ISSN: 0973-7510
E-ISSN: 2581-690X
This study focused on the isolation of laccase enzyme from the fungus Pleurotus ostreatus and its application in the biodegradation of various pollutants present in textile industrial effluent, including chemicals, ions, salts, heavy metals, brittle metals, total suspended solids, total dissolved solids, chemical oxygen demand, biological oxygen demand, minerals, total hardness, total alkalinity, turbidity, electrical conductivity, and dyes. Textile industrial effluent poses a significant threat to the environment, contaminating water bodies and posing risks to human, animal, and plant life. This study employed an economical and ecofriendly biological approach for wastewater treatment, distinguishing it from traditional physical and chemical methods. The optimum temperature of laccase is found to be 30 degree Celsius and pH is 3. Enzyme activity of laccase is 7.25 U/ml. This fugal laccase decolorizes textile Industrial dye effluent containing various dyes, such as Turquoise VG, Black B, Yellow R, Methyl red, Trypan blue, and Acid Orange 7. Laccase depicts maximum decolorization efficacy on Black B dye. Similarly, Laccase from P.ostreatus shows higher decolorization efficacy when compared to other fungal laccase. Additionally, the study assessed the biodegradation of various wastewater quality parameters, including physical and chemical parameters like toxic heavy metals and ions. This research of isolation, characterization, and utilizing laccase from P. ostreatus for the bioremediation of textile industrial effluent wastewater containing dyes, toxic chemicals, ions and metals is effective, economical and ecofriendly.
Laccase, Pleurotus ostreatus, Effluent Treatment, Dye Decolorization, Bioremediation
The field of enzyme manufacturing in biotechnology is experiencing rapid growth, with enzymes being a subject of study since the 1800s.1 Laccase, originally discovered in the exudates of the Japanese lacquer tree, Rhus vernicifera, by scientist Yoshida in 1883,2 is among the enzymes that have been extensively investigated. While laccase can be found in various organisms, fungi, especially basidiomycetes, are significant sources of this enzyme.3
Fungi possess unique enzymes that enable them to thrive in challenging environments. Laccase, an enzyme involved in both an organism’s defense mechanisms and the metabolic breakdown of complex organic compounds like lignin, plays a pivotal role.4 Among basidiomycetes, Pleurotus ostreatus, commonly known as the oyster mushroom, is a noteworthy edible fungus. This mushroom has found commercial use globally and even contains lovastatin, a compound used to lower cholesterol levels.4
Laccase has gained commercial significance due to its widespread applications in depolluting contaminants and phenolic chemicals through bioremediation. These mycelial enzymes can transform various materials, including wood, plastic, paint, and jet fuel, into valuable resources. Several studies have successfully decolorized textile dyes using these enzymes.5
P. ostreatus was selected as the fungal strain for its relatively high laccase activity compared to other laccase-producing fungi. This study focused on the isolation and characterization of laccase from P. ostreatus and its application in addressing environmental challenges, particularly the degradation of lignin.
Laccase, which catalyzes oxidation reactions using four electrons to reduce molecular oxygen to water, can be extracted from various sources, including higher plants, bacteria, insects, and fungi.5 Many basidiomycetes, including white-rot and brown-rot fungi, produce laccases, which play a role in lignin degradation.
Solid-state fermentation was employed in this process to produce laccase, and was selected based on its effectiveness in generating the enzyme. Laccase’s importance in degrading lignin is crucial for addressing current environmental concerns. Its commercial applications span multiple industries, including nutrition, textiles, pulp and paper, synthetic chemicals, cosmetics, and environmental bioremediation.5,6
Research into laccase production through solid-state fermentation has been conducted extensively in recent years.7 Solid-state fermentation involves microbial growth on a solid, moist substrate supplemented with essential nutrients.8 This technique has been applied to support materials, including inert substances like plastic, glass, and stainless steel, as well as lignocellulosic residues, which serve as substrates for growing white-rot fungi.9
Bioremediation, employing the biological activity of microorganisms to break down harmful substances in the environment, is gaining traction as a method to address environmental pollution. The textile industry, known for its extensive water consumption, significantly impacts water quality and attributes, contributing a substantial portion of biochemical oxygen demand.10
Traditional methods, including physical and chemical procedures, have been employed to remove dye-containing effluents. However, these physiochemical approaches have notable limitations, including inflexibility, high costs, slow processing, and the generation of sludge.10 In contrast, biological dye degradation technologies are considered cost-effective and environmentally friendly. Various microorganisms, such as bacteria and fungi, can be used for the biodegradation of textile dyes. Among these microorganisms, white-rot fungi are particularly effective at breaking down textile dyes due to their ability to degrade a wide range of refractory organic compounds, including numerous dyes.10
