Abstract
Keywords
Access Affordability Rational use of medicines Quality Pharmaceutical policy
Introduction
Since pharmaceutical services are of prime importance and play a key role in health system, improving quality services have always been one of policymakers′ main priorities in pharmaceutical sector. Ensuring the quality and safety of medicines, on-time provision of medicines, observing patients′ affordability, and improving rational use of medicines are all among the goals of this system. Policy making in pharmaceutical system necessitates providing scientific evidence drawn from the present conditions and the impacts of previous decisions. The evaluation of pharmaceutical system highlights the effectiveness of previous policies and their positive and negative consequences as well as the need for adopting new policies (1).
Due to the vital role of medicines in controlling burden of diseases and in decreasing their mortality rates, it should be noted that this aim can be achieved when medicines are available to all walks of life. High prices of medicines and their unavailability in pharmacies are the factors which can deteriorate patients′ health. Access generally means medicines are physically available in pharmacies and affordable for all citizens (2). Likewise, according to the laws of health systems in most countries, people are entitled to have access to essential medicines, and governments are to fulfill this aim (3). According to the world health organization (WHO)′s reports, around one-third of people in the world do not have assured access to essential medicines (4); that fifty percent of them live in Africa and Asia (5). In addition, according to WHO recommendations, to warrantee patients’ safety and promote community health level, it is necessary to evaluate pharmaceutical service providers in terms of “quality” (6). Quality is defined as “being free from defects, deficiencies, and significant variations” in service provision (7). As a result, quality, as a commitment to national or international standards, can promote health indicators, increase health system reliability, and enhance patients’ trust to pharmaceutical service providers. Furthermore, in the past decades, development of rational use of medicines was continuously one of the major priorities in pharmaceutical policy-making. According to the WHO′s report more than fifty percent of medicines are prescribed, dispensed, and sold irrationally (8). Economic losses on patients and health system, occurrence of adverse drug reactions, increased medicine resistance, prolonged illness or finally death can be the consequences and harms of irrational use of medicines (9). Rational use of medicines is defined as ″patients should receive medications commensurate with their clinical needs, in appropriate dosage that meet their individual requirements, for an adequate period of time, and at lowest cost to them and their community″ (10). Physicians and other health sectors’ professions, therefore, play the main role in the development of rational use of medicines. The average number of medicines per prescription, the percentage of antibiotics and injectable medicines in prescriptions, medicines prescribed by generic name, and prescription based on essential medicines list as well as standard therapeutic guidelines (STGs) are the main criteria to evaluate rational use of medicines (6).
This study aims to evaluate the performance of Iran′s pharmaceutical service providers to patients in terms of access to key medicines which were selected by research team according to WHO’s recommended list and national clinical practices, quality of drugs, and the status of rational use of medicines accordance with the WHO instructions. Despite the WHO′s advice concerning conducting such periodical evaluations, in Iran, some thinly scattered studies were carried out in each of these dimensions past few years (11, 12), yet the present study covers all the indicators related to these three dimensions all inclusively. The results of this study can indicate whether the aims of pharmaceutical system have been fulfilled or not and can clearly reveal the existing gaps to the policy-makers.
Methods
Study design
This research has been carried out based on the level II of “WHO′s operational package for assessing, monitoring and evaluating country pharmaceutical situations” (6). Dimensions and indicators investigated in this study are provided in Table 1. In the primary stage of designing study, the checklists had been exactly extracted from WHO guideline (by adjusting some items such as the list of key medicines). However, due to lack of IT capacity in Iran PHFs, missing of medicines’ shortage history recording, and lack of diseases diagnosis records in prescriptions, three survey forms (about average stock out duration, availability of standard treatment guidelines, and tracer cases treated according to recommended treatment protocols) were excluded in this survey. Similarly, three forms which are related to wholesalers’ data were omitted since they are not directly related to the aims of this study, analyzing service providers to end-users. Based on this instruction for evaluating these indicators, eleven separate checklists were developed and were scrutinized and certified by nine pharmaceutical sector experts, in terms of translation, content, and face validity. The experts, with minimum five-years experiences in pharmaceutical policy and management, were invited for face to face interview to validate checklists.
