higher the antioxidant capacity.58
The results of the present study demonstrated significant scavenging activity of all four extracts (Figure 1 c-f). TA extract exhibited the highest DPPH radical scavenging activity with the lowest EC50 value (1.14 ± 0.06 mg/ml), followed by BM (2.20 ± 0.07 mg/ml), AM (2.34 ± 0.05 mg/ml), and TB (4.15 ± 0.25 mg/ml) (Figure 1c). Comparing these results with those of previous studies, it is evident that the DPPH scavenging potentials of these extracts vary. For example, one study reported an IC50 value of 10.3 µg/ml in the aqueous fraction of TA bark extract. The IC50 values were generally lower for the other solvent extracts, indicating their strong DPPH scavenging potential.6
Another study reported an IC50 value of 13.3 µg/ml in the methanolic fraction of TA bark extract.45 For BM, the ethanolic extract showed an IC50 value of 90 mg/l for DPPH scavenging ability.59
Similar results were obtained by Nampoothiri et al., who found that the methanolic extract of TB showed a higher radical scavenging effect than the aqueous fraction. In the case of methanolic extract, 1.2 µg/ml IC50 value was obtained, while in the case of water extract, 2.43 µg/ml IC50 value was calculated. However, the antioxidant effect of the hexane extract was much lower than that of water.60 For the aqueous extract of TB, 3.5 an IC50 value was reported.61 The methanolic extract of AM exhibited 961.53 µg/ml IC50 value.62 Vardhini et al.63 reported that the aqueous AM exhibited 183.58 an IC50 value. The IC50 of the methanolic fraction of the leaf extract was 63.52 µg/mL.64
The extract of TA showed the highest activity for ABTS+ with an EC50 (1.02 ± 0.079 mg/ml), BM (1.09 ± 0.027 mg/ml), AM (1.13 ± 0.048 mg/ml) and TB (3.71 ± 0.18 mg/ml) (Figure 1d). In the ABTS assay, 52.6 µg/ml IC50 value for the aqueous extract of TB was reported, which was quite high as compared to solvent extracts, suggesting the low antioxidant potential of water extract.61 In another study, 10.81 µg/mL IC50 value for the aqueous extract of AM was reported.63 Similar to the DPPH assay, the antioxidant effect of the water extract was quite low compared to that of the other solvent extracts in the ABTS assay of TA.46 Leaf extract of TB exhibited 11 µg/ml IC50 value from the aqueous fraction, indicating a better antioxidant effect as compared to the result of the present study.65 The inhibition of ABTS with the water fraction of TA was observed with an IC50 value of 251 µg/ml which was quite low as compared to ethanolic extract.66
The extracts of the selected plants showed significant electron-donating activity in a concentration-dependent manner by reducing ferric ions to ferrous ions. BM exhibited the highest reducing power was shown by BM followed by TA, AM, and TB (Figure 1e). The reducing power of an antioxidant corresponds to the color change from yellow to green or blue. A higher absorbance indicates a higher ferric-reducing power of the plant extracts. Phenols and tannins in the extracts act as radical scavengers by donating electrons and hydrogen. Researchers have reported that the ethanolic bark extract of TA possesses a strong potential to reduce Fe2+ compared with the distilled water extract. Significantly better FRAP activity was observed in the ethanolic extract than in the distilled water extract, as reported by Meena et al.46 This difference in reducing potential may be due to the adequacy of the solvent used for extraction. Other studies have also reported higher FRAP activities in alcoholic extracts.67 In this study, the antioxidant potential of TB was significantly lower than that of TA. Similar results were obtained by Ayra et al., where the EC50 value for TA was 232 µg/mL and for TB, it was 265 µg/ml. This improved ability to reduce iron may be due to the high amount of polyphenols with hydroxyl groups.68
Figure 1. (a) Extraction yield percentage of aqueous plant extracts, (b) total phenolic and tannin content in extracts, (c) DPPH percentage inhibition, (d) ABTS percentage inhibition, (e) Reducing power assay (FRAP), and (f) effective concentration (EC50) values of T. arjuna, T. bellirica, A. marmelos, and B. monnieri. Value represents the means of triplicate values and bars represents the standard deviation
UV-visible spectroscopy
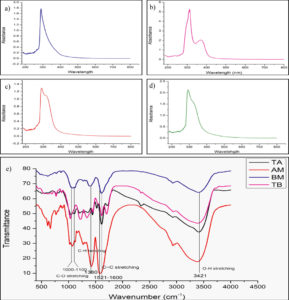
The qualitative UV-vis profiles of the plant extracts (TA, TB, AM, and BM) were recorded to identify chromophores, aromatic rings, and compounds containing σ and π-bonds. Sharp peaks were observed in the wavelength range of 200–800 nm. The profile showed peaks at 290 and 320 nm with absorptions of 1.3, 1.1, 311.5, and 370 nm with absorptions of 5.256 and 2.046 for TB, and 280 nm with an absorption of 1.45 for AM, and 292.5 nm with an absorption of 1.774 for BM, respectively (Figure 2a, b, c, and d).