Laccase, being produced in significant quantities, offers potential advantages for decolorization treatments and bioremediation technologies.11 The capability of P. ostreatus to biodegrade fiber-reactive dyes from textile mill effluents has been investigated in this study. Laccase enzymes have demonstrated the ability to degrade dyes, such as Acid Red 27, Reactive Orange 16, Indigo Carmine, Remazol Brilliant Blue R, and more, with over 80% degradation observed in less than one hour.11 This study aimed to identify fungi capable of synthesizing laccase enzymes to facilitate both the decolorization of industrial dyes and the biodegradation of heavy metals, such as Cd, Cr, Ni, and Pb, with a focus on survivability within these classes and the activation of functional groups in the presence of heavy metals.12
Similarly, in the biological treatment of wastewater, microbial coagulation and the removal of non-soluble organic colloidal particles effectively reduce pollutant concentrations. Physiologically stabilized organic matter no longer exerts an oxygen demand. For effective biological treatment, contact between biomass and substrate is crucial. Advancements in anaerobic reactor design have led to the development of suspended growth systems, attached growth systems, fixed film systems, or combinations of these approaches to achieve variations in contact time and mode of contact. While anaerobic waste treatment systems have been in use since the late 19th century, they were initially considered to have low treatment efficiency and were too slow to cope with the increasing volume of wastewater, especially in industrialized and densely populated area.13
Simultaneously, assessing the water quality parameters of textile industrial effluent wastewater involves analyzing its physical and chemical characteristics. This is essential to address water pollution caused by toxic chemicals, heavy metals, ions, salts, pH variations, and the concentrations of various elements, such as NO3, B, Mn, Pb, Cd, Zn, Cu, As, Fe, F, oil and grease, NH3N, biological oxygen demand (BOD), chemical oxygen demand (COD), SO4, Mg, Ca, CaCO3, Cl, total alkalinity, and the solubility of total dissolved solids (TDS) and total suspended solids (TSS). Additionally, parameters such as turbidity and electrical conductivity are evaluated.14 These assessments are crucial for preserving the environment, particularly water bodies, and safeguarding the health of humans and animals that rely on this water for various purposes. Therefore, laccase enzymes from the fungus P. ostreatus hold promise for the biodegradation of toxic chemicals, metals, and ions in textile industrial wastewater, contributing to environmental protection.15
Fungal strain collection
A locally isolated fungus strain of P. ostreatus was collected from the culture collection of local mushroom farms.
Authentication
The mushroom specimen obtained from the farm was submitted for authentication at the Botanical Survey of India (BSI) in Tamil Nadu Agricultural University (T.N.A.U.) Campus. The specimen was identified as belonging to the Pleurotaceae family and confirmed to be P. ostreatus.
Preparation of fermentation medium
The bioprocess medium was prepared in a 250 mL conical flask by adding approximately 3.95 g of potato dextrose agar (PDA) and 0.75 g of Agar to 100 mL of distilled water (Table 1), which was then thoroughly mixed. The pH of the medium was adjusted to 5.
Table (1):
Citrate phosphate buffer.
No |
Ingredients |
Amount Required |
|---|---|---|
1 |
Dibasic sodium phosphate |
0.9149 g |
2 |
Citric acid |
0.4668 g |
3 |
Distilled water |
100 ml |
Sterilization of media
The prepared PDA medium in the conical flask was sterilized in an autoclave at 121°C for 20 min.
Mushroom tissue inoculation
Fungal (mushroom) tissues were aseptically inoculated into PDA inside a laminar flow hood and incubated at room temperature without agitation.
Subculture of mushroom tissues
After microscopic identification, the remaining mushroom tissues were subcultured in PDA and stored at 4°C to maintain their viability.
Preparation of citrate phosphate buffer
A solution of citrate phosphate buffer was prepared by dissolving 0.9149 g of dibasic sodium phosphate and 0.4668 g of citric acid in 100 mL of distilled water. The buffer was adjusted to a pH of 5 (Table 2).
Table (2):
Potato Dextrose Agar medium
No |
Ingredients |
Amount Required |
|---|---|---|
1 |
Potato Dextrose Agar (PDA) |
3.95 g |
2 |
Agar Agar |
0.75 g |
3 |
Distilled water |
100 ml |
Extraction of laccase
After 7–10 days, the entire contents of the flask were immersed for 2 h in 100 mL of 1 mM citrate phosphate buffer (pH 5) and placed in an orbital shaker at 200 rpm overnight. The extract was then filtered using Wattman filter paper and centrifuged for 10 min at 6°C at 40,000 rpm to remove particulate matter.
Partial purification of laccase
Ammonium sulphate was added to the free filtrate from P. ostreatus to achieve saturation. The mixture was kept at 4°C, and the supernatant was separated.
