ISSN: 0973-7510
E-ISSN: 2581-690X
The leaves essential oil of Eupatorium odoratum (L.) obtained by the hydro-distillation method was light yellowish in colour, having pungent aromatic fragrance with the yield of 0.010%. Fifteen secondary metabolites were identified via the GC-MS analysis of the essential oil and tau-Cadinol (20.10%) was found to be the major secondary metabolite in the essential oil followed by α Bisabolol (15.33%), cis-muurola-4 (14) 5- diene (10.79%), Isobornyl acetate (6.40%), and Isocaryophyllene (5.39%). The antimicrobial efficacy was investigated via agar well diffusion technique and essential oil was most effective against P. aeruginosa with inhibition zone 22.00±0.57 mm, which was followed by E. coli (19.00±0.57 mm), S. aureus (18.33±0.33 mm), K. pneumoniae (16.50±0.33 mm), and S. pyogenes (11.00±0.33 mm). MIC and MBC values were observed lowest against P. aeruginosa, i.e. 3.12µl/ml and 6.24µl/ml, respectively. Antioxidant activity of essential oil was investigated by DPPH and H2O2 scavenging assay and IC50 value was found very low, i.e. 10.58µl/ml and 11.76µl/ml, respectively, so the essential oil was considered as a potential antioxidant agent. The results validate scientifically the traditional utilization of leaves essential oil of E. odoratum in traditional medicinal system and confirmed that it can be used as modern phytomedicines as an antimicrobial as well as antioxidant agent.
Eupatorium odoratum, Essential Oil, GC-MS, Antimicrobial Activity, Antioxidant Activity
Medicinal plant produces a variety of bioactive phytochemicals, offering a valuable source of various herb-based medicines. Medicinal and aromatic plants have long been served as vital therapeutic agents with pharmacological and economic significance. Due to economic considerations, approximately 80% of the population in developing nations still uses medicinal plants as a source of their treatments.1 Herbal drugs have several advantages, such as low cost, fewer side effects, various molecular targets, etc.; due to these benefits, these are considered as good and safe alternatives of synthetic drugs.2
The essential oils are the composite of aromatic, volatile, and lipophilic secondary plant metabolites and the major constitutes include mono and sesquiterpenes that are synthesised from isoprenoid pathway as well as their oxygenated derivatives as alcohols, oxides, aldehydes, ketones, esters, and phenols. Aromatic medicinal plants are thoroughly used in conventional medicinal system due to the bioactivity of their essential oils. From the last few years, it was observed that the use of aromatic medicinal herbs and their essential oils has been increased in the field of scientific research as well as in industries such as pharmaceutical, nutritional, and cosmetics.3 Essential oils and their constituents are used in sanitary products, cosmetics, medicines, aromatherapy, agriculture, food additives, food preservation, and natural medicine. As a result, with yearly earnings in the billions, essential oils became a far more interesting topic for both study and business.4
Genus Eupatorium belongs to Asteraceae family is perennial weed, native to America and distributed to different parts of the world. It includes approximately 1200 species out of which 7 are reported in India. E. odoratum is synonymously well recognized as Chromolaena odorata (L.), commonly called Siam weed, etc. and known for its pharmacological and ethnobotanical properties.5 Pungent aromatic fragrance of crushed leaves, colour of flower represents its signature morphology. Decoction of this plant is very useful for skin disorders, curing cough, and cold, used as wound healing agent around the globe. As this plant is highly diverged, its essential oil is used in several pharmacological purposes such as antimicrobial, antidepressant, sedative, analgesic etc., these properties are probably because of the presence of flavonoids, alkaloids, phenols, and tannins, etc.6 It has hepatoprotective, anti-diabetic, anti-hypertensive, and immunomodulatory properties. Mainly terpenoids and flavonoids present in essential oils are responsible for these broad-spectrum bioactivities.7 According to Amaliah et al.8 leaf extract at dosage concentrations of 5%, 10%, and 20% can lower blood glucose levels in hyperglycemic mice, with 20% being the most effective.