Figure 2. UV-Vis absorption spectra of (a) T. arjuna (Bark), (b) T. bellirica (fruit), (c) A. marmelos (leaves), (d) B. monnieri (leaves) aqueous extracts. Fourier transform infrared (FTIR) spectrum (e) shows the presence of amine, alkanes, carboxylic acid, alcohol and phenol functional groups in aqueous extracts
FTIR
The FTIR spectroscopic analysis confirmed the presence of various functional groups in the aqueous extracts of the selected plants, as indicated by the following groups: O-H, O=C=O, C=O, C=C, N=O, C-O, C=S, C-H, C-F, C-O, and C-Br (Figure 2e). The presence of carbonyl, amine, and other functional groups in the TA extract has been reported previously.69,70 The TB extract exhibited peaks corresponding to alkanes, carboxylic acids, and alcohols, as reported by Patil et al.71 The presence of alcohols, coumarins, tannins, phenols, and aromatic stretching in AM extracts has been confirmed by many studies.72,73 The BM extract contained alcoholic O-H, carbonyl, carboxylic acid, and aromatic groups.74 The groups present in the selected plants are listed in Table 3.
Table (3):
Functional groups obtained through IR spectrum analysis of the aqueous extracts of the plants used in the present study
| Plant | Functional Group | Wavenumber cm-1 |
|---|---|---|
|
T. arjuna
|
O-H stretching | 3408 |
| O=C=O stretching | 2336 | |
| C=O stretching (Carboxylic acid) | 1718 | |
| C=C stretching | 1611 | |
| N=O stretching | 1521 | |
| N=O stretching | 1444 | |
| C-O stretching (Phenol) | 1205 | |
| C=S stretching | 1043 | |
| C-H bending (out of plane) | 767 | |
|
T. bellerica |
O-H stretching | 3388 |
| C-H stretching | 2932 | |
| C-H Vibration (polyols) | 1449 | |
| C=C stretching | 1704 | |
| C=O stretching | 1614 | |
| C-F stretching | 1342 | |
| C-O Vibration(polyols) | 1220 | |
| C-O stretching | 1034 | |
| C-H bending (out of plane) | 770 | |
|
A. marmelos |
O-H stretching | 3381 |
| C-H (Alkane) | 2932 | |
| N-O stretching | 1574 | |
| O-H bending (Alcohol) | 1417 | |
| N=O stretching | 1268 | |
| C-H bending | 1163 | |
| C-O (Alcohol) | 1072 | |
| C-N (Amine) | 1023 | |
| C-H (Aromatic) | 888 | |
| C=C Bending (Alkene) | 768 | |
| C-Br | 664 | |
| B. monnieri | O-H stretching | 3400 |
| C=C stretching | 1609 | |
| C-H bending (Alkane) | 1386 | |
| C-O stretching (Alcohol) | 1080 | |
| C-Br | 616 |
LC-MS/MS analysis
The present study employed LC-MS/MS to elucidate the chemical profiles of medicinal plant extracts. In particular, the aqueous extract of Aegle marmelos (AM) was found to contain a twenty two compounds. The compounds identified in the AM extract are listed in
Table S1, along with their molecular weight, chemical formula, retention time, and chemical structure. The notable compounds identified included levomefolic acid, commonly used as a folate supplement for food fortification; triamterene, a phenolic compound used in the treatment of edema and liver cirrhosis, and as a diuretic; and balapiravir, an example of a direct-acting antiviral that targets both structural and non-structural viral proteins.75-77 Additionally, Threonic acid, a phenolic acid, was identified for its potential to inhibit TNF-a, thus potentially offering benefits in memory preservation. Neosaxitoxin, which is known for its local anesthetic properties, and methyldilazine, which exhibits bactericidal activity against Mycobacterium tuberculosis, were also detected. Lucanthone and Remoxipride have emerged as potential antitumor agents with applications in the treatment of conditions such as glioblastoma. Gosogliptin, an anti-diabetic drug, along with Piperoleine B, Altretamine, Azanidazole, and Bortezomib, displays anti-inflammatory and anticancer activities.78-86 Furthermore, Phenothiazine has been recognized for its antibacterial activity, particularly in the treatment of tract infections. The analysis also revealed the presence of compounds such as 2-Phenylaminoadenosine, Lorcainide, Ebastine, (E)-entacapone, and rivastigmine, each with distinct pharmacological properties.87-91
In the case of Bacopa monnieri (BM) extract, LC-MS/MS analysis unveiled the presence of 10 compounds (Table S2). Among these, Aminolevulinic acid, which is recognized for its role in treating actinic keratosis, and propicillin, an antibacterial agent, are noteworthy. Promethazine, an antihistamine, and Ciclazindol, an antidepressant and anorectic drug, were also identified. Morclofone, with its antitussive properties, and Pexacerfont, used in the treatment of alcoholism and anxiety disorders, were present. Tenofovir, known for its antiviral activity, and avenanthramides D, which exhibits antineoplastic properties, were detected.92-97
LC-MS/MS analysis of Terminalia arjuna (TA) extract revealed the presence of five compounds (Table S3). Notably, Thiethylperazine (TEP), known for its anti-nausea properties, was among the identified compounds. TEP is currently undergoing clinical trials (phase 2) for potential applications in Alzheimer’s Disease treatment. Additionally, the extract contains compounds such as oxyphenonium, which is used to treat visceral spasms, and octinoxate, a sunscreen ingredient. Altretamine, which is known for its antineoplastic properties, was also detected.98,99 (https://pubchem.ncbi.nlm.nih.gov/compound/5749).