Ammonium salt precipitation (salting out method)
For ammonium salt precipitation, 2 mL of the crude extract was set aside for preservation. The remaining crude extract was placed in a small beaker within a larger beaker filled with ice. Ammonium sulphate was added gradually until the first portion completely dissolved while the beaker was subjected to magnetic stirring. The process was continued until all the weighed ammonium sulphate was dissolved. After complete dissolution, the mixture was refrigerated for 24 h. Subsequently, it was transferred to a centrifuge tube and spun at 2,000 rpm for 10 min at 4°C to separate the pellet from the supernatant. The pellet obtained was dissolved in 10 mL of Tris buffer, and both the raw enzyme and the pellet were stored in the refrigerator.
Dialysis bag
To remove low molecular weight compounds and other ions that could interfere with enzyme activity, the pellets were desalted using a dialysis bag. The dialysis bag was activated by boiling it in distilled water for two 1 min intervals. After activation, it was transferred to warm distilled water and boiled for 1 min. The desalting process involved placing the pellet in the dialysis bag, sealing it, and subjecting it to dialysis in a 100 mM Tris buffer at pH 7 for 90 min, with buffer changes during this period.
Enzyme activity
Enzyme activity depends on certain conditions, which should be clearly specified, as it measures the amount of active enzyme present. The following formula can be used to calculate enzyme activity:
Enzyme activity = (A x V) / (t x e x v)
Where A is the absorbance, t is the incubation time, e is the extinction coefficient, V is the total mixture volume and v is the enzyme volume.
Characterization of laccase
Effects on pH and temperature
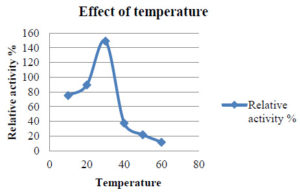
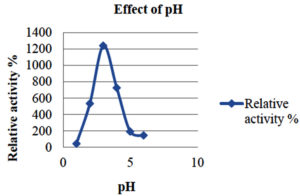
To investigate how pH and temperature affect enzyme activity, the partly purified enzyme was incubated under different conditions. The pH was maintained at 3–5 using citrate phosphate buffer and at 6–8 using sodium phosphate buffer. Temperature effects on laccase enzyme activity were assessed by incubating the enzyme in a water bath at temperatures ranging from 30°C to 90°C, with a 10°C increase every 15 min. The impact of pH on laccase activity was investigated by incubating the enzyme with different pH ranges (1–6) (Table 3 and 4).
Table (3):
Effect of Temperature
No |
Temperature |
Relative activity % |
|---|---|---|
1 |
10 |
75.177 |
2 |
20 |
89.523 |
3 |
30 |
149.27 |
4 |
40 |
37.5 |
5 |
50 |
21.793 |
6 |
60 |
11.68 |
Table (4):
Effect of pH
No |
pH |
Relative activity % |
|---|---|---|
1 |
1 |
44.81 |
2 |
2 |
534.95 |
3 |
3 |
1238.57 |
4 |
4 |
725.42 |
5 |
5 |
192.896 |
6 |
6 |
145.83 |
Guaiacol assay
The laccase enzyme’s oxidation of guaiacol, as described by Kalra et al., was used to assess enzyme activity. The reddish-brown hue resulting from the oxidation of guaiacol by laccase was measured at 450 nm using a UV spectrometer. The composition for the guaiacol assay is provided in the table.15
Types and concentration of dye samples used
Several dye samples were employed in the study, including Turquoise VG, Black B, Yellow R, Methyl red, Trypan blue, and Acid Orange 7. The concentrations of these dye samples were as follows (Table 5):
- Turquoise VG: 1 g in 25 mL of distilled water
- Black B: 1 g in 25 mL of distilled water
- Yellow R: 1 g in 25mL of distilled water
- Methyl red: two drops in each 25 mL of distilled water
- Trypan blue: 1 g in 500 mL of 60% alcohol
- Acid Orange 7: two drops in each 25 mL of distilled water
Table (5):
Wavelength of textile dyes
No |
Textile dye colour |
Wavelength(nm) |
|---|---|---|
1 |
Turquoise VG |
465 |
2 |
Black B |
700 |
3 |
Yellow R |
590 |
4 |
Methyl Red |
409 |
5 |
Trypan Blue |
607 |
6 |
Acid Orange 7 |
484 |
Decolorization of dyes
To evaluate the enzyme’s ability to remove dyes, Turquoise VG, Black B, Yellow R, Methyl red, Trypan blue, and Acid Orange 7 were used. The percentage drop in color was measured spectrophotometrically by observing the absorbance at the specific wavelength of each dye every hour. A stock solution (20 ppm) of each dye was added to 2 mL of the partially purified enzyme extract and 2 mL of distilled water.
Dye decolourization percentage = [ Initial absorbance – Final absorbance / Initial absorbance ] × 100
Textile Industrial wastewater sample collection
A 1 L sample of textile industrial wastewater was collected from an effluent outlet in Tiruppur. The sample was submitted to Enviro Farmers Labs and Technologies lab in Coimbatore for analysis, both before and after treatment with laccase enzyme.