The objective of the present investigation is to extract essential oil from leaves of E. odoratum and characterize its phytoconstituents via GC-MS, along with the evaluation of in vitro antimicrobial activity against some wound infection causing bacteria.
Plant material
For the selection of sampling area, firstly the field survey was done for the identification of plant habitat. Then the plant material (leaves) was collected from Sahastradhara road, Dehradun in the month of December when the plant was in flowering period. The leaves were then packed, brought to laboratory, and left to dry in the shade then crushed and stored till further processing. Plant material was authenticated in Botanical Survey of India, Dehradun, Uttarakhand and the herbarium sheet was submitted with the accession number 911.
Essential oil extraction
Freshly dried leaves of E. odoratum were hydro-distilled for approximately 3 hours by using Clevenger-type appliance. The distillate has been saturated and n-hexane was used for the oil extraction and dehydrate with the help of anhydrous sodium sulphate to remove moisture content and stored in glass vials with tight lid in refrigerator at 4°C for further use.9 The formula was used to calculate the yield of E. odoratum leaves essential oil:
Yield (%) = [ (Weight of the essential oil) (Weight of initial sample taken) ] × 100
GC-MS characterization
The analysis was performed in Sophisticated Analytical Instrumentation Facility, SAIF Panjab University, Chandigarh India. GC-MS characterization of E. odoratum leaves essential oil was performed on Trace 1300 GC coupled with Thermo TSQ8000 Triple Quadrupole MS, Thermo Scientific, US. Column was consist of triple quadrupole30 mm 0.25 mm ID 0.25 m dimensions film of a DB 35-MS Capillary Standard non-polar column and Helium carrier gas(flow rate: 1.0 ml/min) was used. Ion Source temperature is 350°C and its mass range is 2.1100amu. It maintained temperature of oven at 60°C-280°C for approximately 15-30 min. The constituents were analysed by comparing their retention time and mass spectra of peaks to that of standard computational database of NIST and Willey libraries.10
Antimicrobial studies
The antimicrobial activity was assessed by using two Gram-positive human pathogenic bacteria, i.e. Staphylococcus aureus (MTCC 1144), Streptococcus pyogenes (MTCC 442) and three Gram-negative bacteria, i.e. Klebsiella pneumoniae (MTCC 4030), Escherichia coli (MTCC 40), and Pseudomonas aeruginosa (MTCC2474). The agar well diffusion technique was utilized to assess the antimicrobial potency of the essential oil. Essential oil was diluted with dimethyl sulfoxide (DMSO) to prepare final concentration (100µl/ml) for the in vitro assay and the bacterial inoculum turbidity was maintained at 1.5×108 CFU/ml (0.5 McFarland standards).11 The diameter of well was 6 mm and 50µl of essential oil dilution was suspended into the well for the antimicrobial assay. Plates were incubated for 24 h in a BOD incubator at 37°C and experiment was conducted in triplicates.12 DMSO was used as negative control and three antibiotic disks [streptomycin (10µg), clindamycin (2µg) and ciprofloxacin (10µg)] were taken as positive control to compare the antibacterial efficacy of the essential oil by disk diffusion method.
MIC and MBC evaluation
Minimum inhibitory concentration (MIC)of the essential oil was carried out using the broth micro dilution method using 96 well microtiter plate method.13 For the preparation of stock solution essential oil suspended into the DMSO and different oil concentrations were made ranges from 100, 50, 25, 12.5, 6.25 and 3.12µl/ml. The smallest concentration of the essential oil that can inhibit the bacterial growth is noted as MIC. For Minimum bactericidal concentration (MBC) calculation, 10µl inoculum from MIC plate with no visible growth was transferred to the nutrient agar plates and incubated. The concentration, that resulted in no apparent bacterial growth on the plate was noted as MBC. DMSO and Ciprofloxacin were utilized as negative and positive control respectively for the experiment.