In order to investigate the affordability of medicines, standard treatments of diseases, including diabetes, asthma, hypertension, hyperlipidemia, pulmonary, and out-patient pneumonia were examined. By dividing the patients′ payment for a course of pharmacotherapy or the needed medicines for a one-month treatment of chronic diseases into the daily wage of the lowest paid unskilled government worker (LPGW), the affordability of such periods have been calculated (13). Should the result of such division be less than one (≤1), the purchase of medicines for a treatment period can be affordable. In 2018, the minimum daily wage in Iran was 380000 Rials (9 USD; exchange rate 42000 Rials). Regarding the indicator of geographic accessibility to pharmacies, the percentage of patients who get to a pharmacy by walking within maximum thirty minutes was investigated.
In order to compare the price of essential medicines, median price ratio (MPR) was considered, which is gained by comparing the price of each medicine with its international price. According to Health Action International (HAI) (14), the source utilized as a reference for exchange rates was the websites of “World Bank”, “IMF”, and “Central Bank of Iran”. According to these references, each U.S dollar was equal to 42000 Iranian Rials. By dividing the price of a given medicine in Iran into its reference price, MPR can be gained. This indicator should be less than 1.5 and 2 in public and private sector, respectively (15).
Concerning the quality, as WHO guideline recommends, the existence of expired medicines in pharmacies and observing the standards of storing medicines were considered as evaluation indicators. Standards concerning storing medicines based on the WHO guideline are: controlling the pharmacy temperature and appropriate thermal insulation, proper ventilation, not being exposed to direct sunlight, controlled humidity of the pharmacy, having air-conditioner and temperature log-sheets, sorting of medicines based on the expiry dates, having pest control program, not manipulation of tablets/capsules by naked hands, and not storing medicines directly on the floor. Since there is no list of essential medicines in Iran, a list of key medicines was prepared so that some indicators such as availability of medicines, the percentage of expired medicines, and the price of key medicines could be provided. The list of key medicines was developed considering the list of 15 medicines recommended by WHO, expanding to 25 medicines based on clinical expert opinion. The 25 selected medicines are prescribed in primary and secondary health care levels for common diseases and such medicines are expected to be always available in pharmacies. In this study, only the lowest price of generic (LPG) medicines have been taken into account, not the brand or brand-generic ones. To clear more, the list of selected medicines was presented in Table 2.
Sampling and Data Collection
To carry out this research, 30 public pharmacies and 30 private pharmacies from five cities were selected. According to the data provided by Iran Central Bank, Tehran, as the most privileged, and Zahedan, as the least privileged cities were selected. Then, based on clustered sampling technique, to cover different geographic regions, three more cities, namely Mashhad, Yazd, and Tabriz, were randomly chosen. The research sample included pharmacies and pharmaceutical centers which provided out-patient services in public (general) levels and had a specific place to dispense medicines to the patients. Therefore, specialized services were excluded. The first medical service-providers were the biggest out-patient pharmacies belonging to public hospitals in each city. The second samples were the smallest pharmaceutical service-providers in one of health centers located in the least privileged part of the city. These centers were selected by vice-chancellor for food and drug in medical universities of each province. Four more pharmacies were selected randomly in different parts of the cities. Pharmacies belonging to universities, social security organization, charities, and the armed forces were assumed as public ones. Having selected public pharmacies, the researchers chose the nearest private centers to the selected public pharmacies as the samples of private sectors.
In data collection phase, some appointments were made with pharmacy managers so that data collectors could collect the necessary information. Furthermore, As the WHO guidelines recommend, both retrospective and prospective methods were employed in patients sampling. For example, to measure some indicators related to rational use of medicines, prospective sampling was randomly carried out from among thirty patients who referred to the pharmacy. In order to achieve comparable data from different cities, uniformity in data collection was necessary. Therefore, data collectors were provided with some information, such as key medicines list, treatment guidelines, checklists, official letter of introduction to the local health authorities, and identification card. According to the WHO guideline, the selected patients were suffering from common diseases such as non-bacterial diarrhea, mild/moderate (outpatient) pneumonia, non-pneumonia acute respiratory tract infection, diabetes, asthma, and hyperlipidemia. It is worth mentioning that patients who referred for prenatal and postnatal care, the elderly and the children′s healthcare, and specialized counseling were excluded due to the difference in their treatments. The data were collected from April to November 2018.
Data Analysis
During the data gathering process, the non-qualified data were modified and the biased data were deleted. SPSS 24 was employed to analyze the data. Depending on proving data normality based on the Kolmogorov-smirnov test, Independent sample t-test and one-way ANOVA test were performed to test significant differences among various sectors with P-value <0.05 or <0.1.