Textile Industrial wastewater sample analysis
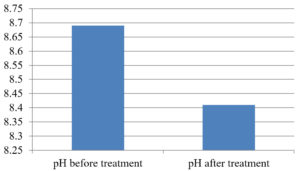
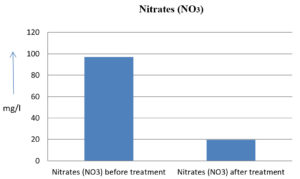
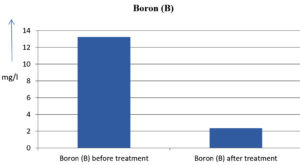
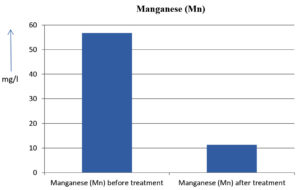
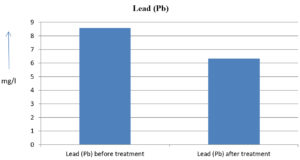
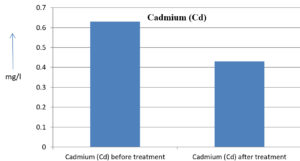
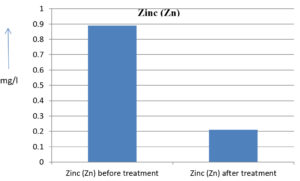
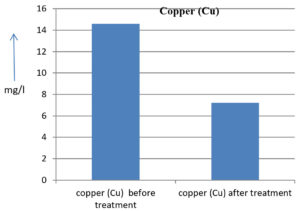
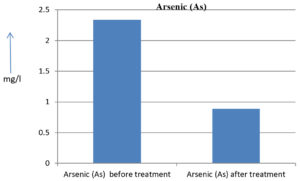
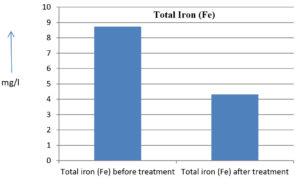
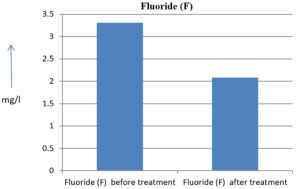
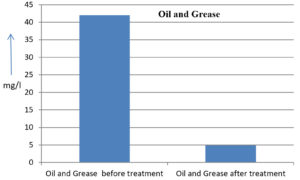
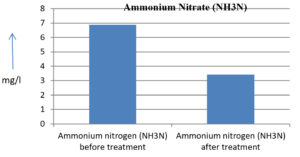
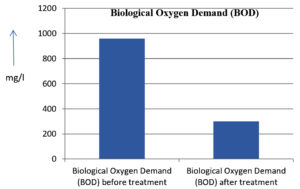
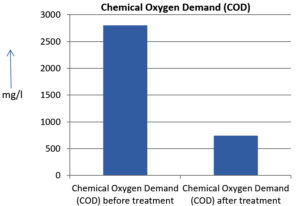
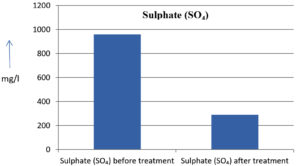
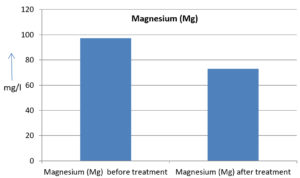
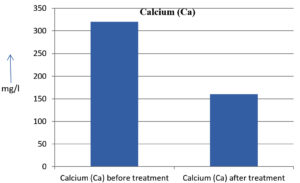
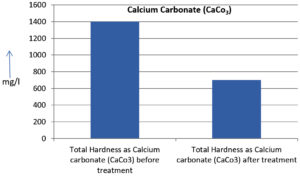
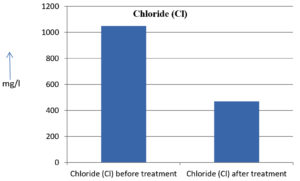
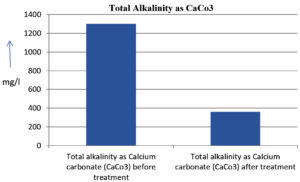
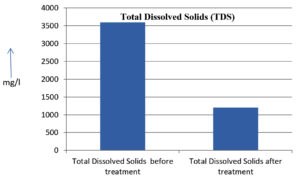
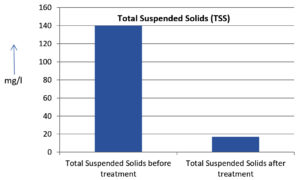
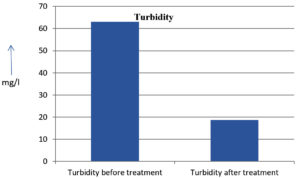
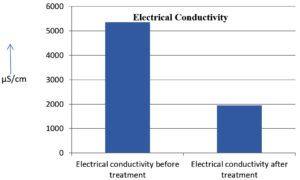
The analysis of the textile industrial wastewater sample yielded the following results for vital parameters before treatment with the laccase enzyme: pH, 8.69; nitrates (NO3), 96.84 mg/L; B, 13.24 mg/L; Mn, 56.84 mg/L; Pb, 8.57 mg/L; Cd, 0.63 mg/L; Zn, 0.89 mg/L; Cu, 14.58 mg/L; As, 2.34 mg/L; Total Fe, 8.74 mg/L; F, 3.31 mg/L; oil and grease, 42 mg/L; ammonium nitrogen (NH3N), 6.88 mg/L; BOD, 960 mg/L; COD, 2,800 mg/L; sulfate (SO„ ), 960 mg/L; Mg, 97.28 mg/L; Ca, 320.64 mg/L; Total hardness as calcium carbonate (CaCo3), 1,400 mg/L; Cl, 1,050 mg/L; total alkalinity as CaCo3, 1,300 mg/L; TDS, 3,600 mg/L; TSS, 140 mg/L; turbidity, 63 mg/L; electrical conductivity, 5,360µS/cm.