Antioxidant activity
DPPH free radical scavenging assay
To evaluate the antioxidant potential of the essential oil 2,2-diphenyl1-picrylhydrazyl (DPPH) free radical scavenging assay was carried out.14 4 mg of DPPH was dissolved in 100 ml of ethanol to prepare 0.004% w/v fresh DPPH working solution. 2 ml of different concentrations of essential oil (15.62, 31.25, 62.5, 125, 250, 500, 1000 µg/ml) was suspended in3 ml aliquot of working solution and incubated for 30 min in dark at room temperature. 2 mlethanol + 3 ml DPPH working solution was taken as negative control and ascorbic acid was used as positive control. The absorbance of essential oil was compared to the corresponding absorbance of ascorbic acid at 517 nm in triplicates. The percentage inhibition of DPPH was measured by the formula:
Percentage Inhibition = [((OD of Control – OD of Sample))/ ((OD of Control) )] × 100
Hydrogen peroxide scavenging assay
The method was carried out to assess the capacity of essential oil to scavenge hydrogen peroxide (H2O2) according to Bhatti et al.15 Aliquot of 0.1 mL of oil (25-400µg/mL) was transferred into the Eppendorf tubes and their volume was made up to 0.4 mL with 50 mM phosphate buffer (pH 7.4) followed by the addition of 0.6 mL of H2O2 solution (2 mM). After 10 minutes of reaction time, the reaction mixture was vortexed, and its absorbance at 230 nm was determined. Ascorbic acid was served as positive control. Each experiment was performed out in triplicates. The following equation was used to determine the capacity of extracts to scavenge H2O2:
Percentage of H2O2 scavenging activity = ((A0-A1))/A0 × 100
Where: A0 = Absorbance of control, A1 = Absorbance of sample.
Statistical analysis
All the in vitro biological experiments were completed in triplicates, and results were evaluated as mean standard error. Data was statistically checked using the one-way analysis of variance (ANOVA) by using Microsoft Excel 2010 and differences between the means were checked for the significance at P ≥ 0.05.
Yield and GC-MS characterization of essential oil
The physical characteristics of essential oil obtained by the hydro-distillation method was light yellowish in colour, having pungent aromatic fragrance with the yield of 0.010%.
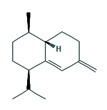
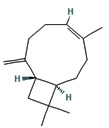
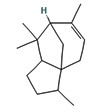
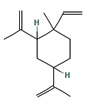
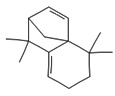
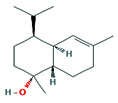
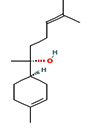
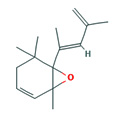
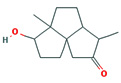
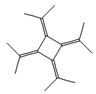
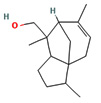
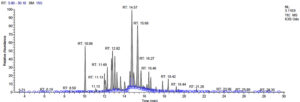
Fifteen bioactive phyto-constituents were identified in the GC-MS analysis of E. odoratum leaves essential oil and displayed in Table 1. The chromatogram of GC-MS is shown in Figure 1. Tau.Cadinol was found to be the major phytochemical with 20.10% peak area followed by a Bisabolol (15.33%); cis-muurola-4(14), 5-diene (10.79%); Isobornyl acetate (6.40%); Isocaryophyllene (5.39%); 2,6 Dimethyl 10 methylene 12 oxatricyclo [ 7.3.1.0(1,6)] tridec2ene ( 3.53%); Cedrene (3.26%); Cyclohexane, 1 ethenyl 1 methyl 2, 4 bis (1 methyl ethenyl), [1S(1α,2α,4α)] (2.80%); Tricyclo [6.3.0.0(1,5)] undecan4one, 5, 9 dimethyl (2.46%); Cyclobutane, tetrakis (1 methyl ethylidene) (2.14%), and others were present in traces.