In the case of Terminalia bellirica (TB) extract, LC-MS/MS analysis identified approximately nine compounds (Table S4). Notably, Tiletamine, an anesthetic and anticonvulsant agent, and (E)-progabide, which are used to treat epilepsy, were among the identified compounds. Tetraphenylphosphonium, which has potential as an antineoplastic agent, and bergenin, which is known for its antifungal and antiviral properties, were also detected. Cyproterone, recognized for its anti-cancer properties, further expands the potential therapeutic applications of TB extract.100–105
The use of herbal products for disease control is an innovative alternative to antimicrobial therapies. This approach offers a non-toxic and environmentally friendly means of managing drug-resistant diseases.106 Owing to the rapid development of resistance against chemotherapeutic drugs (mainly antibiotics), it has become vital to consider any substitute and efficient therapies such as herbs.107 This study explored the potential of herbal products as antibacterial agents against resistant and susceptible bacteria. These findings support the significant role of plant extracts in the treatment of bacterial infections. The active extracts identified in this study offer valuable insights into the development of new compounds that exhibit enhanced activity and effectiveness against both resistant (MDR, XDR, and PDR) and susceptible bacteria responsible for different infections, surpassing the capabilities of currently available antibiotic agents. The aqueous extracts, namely TA, TB, AM, and BM, exhibited significant antimicrobial activity against various clinically important bacterial strains, including multidrug-resistant Escherichia coli 385. In particular, the TB extract demonstrated high inhibitory activity against E. coli. The minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC) of these extracts were determined to determine their efficacy against drug-resistant strains. Furthermore, qualitative analysis of these extracts using UV-visible spectroscopy revealed the presence of chromophores and compounds containing various chemical bonds. FTIR spectroscopy confirmed the presence of functional groups that may have contributed to the medicinal properties of the extracts. LC-MS/MS analysis provided insights into the chemical composition of the extracts, identifying various compounds with potential therapeutic applications. These findings highlight the promising antimicrobial and antioxidant properties of these aqueous plant extracts and suggest their potential utility in medicinal and pharmaceutical applications. Further in silico analyses of the retrieved compounds are warranted to explore the specific bioactive components and their mechanisms of action.
Currently, cutting-edge methods in biotechnology, genomics, proteomics, and metabolomics are used to study medicinal plants, leading to the development of novel natural antimicrobials. Therefore, there is a need to develop integrated technologies that can simultaneously identify active chemical mixtures, characterize the nature of their interactions, and elucidate their probable mechanisms of action. The combination of these evaluation methods with developments in instrument automation will provide significant opportunities to utilize the variety of bioactive chemicals found in medicinal plants in the search for new antimicrobial medications.
The study findings demonstrate the ability of the examined medicinal plants to inhibit the growth of drug-resistant bacteria. These plants have the potential to serve as organic reservoirs of antioxidants. Additional refinement and separation of biologically active substances in these botanical extracts would enable determination of the mode of operation and potential candidate compounds for the advancement of novel pharmaceuticals. Priority should be given to conducting studies that focus on the mechanisms of action, interactions with antibiotics or other medicinal plants or substances, and pharmacokinetic and pharmacodynamic profiles of the extracts. The present study is expected to provide valuable insights into the key problems encountered in this field. These insights will aid in the development of more efficient, successful, and direct techniques to expedite the utilization of new therapeutic medicinal plants against resistant strains.
Additional file: Additional Table S1-S4.
ACKNOWLEDGMENTS
The authors sincerely acknowledge the Central University of Haryana, Mahendergarh, for the necessary facilities.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
MT and VY conceptualized the study. MT, AY and VY applied methodology. AY and MT performed formal analysis. VY performed visualization and supervised the study. MT performed investigation and wrote original draft. K, KKD, VY and TCD wrote, reviewed and edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript and/or in the supplementary files.
ETHICS STATEMENT
Not applicable.
- Thakur M, Kumar Y, Yadav V, Pramanik A, Dubey KK. Understanding resistance acquisition by Pseudomonas aeruginosa and possible pharmacological approaches in palliating its pathogenesis. Biochem Pharmacol. 2023;215:115689.
Crossref - Chassagne F, Samarakoon T, Porras G, et al. A systematic review of plants with antibacterial activities: A taxonomic and phylogenetic perspective. Front Pharmacol. 2021;11:2069.
Crossref - Khan MF, Tang H, Lyles JT, Pineau R, Mashwani ZUR, Quave CL. Antibacterial properties of medicinal plants from Pakistan against multidrug-resistant ESKAPE pathogens. Front Pharmacol. 2018;9:815.