Results
Access
The data related to the availability of key medicines in public and private pharmacies, the percentage of dispensed medicines, and geographical availability of pharmacies in studied cities are provided in Table 3. Based on the results drawn from one-way ANOVA and Independent sample t-test, there was no significant difference in the availability of key medicines in various cities, nor between public and private pharmacies. In comparison with private pharmacies, public pharmacies had dispensed a higher percentage of prescribed medicines. However, in terms of geographical availability of pharmacies, private pharmacies must have enjoyed a better condition.
The medicines’ prices, both in private and public pharmacies, were not different in the studied cities. The prices of key medicines list were calculated and then compared with the average world prices. The median price for one medicine in Iran compared to its world price (MPR) was equal to 1.33, which is acceptable.
As Table 4 demonstrates, for mentioned chronic diseases or common acute infections, the cost of medicines for one period of standard treatment regimens based on STGs is lower than the wage of LPGW. Even though the cost of treatment in private sector is higher than those of public sector, the purchase of the medicines for a period of treatment is affordable in both sectors.
Quality
As mentioned, in this study, quality was evaluated by considering lack of expired medicines in pharmacies and the proper conditions in which medicines are kept. As Table 5 demonstrates, there were no expired medicines in selected pharmacies (except for one in a public pharmacy in Tabriz). Test results drawn from Independent sample t-test revealed that the public sector enjoyed a more favorable condition and made a significant difference with private sector regarding these two indicators, namely ″appropriately storing medicines″ and ″appropriately keeping medicines in dispensing area″. In addition, among public pharmacies, the adequate conservation conditions and handling of medicines in the storeroom and dispensing area in Zahedan is much poorer than those in other cities, while among private pharmacies, these two indicators were much worse in Tehran than in other cities.
Rational use of medicines
Concerning rational use of medicines, prescribing antibiotics and injectable medicines, prescribing drugs using their Generic names and also based on available key medicines in public sector were studied. The average number of medicines per prescription were examined both retrospectively and prospectively. In prospective approach, 30 patients referred to the centers were randomly selected and their prescriptions were examined. In accordance with the WHO′s instructions, retrospective approach was carried out only in public sector; here, we selected randomly 30 general prescriptions registered on previous days and examine them. The average number of medicines per prescription, both retrospectively and prospectively, in public pharmacies was 3.2. Besides, based on prospective sampling this indicator was 3.4 in private pharmacies, which made a significant difference with public pharmacies. Studies in public sector revealed that, on average, 33% of patients received antibiotics and 32% were delivered injectable medicines. Also, 77% of medicines were prescribed using their generic names and 25% of prescriptions were from key medicines list. It is worth mentioning that 92% of medicines delivered to the patients contained adequate label, and 96% of patients precisely knew how to take their medicines. In addition, there was no significant difference between private and public centers concerning appropriately labeling the medicines and informing the patients about medicine consumption. Table 5 demonstrates the results regarding the indicators of rational use of medicines in detail.
Discussion
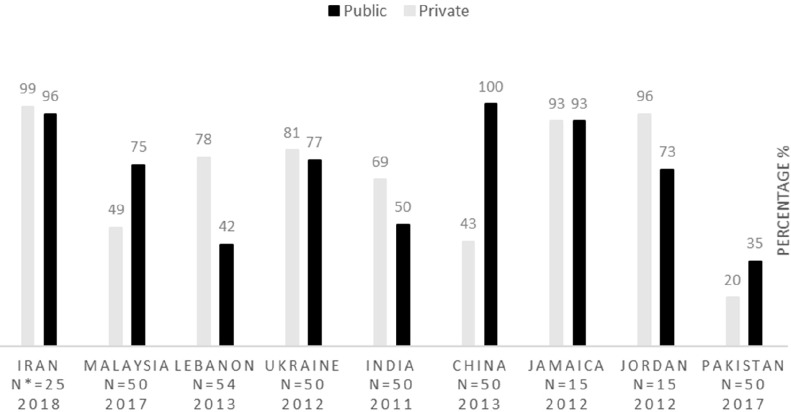
This study intends to evaluate Iran′s pharmaceutical situation in terms of access, quality, and rational use of medicines indicators in accordance with the WHO′s level II instructions. Concerning accessibility, which is upon the government to guarantee that the essential medicines should be available in pharmacies and be affordable for vulnerable groups (2), the results of this study reveal that the lowest price of key generic medicines, both in public and private sectors, are optimally available in Iran. Since the WHO declares that the acceptable level of availability for key medicines is at least 80% (16), this indicator, in Iran, is higher than this acceptable level. Figure 1 demonstrates the key medicines availability in some countries. Three more studies carried out in 2003, 2007, and 2014 in Iran approve these findings; the availability of key medicines in these studies is reported to be higher than 90% (11) (12). These data are compared with that of other countries, including Malaysia (17), Lebanon (18), Ukraine (19), India (20), China (21), Jamaica (22), Jordan (23) and Pakistan (24).