Table (1):
Phytoconstituents of leaves essential oil of E. odoratum identified by GC-MS
No. |
Retention time |
% Peak Area |
Compound Name |
Molecular Structure |
|---|---|---|---|---|
1. |
7.34 |
0.16 |
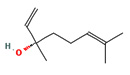
1,6 Octadien3ol, 3,7dimethyl |
|
2. |
8.50 |
0.34 |
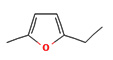
2-Ethyl-5-methylfuran |
|
3. |
10.08 |
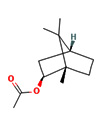
6.40 |
Isobornyl acetate |
|
4. |
11.13 |
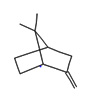
0.78 |
Bicyclo[2.2.1] heptane, 7,7dimethyl2methylene |
|
5. |
12.82 |
10.79 |
cis-Muurola-4(14),5-diene |
|
6. |
13.07 |
5.39 |
Isocaryophyllene |
|
7. |
13.27 |
3.26 |
Cedrene |
|
8. |
13.63 |
2.80 |
Cyclohexane, 1-ethenyl-1-methyl-2,4-bis(1-methylethenyl),[1S(1α,2α,4α)] |
|
9. |
14.57 |
2.39 |
Isolongifolene, 4,5dehydro |
|
10. |
14.77 |
20.10 |
tau.Cadinol |
|
11. |
15.33 |
15.33 |
α Bisabolol |
|
12. |
15.68 |
3.53 |
6-(1,3-Dimethyl-buta-1,3-dienyl)-1,5,5-trimethyl-7- oxa-bicyclo[4.1.0]hept-2-ene |
|
13. |
16.46 |
2.46 |
Tricyclo[6.3.0.0(1,5)]undecan4one, 5,9dimethyl |
|
14. |
18.42 |
2.14 |
Cyclobutane, tetrakis(1-methylethylidene) |
|
15. |
21.26 |
0.46 |
8- Cedren-13-ol, |
Antimicrobial activity
The antimicrobial activity data of E. odoratum leaves essential oil and standard reference antibiotics are shown in Table 2. Essential oil was most effective against P. aeruginosa among all the test bacterial strains with an inhibition zone of 22.00±0.57 mm which was followed by E. coli (19.00±0.57 mm), S. aureus (18.33±0.33 mm), K. pneumoniae (16.50±0.33 mm), and S. pyogenes (11.00±0.33 mm). The reference antibiotic Ciprofloxacin showed the activity against P. aeruginosa with a 19.66±0.33 mm zone of inhibition, i.e. lesser than the zone of inhibition exerted by E. odoratum leaves essential oil.
Table (2):
Zone of inhibitions of leaves essential oils of E. odoratum against some human bacterial pathogens
| Pathogens | Diameter of zone of inhibition (mm) | ||||
|---|---|---|---|---|---|
| Essential oil | Negative control (DMSO) | Positive control | |||
| Clindamycin | Streptomycin | Ciprofloxacin | |||
| Staphylococcus aureus (MTCC 1144) | 18.33±0.33 | – | 11.66±0.33 | 20.00±0.57 | 14.00±0.57 |
| Streptococcus pyogenes (MTCC 442) | 11.00±0.33 | – | 21.00±0.57 | 29.33±0.33 | 24.33±0.33 |
| Klebsiella pneumoniae (MTCC 4030) | 16.50±0.33 | – | – | 14.50±0.28 | – |
| Escherichia coli (MTCC 40) | 19.00±0.57 | – | 19.33±0.33 | 19.66±0.33 | 26.00±0.57 |
| Pseudomonas aeruginosa (MTCC 2474) | 22.00±0.57 | – | – | – | 19.66±0.33 |
DMSO: Dimethyl Sulphoxide, Significant at P ≤ 0.05 level; Diameter of well: 6 mm; – : No zone of inhibition; MTCC: Microbial Type Culture Collection
MIC/MBC evaluation
The MIC of E. odoratum leaves essential oil ranges between 3.12-50µl/ml and MBC ranges between 6.25-100µl/ml against all the test microorganisms. The MIC was observed lowest against P. aeruginosa i.e. 3.12µl/ml which was followed by E. coli (6.25µl/ml), S. aureus (6.25µl/ml), K. pneumoniae (12.5µl/ml), and S. pyogenes (50.0µl/ml). The MBC was reported lowest against P. aeruginosa i.e., 6.25µl/ml which was followed by E. coli (12.5µl/ml), S. aureus (12.5µl/ml), K. pneumoniae (50.0µl/ml), and S. pyogenes (100µl/ml) (Table 3).