Crossref - Wylie MR, Merrell DS. The Antimicrobial Potential of the Neem Tree Azadirachta indica. Front Pharmacol. 2022;13:891535.
Crossref - Chithambharan A, Pottail L, Mirle RM, Rajalakshmi R, Ponnusamy A. Bioinspired Gold Nanoparticle Synthesis Using Terminalia bellerica Fruit Parts and Exploring Their Anti-bacterial Potency In Vitro. Indian J Microbiol. 2021;61(3):298-305.
Crossref - Kumar V, Sharma N, Sourirajan A, Khosla PK, Dev K. Comparative evaluation of antimicrobial and antioxidant potential of ethanolic extract and its fractions of bark and leaves of Terminalia arjuna from north-western Himalayas, India. J Tradit Complement Med. 2018;8(1):100-106.
Crossref - Gopinath K, Venkatesh KS, Ilangovan R, Sankaranarayanan K, Arumugam A. Green synthesis of gold nanoparticles from leaf extract of Terminalia arjuna, for the enhanced mitotic cell division and pollen germination activity. Ind Crops Prod. 2013;50:737-742.
Crossref - Saha A, Pawar VM, Jayaraman S. Characterisation of polyphenols in Terminalia arjuna bark extract. Indian J Pharm Sci. 2012;74(4):339.
Crossref - Dwivedi S, Chopra D. Revisiting Terminalia arjuna-an ancient cardiovascular drug. J Tradit Complement Med. 2014;4(4):224-231.
Crossref - Deb A, Barua S, Das B. Pharmacological activities of Baheda (Terminalia bellerica): a review. J Pharmacogn Phytochem. 2016;5(1):194.
- Devi M, Devi S, Sharma V, Rana N, Bhatia RK, Bhatt AK. Green synthesis of silver nanoparticles using methanolic fruit extract of Aegle marmelos and their antimicrobial potential against human bacterial pathogens. J Tradit Complement Med. 2020;10(2):158-165.
Crossref - Mehta J, Utkarsh K, Fuloria S, et al. Antibacterial Potential of Bacopa monnieri (L.) Wettst. and Its Bioactive Molecules against Uropathogens-An In Silico Study to Identify Potential Lead Molecule (s) for the Development of New Drugs to Treat Urinary Tract Infections. Molecules. 2022;27(15):4971.
Crossref - James DB, Kadejo OA, Nwochiri C, Luca CD. Determination of Phytochemical Constituents of the Aqueous Extracts of the Leaves, Stem Bark and Root Bark of Vitex doniana and its Effects on Lipid Profile of Albino Rats. British Journal of Pharmacology and Toxicology. 2013;4(6):210-214.
Crossref - Okigbo RN, Anuagasi CL, Amadi JE, Ukpabi UJ. Potential inhibitory effects of some African tuberous plant extracts on Escherichia coli, Staphylococcus aureus and Candida albicans. Int J Integr Biol. 2009;6(2):91-98.
- Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564-582.
Crossref - Rajurkar NS, Hande SM. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J Pharm Sci. 2011;73(2):146.
Crossref - Bursal E, Gulcin İ. Polyphenol contents and in vitro antioxidant activities of lyophilised aqueous extract of kiwifruit (Actinidia deliciosa). Food Res Int. 2011;44(5):1482-1489.
Crossref - Ben Nejma A, Znati M, Nguir A, et al. Phytochemical and biological studies of Atriplex inflata f. Muell.: isolation of secondary bioactive metabolites. J Pharm Pharmacol. 2017;69(8):1064-1074.
Crossref - Xiao F, Xu T, Lu B, Liu R. Guidelines for antioxidant assays for food components. Food Front. 2020;1(1):60-69.
Crossref - CK Kokate. Practical Pharmacognosy. 4th ed. Vallabh Prakashan. 1997.
- Harborne JB. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. 2nd ed. Chapman and Hall. 1998.
- Trease GE, Evans WC. Textbook of Pharmacognosy. 12th ed. London: Tindall and Co.; 1983:343-383
- Haile M, Kang WH. Antioxidant activity, total polyphenol, flavonoid and tannin contents of fermented green coffee beans with selected yeasts. Fermentation. 2019;5(1):29.
Crossref - Kalaichelvi K, Dhivya SM. Screening of phytoconstituents, UV-VIS Spectrum and FTIR analysis of Micrococca mercurialis (L.) Benth. Int J Herb Med. 2017;5(6):40-44.
- Caleja C, Barros L, Antonio AL, Carocho M, Oliveira MBPP, Ferreira ICFR. Fortification of yogurts with different antioxidant preservatives: A comparative study between natural and synthetic additives. Food Chem. 2016;210:262-268.
Crossref - Debnath S, Dey D, Hazra S, Ghosh S, Ray R, Hazra B. Antibacterial and antifungal activity of Terminalia arjuna Wight & Arn. bark against multi-drug resistant clinical isolates. J Coast Life Med. 2013;4(4):315-321.