Effect of temperature on enzyme activity
Based on the results, it was determined that the optimum temperature for laccase enzyme activity is 30°C (Figure 1).
Effect of pH on enzyme activity
The results indicated that the optimum pH for laccase enzyme activity is pH 3 (Figure 2).
Biodegradation of textile dyes
Laccase, once extracted and purified, was utilized to decolorize textile dyes.
Textile dye absorbency before laccase enzyme treatment
The absorbance of textile dyes was measured at 1 h intervals to calculate their absorbance.
Textile dye absorbency after laccase enzyme treatment
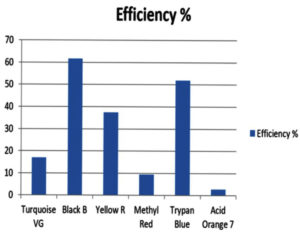
The laccase enzyme was added to the dyes at a ratio of 2:5. The absorbance of textile dyes was measured at 1 h intervals to calculate their absorbance. The percentage reduction was tracked and calculated spectrophotometrically at various wavelengths (Table 6 and Table 7). Figure 3 illustrates the decolorization of dyes by laccase.
Table (6):
Absorbance of the dyes before and after treatment
| No | Time (hr) | INITIAL OD (nm) at 540 nm | FINAL OD (nm) at 540 nm | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| – | – | Turquoise VG | Black B | Yellow R | Methyl Red | Trypan Blue | Acid Orange 7 | Turquoise VG | Black B | Yellow R | Methyl Red | Trypan Blue | Acid Orange 7 | |
| 1 | 0 | 0.327 | 1.375 | 0.742 | 3.297 | 0.055 | 0.074 | 0.312 | 0.85 | 0.670 | 3.293 | 0.035 | 0.059 | |
| 2 | 1 | 0.327 | 1.375 | 0.742 | 3.297 | 0.055 | 0.074 | 0.290 | 0.627 | 0.590 | 3.185 | 0.029 | 0.055 | |
| 3 | 2 | 0.327 | 1.375 | 0.742 | 3.297 | 0.055 | 0.074 | 0.271 | 0.410 | 0.475 | 2.950 | 0.027 | 0.053 | |
| 4 | 3 | 0.327 | 1.375 | 0.742 | 3.297 | 0.055 | 0.074 | 0.253 | 0.392 | 0.376 | 2.857 | 0.022 | 0.051 | |
| 5 | 4 | 0.327 | 1.375 | 0.742 | 3.297 | 0.055 | 0.074 | 0.231 | 0.356 | 0.210 | 2.654 | 0.019 | 0.049 | |
Table (7):
Percentage of degradation of dyes.
Textile dye colours |
Turquoise VG |
Black B |
Yellow R |
Methyl Red |
Trypan Blue |
Acid Orange 7 |
|---|---|---|---|---|---|---|
Efficiency % |
17 |
61.63 |
37.43 |
9.42 |
52 |
2.83 |
Enzyme activity
Enzyme activity was calculated using the formula mentioned above and found to be 7.25 U/mL.
Textile industrial wastewater sample analysis result after laccase treatment
The analysis of textile industrial wastewater after treatment with the laccase enzyme yielded the following results for vital parameters: pH, 8.41; NO3, 19.54 mg/L; B, 2.34 mg/L; Mn, 11.27 mg/L; Pb, 6.33 mg/L; Cd, 0.43 mg/L; Zn, 0.21 mg/L; Cu, 7.22 mg/L; As, 0.89 mg/L; total Fe, 4.32 mg/L; F, 2.08 mg/L; oil and grease, 4.87 mg/L; NH3N, 3.42 mg/L; BOD, 300 mg/L; COD, 740 mg/L; SO„ 290 mg/L; Mg, 72.96 mg/L; Ca, 160.32 mg/L; total hardness as CaCo3, 700 mg/L; Cl, 470 mg/L; total alkalinity as CaCo3, 360 mg/L; TDS, 1,200 mg/L; TSS, 17 mg/L; turbidity, 18.7 mg/L; electrical conductivity, 1,950µS/cm. These results are presented in Figure 4 to Figure 28, and the differences in parameter values before and after laccase treatment are detailed in Table 8.