*N: the number of key medicines
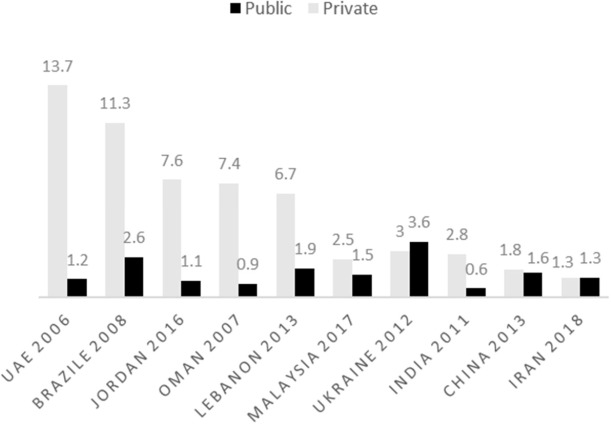
MPR indicator is a way with which the price of a medicine can be compared with that of other countries and its acceptable level is lower than 1.5 and 2 in public and private pharmacies, respectively (15). Figure 2 compares this indicator in Iran and other countries. The results of this chart indicate that medicine price, in public sector of Iran, enjoys an optimal condition, while this indicator in private sector in most countries is much higher than the acceptable level. Studies conducted in 2007 and 2014 confirm the optimal condition of MPR in Iran (12). These data are compared with that of other countries, including United Arab Emirates (25) , Brazil (26), Jordan (27), Oman (28), Lebanon (18), Malaysia (17), Ukraine (19), India (20), and China (21).
The results of the current research indicate that, in terms of affordability, Iran enjoys an optimal condition even if the medicines are not covered in insurance plans. To clarify this point, this indicator for Metformin without insurance coverage is 0.3 in Iran, 0.4 in Lebanon and Oman (18, 28), 0.5 in United Arab Emirates (UAE) (25), 0.6 in Jordan (27), 1.4 in Ukraine (19), and 2.1 in Jamaica (22). Similarly, this indicator for hypertension treatment with Captopril or Enalapril is 0.1 in Iran, 0.3 in China (29), 0.5 in Ukraine (19), 1.6 in Jordan and UAE (25, 27). Affordability for Asthma with inhaled Salbutamol is 0.4 in Iran and India (20); 0.3 in Oman (28), Lebanon (18), and Jordan (27); 0.5 in Ukraine (19); 0.6 in UAE (25) and China (29); and 1.6 in Brazil (26). Finally, this indicator for a seven-day treatment with Amoxicillin is reported 0.3 in Iran, 0.4 in Oman and UAE (25, 28), 0.5 in Ukraine (19), 0.6 in Jordan (27), 0.8 in India and China (20, 29), and 1.2 in Brazil (26). This achievement might root in Iran pharmaceutical policies such as price control, medicines distribution, and generic policies.
As it was mentioned earlier, rational use of medicines is one of the most challenging issues in medical policy making. Since policy makers have no complete control on the ″key prescribers″ and patients, controlling and optimizing the indicators in this field is very difficult (30). The WHO asserts that the acceptable average number of medicines per prescription is 2 or less. Prescribed antibiotics and injectable medicines, according to the WHO guidelines, should be maximally up to 30% and 10% for treated patients, respectively (31). The WHO instructions also maintains that the closer the percentage of prescription using the Generic name and key medicines lists to 100% is, the better it is (31). This study showed that the average number of medicines per prescription in public sector was 3.2, which is in parallel with previous studies conducted in 2003, 2011, and 2013 in Iran (11, 32, 33). Considering the findings of studies in other countries, they enjoy a better condition in this area. So, Iran needs more improvement regarding this indicator. These data are compared with that of other countries, including Jamaica (22), Jordan (23), Kuwait (34), India (35), United Arab Emirates (36), Saudi Arabia (37), Brazil (38), and China (39).