- Aneja KR, Sharma C, Joshi R. Antimicrobial activity of Terminalia arjuna Wight & Arn.: An ethnomedicinal plant against pathogens causing ear infection. Braz J Otorhinolaryngol. 2012;78(1):68-74.
Crossref - Gupta D, Kumar M. Evaluation of in vitro antimicrobial potential and GC-MS analysis of Camellia sinensis and Terminalia arjuna. Biotechnol Rep. 2017;13:19-25.
Crossref - Jaiswal P, Kumar P. Antimicrobial screening of free and bound flavonoid from the bark of Terminalia arjuna. J Phytopharmacol. 2015;4(6):303-306.
Crossref - Mandal S, Patra A, Samanta A, et al. Analysis of phytochemical profile of Terminalia arjuna bark extract with antioxidative and antimicrobial properties. Asian Pac J Trop Biomed. 2013;3(12):960-966.
Crossref - Khan R, Islam B, Akram M, et al. Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecules. 2009;14(2):586-597.
Crossref - Aqil F, Khan MSA, Owais M, Ahmad I. Effect of certain bioactive plant extracts on clinical isolates of β-lactamase producing methicillin resistant Staphylococcus aureus. J Basic Microbiol. 2005;45(2):106-114.
Crossref - Devi PN, Kaleeswari S, Poonkothai M. Antimicrobial activity and phytochemical analysis of fruit extracts of Terminalia bellerica. Int J Pharm Pharm Sci. 2014;6(5):639-642.
- Sumathi P, Parvathi A. Antibacterial potential of the three medicinal fruits used in Triphala: An Ayurvedic formulation. J Med Plant Res. 2010;4(16):1682-1685.
- Dharmaratne M, Manoraj A, Thevanesam V, et al. Terminalia bellirica fruit extracts: in-vitro antibacterial activity against selected multidrug-resistant bacteria, radical scavenging activity and cytotoxicity study on BHK-21 cells. BMC Complement Altern Med. 2018;18(1):1-12.
Crossref - TA H, AS A, Suseelan S, RK JC, PV D. Antimicrobial activity of five South Indian medicinal plants against clinical pathogens. International Journal of Pharma and Biosciences. 2013;4(1):70-80.
- Haque SM, Chakraborty A, Dey D, Mukherjee S, Nayak S, Ghosh B. Improved micropropagation of Bacopa monnieri (L.) Wettst.(Plantaginaceae) and antimicrobial activity of in vitro and ex vitro raised plants against multidrug-resistant clinical isolates of urinary tract infecting (UTI) and respiratory tract infecting (RTI). Clinical Phytoscience. 2017;3(1):1-10.
Crossref - Kothari S, Mishra V, Bharat S, Tonpay SD. Antimicrobial activity and phytochemical screening of serial extracts from leaves of Aegle marmelos (Linn.). Acta Pol Pharm. 2011;68(5):687-692.
- Ganapathy S, Karpagam S. In vitro antibacterial and phytochemical potential of Aegle marmelos against multiple drug resistant (MDR) Escherichia coli. J Pharmacogn Phytochem. 2016;5(1):253.
Crossref - Abirami SKG, Vivekanandhan K, Hemanthkumar R, Prasanth S, Kumar JR. Study of antimicrobial potential of Aegle marmelos. J Med Plants. 2014;2(2):113-116.
- Bhalodia NR, Shukla VJ. Antibacterial and antifungal activities from leaf extracts of Cassia fistula l.: An ethnomedicinal plant. J Adv Pharm Technol Res. 2011;2(2):104.
Crossref - Kisiriko M, Anastasiadi M, Terry LA, Yasri A, Beale MH, Ward JL. Phenolics from medicinal and aromatic plants: Characterisation and potential as biostimulants and bioprotectants. Molecules. 2021;26(21):6343.
Crossref - Majnooni MB, Fakhri S, Shokoohinia Y, et al. Phytochemicals: potential therapeutic interventions against coronavirus-associated lung injury. Front Pharmacol. 2020;11:588467.
Crossref - Marcinczyk N, Gromotowicz-Poplawska A, Tomczyk M, Chabielska E. Tannins as Hemostasis Modulators. Front Pharmacol. 2022;12:4055.
Crossref - Abo-Elghiet F, Abd-elsttara A, Metwaly AM, Mohammad AE. Antioxidant, anti-inflammatory, and antimicrobial evaluation of Terminalia arjuna leaves, fruits, and bark. Azhar Int J Pharm Med Sci. 2022;2(2):148-158.
Crossref - Meena DK, Sahoo AK, Srivastava PP, et al. On valorization of solvent extracts of Terminalia arjuna (arjuna) upon DNA scission and free radical scavenging improves coupling responses and cognitive functions under in vitro conditions. Sci Rep. 2021;11(1):1-20.
Crossref - Kumar V, Chandel SR, Guleria S, et al. Comparative analysis of phytochemicals, antimicrobial and antioxidant activity of different species of Terminalia from Himachal Pradesh, India. Vegetos. 2021;34(3):528-539.