Table (8):
Test Report of Textile Industrial Effluent (Waste Water) Analysis
No |
Characteristics |
Before Laccase Treatment |
After Laccase Treatment |
|---|---|---|---|
1 |
pH |
8.69 |
8.41 |
2 |
Nitrates (NO3) |
96.84 mg/l |
19.54 mg/l |
3 |
Boron (B) |
13.24 mg/l |
2.34 mg/l |
4 |
Manganese (Mn) |
56.84 mg/l |
11.27 mg/l |
5 |
Lead (Pb) |
8.57 mg/l |
6.33 mg/l |
6 |
Cadmium (Cd) |
0.63 mg/l |
0.43 mg/l |
7 |
Zinc (Zn) |
0.89 mg/l |
0.21 mg/l |
8 |
copper (Cu) |
14.58 mg/l |
7.22 mg/l |
9 |
Arsenic (As) |
2.34 mg/l |
0.89 mg/l |
10 |
Total iron (Fe) |
8.74 mg/l |
4.32 mg/l |
11 |
Fluoride (F) |
3.31 mg/l |
2.08 mg/l |
12 |
Oil and Grease |
42 mg/l |
4.87 mg/l |
13 |
Ammonium nitrogen (NH3N) |
6.88 mg/l |
3.42 mg/l |
14 |
Biological Oxygen Demand (BOD) |
960 mg/l |
300 mg/l |
15 |
Chemical Oxygen Demand (COD) |
2800 mg/l |
740 mg/l |
16 |
Nitrate (NO3) |
96.84 mg/l |
19.54 mg/l |
17 |
Sulphate (SO₄) |
960 mg/l |
290 mg/l |
18 |
Magnesium (Mg) |
97.28 mg/l |
72.96 mg/l |
19 |
Calcium (Ca) |
320.64 mg/l |
160.32 mg/l |
20 |
Total Hardness as Calcium carbonate (CaCo3) |
1400 mg/l |
700 mg/l |
21 |
Chloride (Cl) |
1050 mg/l |
470 mg/l |
22 |
Total alkalinity as Calcium carbonate (CaCo3) |
1300 mg/l |
360 mg/l |
23 |
Total Dissolved Solids (TDS) |
3600 mg/l |
1200 mg/l |
24 |
Total Suspended Solids (TSS) |
140 mg/l |
17 mg/l |
25 |
Turbidity |
63 mg/l |
18.7 mg/l |
26 |
Electrical conductivity |
5360 μS/cm |
1950 μS/cm |
Biodegradation percentage (%)
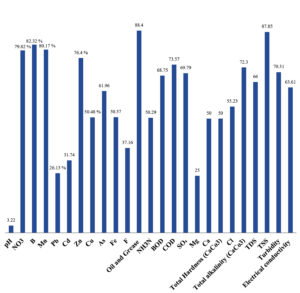
The bioremediation percentages for various physical and chemical parameters, heavy metals, ions, salts, and chemicals in textile industrial effluent wastewater are as follows: pH, 3.22%; NO3, 79.82%; B, 82.32%; Mn, 80.17%; Pb, 26.13%; Cd, 31.74%; Zn, 76.40%; Cu, 50.48%; As, 61.96%; total Fe, 50.57%; F, 37.16%; oil and grease, 88.40%; NH3N, 50.29%; BOD, 68.75%; COD, 73.57%; SO4, 69.79%; Mg, 25%; Ca, 50%; total hardness as CaCo3, 50%; Cl, 55.23%; total alkalinity as CaCo3, 72.30%; TDS, 66%; TSS, 87.85%; turbidity, 70.31%; electrical conductivity, 63.61% (Table 9 and Figure 29 illustrate these percentages).