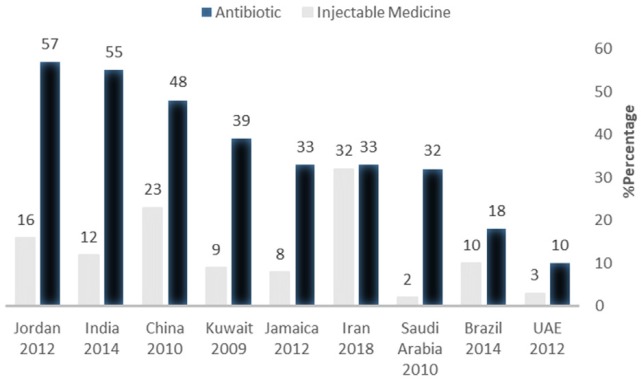
The percentage of patients for whom Antibiotics or injectable medicines are prescribed is another indicator demonstrating rational use of medicines because overconsumption of antibiotics brings about microbial resistance and injectable medicines increase risk of some complications. Figure 4 demonstrates the percentage of patients for whom antibiotics and injectable medicines have been prescribed is higher than other countries. These data are compared with that of other countries, including Jordan (23), India (35), China (39), Kuwait (34), Jamaica (22), Saudi Arabia (37), Brazil (40), and United Arab Emirates (36).
As it can be seen, injectable medicines consumption is much higher than the accepted level in the current study, as well as the studies conducted in 2003, 2011, and 2013 (11, 32, 33). There is a law in many public hospitals in UAE and Saudi Arabia stipulating that physicians are not allowed to prescribe an injectable medicine if its non-parenteral form is available (36, 37). Cultural factors and attitudes of both physicians and patients are important factors that may be affect this issue (41). Furthermore, poor-quality prescribing is taken by general practitioners more than specialists (42). However, in the case of number of items in a prescription and the percentage of antibiotics and injectable medicines in prescription developed countries, thanks to more optimal policy-making and governance, are in a more suitable condition (43).
Summary list of indicators used in present study
| dimensions | indicators | |
|---|---|---|
| 1 | Access | Availability of key medicines |
| % of prescribed medicines dispensed | ||
| Affordability of standard treatment regimens | ||
| Median price ratio of key medicines | ||
| Geographical accessibility to pharmacies | ||
| 2 | Quality | % expired medicines in pharmacies |
| Adequacy of conservation conditions and handling of medicines in dispensing facilities | ||
| Proper conditions and good handling of medicines in storerooms | ||
| 3 | Prescription and rational use of medicines | Average number of medicines per prescription (retrospective) |
| Average number of medicines per prescription (prospective) | ||
| % patients prescribed antibiotics in public health facilities | ||
| % patients prescribed injectable medicines in public health facilities | ||
| % medicines prescribed by generic name at public health facilities | ||
| % prescribed medicines on the key medicines list at public health facilities | ||
| % medicines adequately labelled at health facility dispensaries and private pharmacies | ||
| % patients knowing how to take medicines at public and private pharmacies | ||
The key medicines list
| Medicine | Dosage form | Medicine | Dosage form | ||
|---|---|---|---|---|---|
| 1 | Fluoxetine | 20 mg cap | 14 | Atorvastatin | 20 mg tab |
| 2 | Amoxicillin | 500 mg cap | 15 | Sodium valproate | 200 mg tab |
| 3 | Metoprolol | 50 mg tab | 16 | ASA | 80 mg tab |
| 4 | Losartan | 25 mg tab | 17 | Prednisolone | 5 mg tab |
| 5 | Ceftriaxone | 1 g vial | 18 | Metformin | 500 mg tab |
| 6 | Ciprofloxacin | 500 mg tab | 19 | Folic acid | 1 mg tab |
| 7 | Co-trimoxazole | 480 mg tab | 20 | Mebendazole | 100 mg tab |
| 8 | Alprazolam | 0.5 mg tab | 21 | Povidone iodine | 10% solution 250 mL |
| 9 | Ibuprofen | 400 mg tab | 22 | Oral rehydration salts (ORS) | 1 sachet |
| 10 | Glibenclamide | 5 mg tab | 23 | Normal Saline | 500 ml IV Fluid |
| 11 | Omeprazole | 20 mg cap | 24 | Captopril | 25 mg tab |
| 12 | Acetaminophen | 25 mg/mL syrup | 25 | Dexamethasone | 8 mg Injection |