Crossref - Guleria S, Kumar V, Chandel SR, et al. Antioxidant and antimicrobial activity of ethanolic extract and its fractions from fruit and leaves of Terminalia chebulafrom himachal pradesh, india. Plant Arch. 2020;2(2):4753-4761.
- Gupta A, Kumar R, Pandey AK. Antioxidant and antidiabetic activities of Terminalia bellirica fruit in alloxan induced diabetic rats. S Afr J Bot. 2020;130:308-315.
Crossref - Petridis A, Therios I, Samouris G, Tananaki C. Salinity-induced changes in phenolic compounds in leaves and roots of four olive cultivars (Olea europaea L.) and their relationship to antioxidant activity. Environ Exp Bot. 2012;79:37-43.
Crossref - Trendafilova A, Ivanova V, Rangelov M, et al. Caffeoylquinic acids, cytotoxic, antioxidant, acetylcholinesterase and tyrosinase enzyme inhibitory activities of six Inula species from Bulgaria. Chem Biodivers. 2020;17(4):e2000051.
Crossref - Rakholiya K, Vaghela P, Rathod T, Chanda S. Comparative study of hydroalcoholic extracts of Momordica charantia L. against foodborne pathogens. Indian J Pharm Sci. 2014;76(2):148-156.
- Chandel SR, Kumar V, Guleria S, et al. Sequential fractionation by organic solvents enhances the antioxidant and antibacterial activity of ethanolic extracts of fruits and leaves of Terminalia bellerica from North Western Himalayas, India. Pharmacognosy Journal. 2019;11(1):94-101.
Crossref - Charoenphon N, Kangwanrangsan N, Jiraungkoorskul W. Artemia salina lethality and histopathological studies on Bacopa monnieri leaf extract. Indian J Anim Res. 2018;52(4):610-614.
Crossref - Ahmad W, Amir M, Ahmad A, et al. Aegle marmelos Leaf Extract Phytochemical Analysis, Cytotoxicity, In Vitro Antioxidant and Antidiabetic Activities. Plants. 2021;10(12):2573.
Crossref - Singh A, Bajpai V, Kumar S, Kumar B, Srivastava M, Rameshkumar KB. Comparative profiling of phenolic compounds from different plant parts of six Terminalia species by liquid chromatography-tandem mass spectrometry with chemometric analysis. Ind Crops Prod. 2016;87:236-246.
Crossref - Bhakta D, Ganjewala D. Effect of leaf positions on total phenolics, flavonoids and proanthocyanidins content and antioxidant activities in Lantana camara (L). J Sci Res. 2009;1(2):363-369.
Crossref - Singh G, Passsari AK, Leo VV, et al. Evaluation of phenolic content variability along with antioxidant, antimicrobial, and cytotoxic potential of selected traditional medicinal plants from India. Front Plant Sci. 2016;7:407.
Crossref - Eze FN, Ingkaninan K, Prapunpoj P. Transthyretin anti-amyloidogenic and fibril disrupting activities of Bacopa monnieri (L.) Wettst (Brahmi) extract. Biomolecules. 2019;9(12):845.
Crossref - Nampoothiri S V, Prathapan A, Cherian OL, Raghu KG, Venugopalan V V, Sundaresan A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food Chem Toxicol. 2011;49(1):125-131.
Crossref - Shah S, Dhanani T, Kumar S. Comparative evaluation of antioxidant potential of extracts of Vitex negundo, Vitex trifolia, Terminalia bellerica, Terminalia chebula, Embelica officinalis and Asparagus racemosus. Innovations in Pharmaceuticals and Pharmacotherapy. 2013;1(1):44-53.
- Nemkul CM, Bajracharya GB, Shrestha I, Gan C, Bajracharya B. Phytochemical, antibacterial and DPPH free radical scavenging evaluations of the barks of Aegle marmelos (L.) Correa. J Pharmacogn Phytochem. 2018;7(4):1637-1641.
- Vardhini SP, Sivaraj C, Arumugam P, Ranjan H, Kumaran T, Baskar M. Antioxidant, anticancer, antibacterial activities and GCMS analysis of aqueous extract of pulps of Aegle marmelos (L.) Correa. J Phytopharmacol. 2018;7(1):72-78.
Crossref - Andleeb R, Ijaz MU, Rafique A, et al. Biological activities of methanolic extract of aegle marmelos against HN protein of newcastle disease virus. Agronomy. 2021;11(9):1784.
Crossref - Rakholiya K, Kaneria M, Nagani K, Patel A, Chanda S. Comparative analysis and simultaneous quantification of antioxidant capacity of four Terminalia species using various photometric assays. World J Pharm Res. 2015;4(4):1280-1296.
- Kumar GP, Navya K, Ramya EM, Venkataramana M, Anand T, Anilakumar KR. DNA damage protecting and free radical scavenging properties of Terminalia arjuna bark in PC-12 cells and plasmid DNA. Free radicals and antioxidants. 2013;3(1):35-39.
Crossref - Kumar V, Dev K, Sourirajan A, Khosla PK. Comparative antioxidant potential of bark and leaves of Terminalia arjuna (Roxb) Wight & Arn from Himachal Pradesh. Int J Pharm Phytopharmacol Res. 2016;6(1):27-33.