Table (9):
Biodegradation % of Heavy Metals, Toxic Chemicals, Ions, Physical and Chemical Parameters of Textile Industrial Effluent
No |
Parameters |
Biodegradation % |
|---|---|---|
1 |
pH |
3.22 % |
2 |
Nitrates (NO3) |
79.82 % |
3 |
Boron (B) |
82.32 % |
4 |
Manganese (Mn) |
80.17 % |
5 |
Lead (Pb) |
26.13 % |
6 |
Cadmium (Cd) |
31.74 % |
7 |
Zinc (Zn) |
76.40 % |
8 |
copper (Cu) |
50.48 % |
9 |
Arsenic (As) |
61.96 % |
10 |
Total iron (Fe) |
50.57 % |
11 |
Fluoride (F) |
37.16 % |
12 |
Oil and Grease |
88.40 % |
13 |
Ammonium nitrogen (NH3N) |
50.29 % |
14 |
Biological Oxygen Demand (BOD) |
68.75 % |
15 |
Chemical Oxygen Demand (COD) |
73.57 % |
16 |
Nitrate (NO3) |
79.82 % |
17 |
Sulphate (SO₄) |
69.79 % |
18 |
Magnesium (Mg) |
25 % |
19 |
Calcium (Ca) |
50 % |
20 |
Total Hardness as Calcium carbonate (CaCo3) |
50 % |
21 |
Chloride (Cl) |
55.23 % |
22 |
Total alkalinity as Calcium carbonate (CaCo3) |
72.3 % |
23 |
Total Dissolved Solids (TDS) |
66 % |
24 |
Total Suspended Solids (TSS) |
87.85 % |
25 |
Turbidity |
70.31 % |
Figure 29. Laccase Enzyme Biodegradation % of Metals, Ions, Salts, Chemicals, Physical & Chemical Parameters of Textile Industrial Effluent Waste Water
Time vs laccase concentration vs dye decolorization
The relationship between time, laccase concentration, and dye decolorization is presented in Table 10.
Table (10):
Different laccase concentrations and incubation times on degradation efficiency of dyes
| No | Laccase Concentration | Incubation time | Dyes | Dye Degradation % |
|---|---|---|---|---|
|
1 |
1 ml | 1 hr | Turquoise VG [0.1 ml Dye (20 ppm)] + 2 ml dH2O | 2.1 % |
| 2 hr | 4.2 % | |||
| 3 hr | 7.5 % | |||
| 4 hr | 9 % | |||
| 2 ml | 1 hr | 4.1 % | ||
| 2 hr | 8.3 % | |||
| 3 hr | 12.5 % | |||
| 4 hr | 17 % | |||
|
2 |
1 ml | 1 hr | Black B [0.1 ml Dye (20 ppm)] + 2 ml dH2O | 3.65 |
| 2 hr | 7.7 % | |||
| 3 hr | 15.25 % | |||
| 4 hr | 30.25 % | |||
| 2 ml | 1 hr | 9 % | ||
| 2 hr | 18 % | |||
| 3 hr | 35 % | |||
| 4 hr | 61.63 % | |||
|
3 |
1 ml | 1 hr | Yellow R [0.1 ml Dye (20 ppm)] + 2 ml dH2O | 2.3 % |
| 2 hr | 4.2 % | |||
| 3 hr | 9 % | |||
| 4 hr | 20 % | |||
| 2 ml | 1 hr | 4.6 % | ||
| 2 hr | 9.3 % | |||
| 3 hr | 18.5 % | |||
| 4 hr | 37.43 % | |||
|
4
|
1 ml | 1 hr | Methyl Red [0.1 ml Dye (20 ppm)] + 2 ml dH2O | 0.67 % |
| 2 hr | 1.1 % | |||
| 3 hr | 2.2 % | |||
| 4 hr | 5.1 % | |||
| 2 ml | 1 hr | 1.15 % | ||
| 2 hr | 2.23 % | |||
| 3 hr | 4.56 % | |||
| 4 hr | 9.42 % | |||
|
5 |
1 ml | 1 hr | Trypan Blue [0.1 ml Dye (20 ppm)] + 2 ml dH2O | 1.65 % |
| 2 hr | 6.3 % | |||
| 3 hr | 12.5 % | |||
| 4 hr | 25 % | |||
| 2 ml | 1 hr | 6.5 % | ||
| 2 hr | 13 % | |||
| 3 hr | 26.5 % | |||
| 4 hr | 52 % | |||
| 6 | 1 ml | 1 hr | Acid Orange [0.1 ml Dye (20 ppm)] + 2 ml dH2O | 0.17 % |
| 2 hr | 0.32 % | |||
| 3 hr | 0.62 % | |||
| 4 hr | 1.21 % | |||
| 2 ml | 1 hr | 0.34 % | ||
| 2 hr | 0.75 % | |||
| 3 hr | 1.41 % | |||
| 4 hr | 2.83 % |
In conclusion, this research demonstrates the successful production of the enzyme laccase by P. ostreatus through solid-state fermentation, which is a simple and effective method. Laccase was found to be capable of successfully decolorizing various synthetic dyes, including Turquoise VG, Black B, Yellow R, Methyl red, Trypan blue, and Acid Orange 7. Notably, laccase exhibited higher efficacy in degrading Black B dye with an efficiency of 61.63% compared to other synthetic dyes. P. ostreatus was found to be the most effective fungus for laccase production, with an enzyme activity of 7.25 U/mL.
Comparatively, when compared to laccase isolated from other fungal species, such as Agaricus bisporus and Trichoderma viride, laccase from P. ostreatus demonstrated higher efficacy and potential. Laccase enzymes hold the potential to replace traditional chemical processes in the degradation of dyes in textile industrial effluent.