Table (3):
MIC and MBC values of leaves essential oils of E. odoratum
| Pathogens | Essential oil (μl/ml) | Ciprofloxacin (μg/ml) | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| Staphylococcus aureus (MTCC 1144) | 6.25 | 12.5 | 5.0 | 10.0 |
| Streptococcus pyogenes (MTCC 442) | 50.0 | 100 | 0.62 | 1.25 |
| Klebsiella pneumoniae (MTCC 4030) | 12.5 | 50.0 | – | – |
| Escherichia coli (MTCC 40) | 6.25 | 12.5 | 0.62 | 1.25 |
| Pseudomonas aeruginosa (MTCC 2474) | 3.12 | 6.25 | 2.50 | 5.0 |
Microbial Type Culture Collection; – : No activity
Antioxidant activity
DPPH free radical scavenging assay
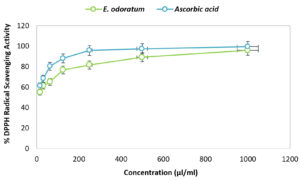
The antioxidant activity of E. odoratum leaves essential oil is shown in Figure 2. At 1000 µl/ml concentration of ascorbic acid showed 99.57±0.57% inhibition of DPPH free radical, and at the same concentration, E. odoratum essential oil showed 95.93±0.33% inhibition, which was almost comparable to that of ascorbic acid. The IC50 value of essential oil was 10.58 µl/ml that is very low, so the essential oil was considered as a potential antioxidant agent.
Hydrogen peroxide scavenging assay
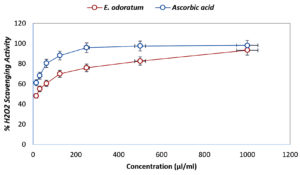
Antioxidant activity evaluated by H2O2 scavenging assay is shown in Figure 3 where ascorbic acid at 1000 µl/ml concentration showed 98.12±0.57% inhibition, whereas E. odoratum essential oil showed 95.12±0.33% inhibition at the same concentration, which was nearly as effective as ascorbic acid. The IC50 value was reported very low, i.e.11.76 µl/ml, that suggests remarkable antioxidant activity.
Plants are recognized as a potential source of medicinally important bioactive phytoconstituents which leads to the development of new eco-friendly, having less cost medicines with negligible side effects to that of synthetic drugs. In modern medicine, essential oils, volatile aromatic compounds, and their derivative are thoroughly used in traditional medicines.16,9
In the present study, the major compound identified in the leaves essential oil of E. odoratum were tau. Cadinol followed by a Bisabolol; cis-muurola-4(14) 5- diene, Isobornyl acetate; Isocaryophyllene; 2,6 Dimethyl10methylene12oxatricyclo[ 7.3.1.0(1,6)] tridec2ene; Cedrene; Cyclohexane, 1ethenyl1methyl2,4bis (1methylethenyl),[1S(1a,2a,4a)]; Tricyclo[6.3.0.0(1,5)]undecan4one, 5,9dimethyl; Cyclobutane, tetrakis (1 methyl ethylidene) and others were present in very small amount.
Sharma et al.,5 identified approximately 50 phytocompounds from E. odoratum essential oil that include a-pinene (9.64 %), germacrene D (20.27 %), trans-b-caryophyllene (10.04 %), geijerene (25.10 %) as major components while other were present in trace elements. Another study was performed by Dougnon and Ito,6 in which 15 essential oil components were reported in which caryophyllene oxide with area percent 43.75 present as major compounds.