Crossref - Arya A, Nyamathulla S, Noordin MI, Mohd MA. Antioxidant and hypoglycemic activities of leaf extracts of three popular Terminalia species. E-Journal of Chemistry. 2012;9(2):883-892.
Crossref - Chaudhary SP, Mishra A, Singh AK, Dwivedi KN, Ram B. A FT-IR spectroscopic study of phytoconstituents of prepared formulation of arjuna (Terminalia arjuna lin.) and shilajatu. Int J Sci Res (IJSR). 2015;5:12.
- Swain KK, Mishra PM, Devi AP. Biosorption of praseodymium (III) using Terminalia arjuna bark powder in batch systems: isotherm and kinetic studies. Water Sci Technol. 2018;77(3):727-738.
Crossref - Patil S, Chaudhari G, Paradeshi J, Mahajan R, Chaudhari BL. Instant green synthesis of silver-based herbo-metallic colloidal nanosuspension in Terminalia bellirica fruit aqueous extract for catalytic and antibacterial applications. 3 Biotech. 2017;7(1):1-12.
Crossref - Patil S, Sivaraj R, Rajiv P, Venckatesh R, Seenivasan R. Green synthesis of silver nanoparticle from leaf extract of Aegle marmelos and evaluation of its antibacterial activity. Int J Pharm Pharm Sci. 2015;7(6):169-173.
- Rao KJ, Paria S. Green synthesis of silver nanoparticles from aqueous Aegle marmelos leaf extract. Mater Res Bull. 2013;48(2):628-634.
Crossref - Suganya M, Kavitha S, Mythili Gnanamangai B, Ponmurugan P. Phytofabrication of silver nanoparticles using Bacopa monnieri leaf extract and its antibacterial activity as well as oxidative stress-induced apoptosis of lung cancer. IET Nanobiotechnol. 2018;12(3):318-324.
Crossref - Lam NSK, Long XX, Li X, et al. The potential use of folate and its derivatives in treating psychiatric disorders: A systematic review. Biomed Pharmacother. 2022;146:112541.
Crossref - Zekeya N, Ibrahim M, Mamiro B, et al. Potential of natural phenolic antioxidant compounds from Bersama abyssinica (Meliathacea) for treatment of chronic diseases. Saudi J Biol Sci. 2022;29(6):103273.
Crossref - Shimu MSS, Mahmud S, Tallei TE, et al. Phytochemical compound screening to identify novel small molecules against dengue virus: a docking and dynamics study. Molecules. 2022;27(3):653.
Crossref - Jamnani MJ, Holmelid B, Vedeler A, Parsian HH, Andersen HL, Fossen T. Natural Products from Leaves of the Ancient Iranian Medicinal Plant Echium amoenum Fisch. & CA Mey. Molecules. 2023;28(1):385.
Crossref - Zhou X, Huang Z, Zhang J, et al. Chronic oral administration of magnesium-L-threonate prevents oxaliplatin-induced memory and emotional deficits by normalization of TNF-a/NF-κB signaling in rats. Neurosci Bull. 2021;37:55-69.
Crossref - Lagadinou M, Onisor MO, Rigas A, et al. Antimicrobial properties on non-antibiotic drugs in the era of increased bacterial resistance. Antibiotics. 2020;9(3):107.
Crossref - Radin DP, Smith G, Moushiaveshi V, Wolf A, Bases R, Tsirka SE. Lucanthone targets lysosomes to perturb glioma proliferation, chemoresistance and stemness, and slows tumor growth in vivo. Front Oncol. 2022;12:852940.
Crossref - Weissenrieder JS, Reed JL, Green M V, et al. The dopamine D2 receptor contributes to the spheroid formation behavior of U87 glioblastoma cells. Pharmacology. 2020;105(1-2):19-27.
Crossref - Melnykov KP, Nazar K, Smyrnov O, et al. Mono-and Difluorinated Saturated Heterocyclic Amines for Drug Discovery: Systematic Study of Their Physicochemical Properties. Chemistry. 2023;29(47):e202301383.
Crossref - Phong NV, Anh DTN, Chae HY, et al. Anti-inflammatory activity and cytotoxicity against ovarian cancer cell lines by amide alkaloids and piperic esters isolated from Piper longum fruits: In vitro assessments and molecular docking simulation. Bioorg Chem. 2022;128:106072.
Crossref - Alghazwani Y, Venkatesan K, Prabahar K, El-Sherbiny M, Elsherbiny N, Qushawy M. The Combined Anti-Tumor Efficacy of Bioactive Hydroxyapatite Nanoparticles Loaded with Altretamine. Pharmaceutics. 2023;15(1):302.
Crossref - Robak P, Robak T. Bortezomib for the treatment of hematologic malignancies: 15 years later. Drugs R D. 2019;19:73-92.
Crossref - Li H, Zhuang W, Seo MS, et al. Inhibition of voltage-dependent K+ channels in rabbit coronary arterial smooth muscle cells by the class Ic antiarrhythmic agent lorcainide. Eur J Pharmacol. 2021;904:174158.