Furthermore, the biological use of laccase from P. ostreatus demonstrated significant potential in the biodegradation of toxic heavy metals, ions, and chemicals in textile industrial effluent wastewater. This method proves to be efficient, sustainable, and cost-effective, requiring minimal energy and manpower. The treated water can be reused for agricultural and domestic purposes, such as watering plants and toiletry.
This research presents an effective bioremediation approach through the selection of appropriate fungi, isolation of laccase enzyme, and treatment of textile industrial wastewater. The enzymatic method developed is simple and environmentally friendly, making it suitable for application in underdeveloped nations. Further research is needed to evaluate this treatment system for pathogen removal in wastewater.
ACKNOWLEDGMENTS
The authors would like to thank the Department of Biotechnology at Karunya Institute of Technology and Sciences in Coimbatore, India, for enabling us to perform and complete this academic study on time with adequate lab facilities, guidance and support. We are grateful to all of the professors who helped us and encouraged us to conduct this research.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHORS’ CONTRIBUTION
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
FUNDING
None.
DATA AVAILABILITY
All datasets generated or analyzed during this study are included in the manuscript.
ETHICS STATEMENT
This article does not contain any studies on human participants or animals performed by any of the authors.
- Vishwanath B, Chandra MS, Pallavi H, Reddy BR, Screening and assessment of Laccase producing Fungi isolated from different environmental sample. Afr J Biotechnol. 2008;7(8):1129-1133.
- Muthukumarasamy NP, Jackson B, Joseph Raj A, Sevanan M. Production of extracellular laccase from Bacillus subtilis MTCC 2414 using agro residues as a potential substrate. Biochem Res Int. 2015;765190.
Crossref - El-Batal AI, Elkenaway NM, Yassin AS, Amin MA. Laccase Production by Pleurotus ostreatus and its application in synthesis of gold Nanoparticles. Biotechnol Rep. 2015;5:31-39.
Crossref - Johnnie DA, Issac R, Prabha ML. Bio Efficacy Assay of Laccase isolated and Characterized from Trichoderma viride in Biodegradation of Low Density Polyethylene (LDPE) and Textile Industrial Effluent Dyes. J Pure Appl Microbiol. 2021;15(1):410-420.
Crossref - Kuddus M, Joseph B, Ramteke PW. Production of laccase from newly isolated Pseudomonas putida and its application in bioremediation of synthetic dyes and industrial effluents. Biocatal Agric Biotechnol. 2013;2(4):333-338.
Crossref - Cuoto SR, Herrera HLT. Industrial and biotechnological applications of laccases: A review. Biotechnol Adv. 2006;24(5):500-513.
Crossref - Kanwar RMA, Khan ZM, Farid HU. Development and adoption of wastewater treatment system for peri-urban agriculture in Multan, Pakistan. Water Sci Technol. 2019;80(8):1524-1537.
Crossref - Kalra K, Chauhan RS, Shavez M, Sachdeva S. Isolation Of Laccase Producing Trichoderma Spp. and Effect of PH and Temperature On Its Activity. International Journal of ChemTech Research. 2013; 5(5):2229-2235.
- Devi VM, Inbathamiz L, Ponnu TM, Premalatha S and Divya M. Dye Decolorization using Fungal Laccase. Bulletin of Environment, Pharmacology and Life Sciences. 2012;1(3):67-71.
- Goralczyk-Binkowska A, Jasinska A, Dlugonski A, Plocinski P, Dlugonski J. Laccase activity of the ascomycete fungus Nectriella pironii and innovative strategies for its production on leaf litter of an urban park. Plos One. 2020;15(4):e0231453.
Crossref - Narayanan PM, Murugan S, Eva AS, Devina SU, Kalidass S. Application of Immobilized Laccase from Bacillus subtilis MTCC 2414 on Decolourization of Synthetic Dyes. Res J Microbiol. 2015;10(9):421-432.
- Amutha C, Abhijit M. Screening and Isolation of Laccase Producers, Determination of Optimal condition for Growth, Laccase Production and Choose the Best Strain. J Bioremediat Biodegrad. 2015;6:4.
Crossref - Thakkar AT, Pandya DC, Bhatt SA. Optimization of Laccase Enzyme Production by Amesia atrobrunnea A2: A First Report. Biosci Biotechnol Res Asia. 2020;17(1):65-72.
Crossref - Luo W, Jin X, Yu Y, Zhou S, Lu S. Efficient nitrogen removal via simultaneous nitrification and denitrification in a penicillin wastewater biological treatment plant. Environ Technol. 2014;35(22):2885-2893.
Crossref - Kanwar RMA; Khan ZM, Farid HU. Development and adoption of wastewater treatment system for peri-urban agriculture in Multan, Pakistan. Water Science and Technology. 2019; 80(8):1524-1537.
Crossref
© The Author(s) 2023. Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License which permits unrestricted use, sharing, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.