In other investigation α-pinene, β-pinene, germacrene D were present with area percent 42.2%, 10.6%, 9.7% respectively while others main compounds were ß-copaen-4α-ol (9.4%); (E)-caryophyllene (5.4%); and geijerene/pregeijerene (7.5%).17 In case of leaves essential oil,(+)-camphor (15.46%); α-pinene (19.32%); cadinene (19.09%); cadinol isomer (6.36%); limonene (10.22%); and β-caryophyllene (7.05%) as major compounds by GC/MS analysis.18 E. odorata leaves essential oils were analysed by GC/MS where 20 compounds were detected and include Pregeijerene, dauca-5 (40.60%); 8-diene (16.75%); α-pinene (9.67%); (E)-caryophyllene (6.11%); and β-pinene (5.37%) were found as major components of essential oil.19
The chemical components and yield of leaves essential oil of E. odoratum were reported somehow different from that of previous studies. The difference observed may be due to the plant part that is used for extraction, habitat, distribution, climatic conditions, type of soil, plant age or may be the method of the extraction.
Present investigation reported the E. odoratum leaves essential oil has antibacterial action against a variety of Gram-negative as well as Gram-positive pathogenic bacteria. P. aeruginosa was very well inhibited by the essential oil with a zone of inhibition 22.00±0.57mm followed by E. coli, S. aureus, S. pyogenes and K. pneumoniae.
S. aureus, E. coli, P. aeruginosa, and B. cereus, were screened for the antimicrobial activity using broth microdilution technique and B. cereus was observed most sensitive strain to the leaves essential oil of E. odoratum.17
Inya-Agha et al.18 reported the antimicrobial efficacy of E. odoratum essential oil against bacterial pathogens, namely Bacillus subtilis, E.coli, S. aureus, and K. aerogenes. According to their results, E.coli and S. aureus were noted to be the most sensitive strain that is comparable to the present research work. A recent study reported the remarkable antimicrobial activity of methyl cellulose encapsulated E. odorata methanol extract against E. coli and S. aureus.20
The MIC/MBC values showed that leaves essential oil of E. odoratum inhibited and eradicated the bacterial growth with least concentrations, and this might be because of the biologically active secondary metabolites present in the essential oils.
Antioxidant compounds reduce the detrimental effects of harmful free radicals before they attack the cell or tissue preventing the damage to proteins, enzymes, lipids, DNA carbohydrates. Phytochemicals from medicinal herbs are a potentially vast source of biologically active phyto-components serving as antioxidant agents having negligible side effects in comparison to synthetic chemical drugs.21,22 Present investigation reported the potential antioxidant activity of leaves essential oil of E. odoratum with IC50 value 10.58 µl/ml. Very low IC50 value of essential oil supports the significance of E. odoratum leaves as a promising herbal source of antioxidant compounds, hence can be utilized in nutritional as well as pharmaceutical in industries. Raman et al.,23 reported the DPPH scavenging activity of aqueous and methanol extracts of E. odoratum having IC50 values 10.5µg/ml and 10.2µg/ml, respectively, that are comparable to the present study. Tahir et al.,24 performed antioxidant activity of the leaf extract by DPPH method, and reported n-butanol fraction have maximum activity with IC50 value at 33.535µg/ml. Another study revealed that the DPPH assay of ethyl acetate fraction had the highest antioxidant activity, with IC50 value of 10.05±0.06 mg/ml.25 Amatya and Tuladhar26 reported the antioxidant activity of ethanolic extracts of various parts of E. odoratum (stem, root, leaf, defatted flowers). Leaf and flower extracts showed the lowest IC50, i.e. 44.5 and 50.9µg/ml, respectively, that suggests E. odoratum could be used pharmaceutically.