Crossref - Cataldi M, Maurer M, Taglialatela M, Church MK. Cardiac safety of second-generation H1-antihistamines when updosed in chronic spontaneous urticaria. Clin Exp Allergy. 2019;49(12):1615-1623.
Crossref - Liao X, Wu N, Liu D, Shuai B, Li S, Li K. Levodopa/carbidopa/entacapone for the treatment of early Parkinson’s disease: A meta-analysis. Neurological Sciences. 2020;41:2045-2054.
Crossref - Nguyen K, Hoffman H, Chakkamparambil B, Grossberg GT. Evaluation of rivastigmine in Alzheimer’s disease. Neurodegener Dis Manag. 2021;11(1):35-48.
Crossref - Hussein M, Hu X, Paulin OKA, et al. Polymyxin B combinations with FDA-approved non-antibiotic phenothiazine drugs targeting multi-drug resistance of Gram-negative pathogens. Comput Struct Biotechnol J. 2020;18:2247-2258.
Crossref - Zeitouni NC, Bhatia N, Ceilley RI, et al. Photodynamic therapy with 5-aminolevulinic acid 10% gel and red light for the treatment of actinic keratosis, nonmelanoma skin cancers, and acne: Current evidence and best practices. J Clin Aesthet Dermatol. 2021;14(10):E53-E65.
- Schifano F, Chiappini S, Miuli A, et al. Focus on over-the-counter drugs’ misuse: a systematic review on antihistamines, cough medicines, and decongestants. Front Psychiatry. 2021;12:657397.
Crossref - Nesmerak K, Nemcova I. 6H-Pyrimido [2, 1-a] isoindoles: acid-base and complexation properties and electrooxidation model of metabolic degradation. Monatshefte fur Chemie-Chemical Monthly. 2023;154:1035-1041.
Crossref - Thomas Prasanna K, Pramod J, Vohra Nitin R, Veligandla Krishna C, Anup PU. A newer approach in the man-agement of cough: A review on levodro-propizine. Journal of Respiratory Diseases. 2023;1(3):1-14.
Crossref - Heilig M. Stress-related neuropeptide systems as targets for treatment of alcohol addiction: A clinical perspective. J Intern Med. 2023;293(5):559-573.
Crossref - Aldubayan MA, Elgharabawy RM, Ahmed AS, Tousson E. Antineoplastic activity and curative role of avenanthramides against the growth of ehrlich solid tumors in mice. Oxid Med Cell Longev. 2019;5162687.
Crossref - Latorre M, Revuelta J, García-Junceda E, Bastida A. 6-O-Nucleotidyltransferase: an aminoglycoside-modifying enzyme specific for streptomycin/streptidine. Medchemcomm. 2016;7(1):177-183.
Crossref - Siller A, Blaszak SC, Lazar M, Harken EO. Update about the effects of the sunscreen ingredients oxybenzone and octinoxate on humans and the environment. Plastic and Aesthetic Nursing. 2018;38(4):158-161.
Crossref - Zhu J, Zhang L, Ma D, Gao Y, Mu W, Liu F. A bioactivity and biochemical analysis of iminoctadine tris (albesilate) as a fungicide against Corynespora cassiicola. Pestic Biochem Physiol. 2019;158:121-127.
Crossref - Lachowska S, Antonczyk A, Tunikowska J, Godniak M, Kielbowicz Z. Reduction of greenhouse gases emission through the use of tiletamine and zolazepam. Sci Rep. 2022;12(1):9508.
Crossref - Alam M, Gwon Y, Meza J. Bayesian Conway-Maxwell-Poisson (CMP) regression for longitudinal count data. Commun Stat Appl Methods. 2023;30(3):291-309.
Crossref - Salimo ZM, Yakubu MN, da Silva EL, et al. Chemistry and Pharmacology of Bergenin or Its Derivatives: A Promising Molecule. Biomolecules. 2023;13(3):403.
Crossref - Abdelbaki SA, Al-Falah A, Alhefnawy M, Abozeid A, Fathi A. The effect of short-term use of finasteride versus cyproterone acetate on perioperative blood loss with monopolar transurethral resection of prostate. Afr J Urol. 2021;27:106.
Crossref - Kontoghiorghes GJ, Kleanthous M, Kontoghiorghe CN. The history of deferiprone (L1) and the paradigm of the complete treatment of iron overload in thalassaemia. Mediterr J Hematol Infect Dis. 2020;12(1).
Crossref - Hoseinifar SH, Sun YZ, Zhou Z, Van Doan H, Davies SJ, Harikrishnan R. Boosting immune function and disease bio-control through environment-friendly and sustainable approaches in finfish aquaculture: herbal therapy scenarios. Rev Fish Sci Aquac. 2020;28(3):303-321.
Crossref - Pancu DF, Scurtu A, Macasoi IG, et al. Antibiotics: conventional therapy and natural compounds with antibacterial activity-a pharmaco-toxicological screening. Antibiotics. 2021;10(4):401.
Crossref
© The Author(s) 2024. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.