The present investigation conclusively demonstrates the efficacy of secondary metabolites of leaves essential oil of E. odoratum as a promising antimicrobial and antioxidant agent. Gram-positive and gram-negative pathogenic microorganisms examined showed good antibacterial activity. The leaves of E. odoratum have very good amount of essential oil in which approximately 15 compounds were identified with the help of GC-MS analysis, showing very good biological efficacy against E. coli, K. pneumoniae, P. aeruginosa, S. pyogenes, and S. aureus. In DPPH and H2O2 assay, the IC50 value of leaves essential oil of E. odoratum was found almost equal to the ascorbic acid that indicates it as potential natural antioxidant agent. Hence, this study scientifically concluded and validated the utilization of leaves essential oil of E. odoratum in traditional medicine and suggests it’s use as a resource material in the pharmaceutical industry. In future, some more in vivo experimentations and clinical investigations would be required to validate the potential of essential oil.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Botany and Microbiology, Gurukula Kangri (Deemed to be University), Haridwar, Uttarakhand, India, for providing the resources that allowed this study to be completed.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
NB conceptualized and supervised the study. EC designed and conducted all the experiments. MR evaluated the data. MR wrote the manuscript. TS and PS edited the manuscript. All authors read and approved the final manuscript for publication.
FUNDING
This study was supported by the CSIR-UGC NET [09/1010(0012)/2019-EMR-1] Fellowship provided by the Council of Scientific & Industrial Research (CSIR), New Delhi, India.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
Not applicable.
- Sharifi-Rad J, Hoseini-Alfatemi SM, Sharifi-Rad M, Sharifi-Rad M, Iriti M, Sharifi-Rad M, Sharifi-Rad R, Raeisi S. Phytochemical compositions and biological activities of essential oil from Xanthium strumarium L. Molecules. 2014;20(4):7034-47.
Crossref - Rajput M, Kumar N. Medicinal plants: A potential source of novel bioactive compounds showing antimicrobial efficacy against pathogens infecting hair and scalp. Gene Rep. 2020;21:100879.
Crossref - Mothana RA, Al-Said MS, Al-Yahya MA, Al-Rehaily AJ, Khaled JM. GC and GC/MS analysis of essential oil composition of the endemic Soqotraen Leucas virgata Balf. f. and its antimicrobial and antioxidant activities. Int J Mol Sci. 2013;14(11):23129-39.
Crossref - Mohamed AA, Alotaibi. Essential oils of some medicinal plants and their biological activities: a mini review. Journal of Umm Al-Qura University for Applied Sciences. 2023;9(1):40-49.
Crossref - Sharma K, Saikia AK, Sharma H, Sahariah BJ, Deka S, Das B. Chemical composition and antimicrobial study of essential oil from the leaves of Eupatorium odoratum Linn. from upper Assam region. J Essent Oil-Bear Plants. 2013;6(4):482-488.
Crossref - Dougnon G, Ito M. Essential oil from the leaves of Chromolaena odorata, and sesquiterpene caryophyllene oxide induce sedative activity in mice. Pharmaceut. 2021;14(7):651.
Crossref - Pal A, Banerjee A, Kundu R. Phytochemical analysis and bioactivity reports of ethnomedicinal plants from West Bengal, India. Nat Prod Res. 2023;37(6):1036-1041.
Crossref - Amaliah UN, Johannes E, Hasan MS, Tambaru E. The use extract of siam leaf Eupatorium odoratum L. as alternative material in lowering blood glucose. Int J Appl Biol, 2019;3(1):15-23.
Crossref - Abers M, Schroeder S, Goelz L, et al. Antimicrobial activity of the volatile substances from essential oils. BMC Complement Altern Med. 2021;21(1):1-4.
Crossref - Kanthal LK, Dey A, Satyavathi K, Bhojaraju P. GC-MS analysis of bio-active compounds in methanolic extract of Lactuca runcinata DC. Pharmacogn Res. 2014;6(1):58-61.
Crossref - El Hadidy D, El Sayed AM, El Tantawy M, El Alfy T. Phytochemical analysis and biological activities of essential oils of the leaves and flowers of Ageratum houstonianum Mill. cultivated in Egypt. J Essent Oil-Bear Plants. 2019;22(5):1241-51.
Crossref - Rajput M, Bithel N, Vijayakumar S. Antimicrobial, antibiofilm, antioxidant, anticancer, and phytochemical composition of the seed extract of Pongamia pinnata. Arch Microbiol. 2021;203(7):4005-24.
Crossref - Rajput M, Kumar N. In vitro Antimicrobial and antibiofilm efficacy of medicinal plant extracts against clinical MDR isolates from scalp infection cases. Int J Sci Technol Res. 2020;9(2):4218-28.
- Gupta S, Bisnoi JP, Singh D, Singh R. Effect of different drying technique on the bioactive components of Terminalia arjuna bark. Res J Pharm Technol. 2019;12(5):2372-2378.
Crossref - Bhatti MZ, Ali A, Ahmad A, Saeed A, Malik SA. Antioxidant and phytochemical analysis of Ranunculus arvensis L. extracts. BMC research notes, 2015; 8: 1-8.
Crossref - Sharma K, Sharma S, Kumar N, Singh R, Chauhan N. Profiling of polyphenolic compounds of Ficus palmata fruits via Ultra high performance liquid chromatography with diode array detector spectrometry. Med Plants – Int J Phytomed Relat Ind. 2021;13(3):524-528.
Crossref - Owolabi MS, Ogundajo A, Yusuf KO, et al. Chemical composition and bioactivity of the essential oil of Chromolaena odorata from Nigeria. Rec Nat Prod. 2010;4(1):72-78.
- Inya-Agha SI, Oguntimein BO, Sofowora A, Benjamin TV. Phytochemical and antibacterial studies on the essential oil of Eupatorium odoratum. Int J Crude Drug Res. 1987;25(1):49-52.
Crossref - Pitakpawasutthi Y, Thitikornpong W, Palanuvej C, Ruangrungsi N. Chlorogenic acid content, essential oil compositions, and in vitro antioxidant activities of Chromolaena odorata leaves. J Adv Pharm Technol Res. 2016;7(2):37-42.
Crossref - Hu J, Qi Q, Zhu Y, Wen C, Olatunji OJ, Jayeoye TJ, Eze FN. Unveiling the anticancer, antimicrobial, antioxidative properties, and UPLC-ESI-QTOF-MS/GC-MS metabolite profile of the lipophilic extract of siam weed (Chromolaena odorata). Arab J Chem. 2023;16(7): 104834.
Crossref - Raju Ilavarasan MM, Venkataraman S. Anti-inflammatory and antioxidant activities of Cassia fistula Linn bark extracts. Afr J Trad Complement Altern Med. 2005;2(1):70-85.
Crossref - Walia A, Kumar N, Singh R, Kumar H, Kumar V, Kaushik R, Kumar AP. Bioactive Compounds in Ficus Fruits, Their Bioactivities, and Associated Health Benefits: A Review. J Food Qual. 2022;1-19.
Crossref - Raman BV, Samuel LA, Saradhi MP, Rao BN, Krishna NV, Sudhakar M, Radhakrishnan TM. Antibacterial, antioxidant activity and GC-MS analysis of Eupatorium odoratum. Asian J Pharm Clin Res. 2012;5(2):99-106.
- Tahir KA, Miskad UA, Djawad K, Djide S, Khaerani K, Indrisari M. Evaluation of Antioxidant Activity of Botto-Botto Leaf Fraction (Chromolaena odorata L.) Using DPPH and ABTS Methods. Maced J Med Sci. 2021;9(A):183-188.
Crossref - Sabarudin, Nuralifah, Zubaydah WOS, Sahumena MH, Sari FN, Nelisa, Yamin. Phytochemical content and antioxidant activity of Komba-komba (Eupatorium odoratum L). Food Res. 2022;6(3):335-341.
Crossref - Amatya S, Tuladhar SM. In vitro antioxidant activity of extracts from Eupatorium odoratum L. Res J Med Plant. 2011;5(1):79-84.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.