| 13 | Salbutamol | 0.1 mg/dose inhaler |
Access to key medicines
| Tehran | Zahedan | Tabriz | Mashhad | Yazd | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Public | Private | Public | Private | Public | Private | Public | Private | Public | Private | |
| % key medicines available | 96 | 99 | 96 | 100 | 93 | 97 | 99 | 100 | 96 | 100 |
| % of prescribed medicines dispensed | 98 | 85 | 98 | 99 | 95 | 75 | 97 | 90 | 95 | 86 |
| Geographical accessibility to pharmacies % | 60 | 95 | 88 | 77 | 55 | 89 | 48 | 70 | 91 | 71 |
| Median price ratio of key medicines* | 1.33 | .33 | 1.33 | 1.33 | 1.33 | 1.33 | 1.33 | 1.33 | 1.33 | 1.33 |
The affordability of standard treatment regimens
| Reimbursed* | Not reimbursed | |||
|---|---|---|---|---|
| Public | Private | Public | Private | |
| Diabetes | ||||
| Glibenclamide 5 mg tab BD | 0.03 | 0.07 | 0.07 | 0.1 |
| Metformin 500 mg tab TDS | 0.08 | 0.1 | 0.28 | 0.31 |
| Gliclazide 80 mg tab QD | 0.04 | 0.08 | 0.1 | 0.15 |
| Insulin NPH OR Regular 2 Vial+15 syringe | 0.33 | 0.39 | 1.3 | 1.35 |
| Asthma | ||||
| Salbutamol 100mcg/dose Inhaler | 0.13 | 0.19 | 0.35 | 0.42 |
| Beclomethasone 250mcg | 0.17 | 0.24 | 0.45 | 0.52 |
| Blood pressure | ||||
| Atenolol 50 mg QD | 0.02 | 0.04 | 0.08 | 0.1 |
| Captopril 25 mg BD | 0.04 | 0.06 | 0.1 | 0.13 |
| Amlodipine 5 mg QD | 0.04 | 0.06 | 0.08 | 0.1 |
| Enalapril 10 mg BD | 0.15 | 0.22 | 0.15 | 0.22 |
| Losartan 50 mg QD | 0.04 | 0.06 | 0.1 | 0.13 |
| Hydrochlorothiazide 25 mg QD | 0.02 | 0.04 | 0.06 | 0.08 |
| Hyperlipidemia | ||||
| Simvastatin 20 mg QD | 0.07 | 0.13 | 0.16 | 0.23 |
| Upper respiratory tract infectious | ||||
| Amoxicillin 500 mg tab TDS for 7 days | 0.08 | 0.14 | 0.2 | 0.3 |
| Ciprofloxacin 500 mg tab BD for 7 days | 0.05 | 0.1 | 0.1 | 0.2 |
| Moderate pneumonia (without hospitalization) | ||||
| Azithromycin cap 250 mg 6 tab/cap | 0.07 | 0.14 | 0.18 | 0.25 |
| For children: Azithromycin suspension 200 mg/5mL | 0.1 | 0.17 | 0.17 | 0.23 |
The Quality of medicines
| Tehran | Zahedan | Tabriz | Mashhad | Yazd | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Public | Private | Public | Private | Public | Private | Public | Private | Public | Private | |
| medicines expired in outlets (%) | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Adequacy of conservation conditions and handling of medicines in dispensing facilities (%) | 88 | 68 | 76 | 73 | 82 | 79 | 92 | 71 | 76 | 82 |
| Proper conditions and good handling | 94 | 71 | 77 | 74 | 84 | 86 | 92 | 71 | 89 | 80 |
The status of rational use of medicines indicators
| Tehran | Zahedan | Tabriz | Mashhad | Yazd | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Public | Private | Public | Private | Public | Private | Public | Private | Public | Private | |
| Average number of medicines per prescription (prospective) | 3 | 3.7 | 3.5 | 3.8 | 2.9 | 3.4 | 3.5 | 2.8 | 3.1 | 3.6 |
| Average number of medicines per prescription (retrospective) | 3 | 3.4 | 2.8 | 3.3 | 3.2 | |||||
| % patients prescribed antibiotics in public facilities | 32 | 43 | 17 | 26 | 45 | |||||
| % patients prescribed injections in public facilities | 37 | 34 | 19 | 40 | 32 | |||||
| % medicines prescribed by generic name at public facilities | 75 | 92 | 61 | 76 | 79 | |||||
| % prescribed medicines on the key medicines list at public facilities | 26 | 32 | 17 | 24 | 25 | |||||
| % medicines adequately labelled at public and private facilities | 97 | 91 | 100 | 99 | 100 | 99 | 92 | 81 | 79 | 79 |
| % patients knowing how to take medicines at public and private facilities | 99 | 98 | 99 | 99 | 100 | 100 | 99 | 97 | 87 | 93 |

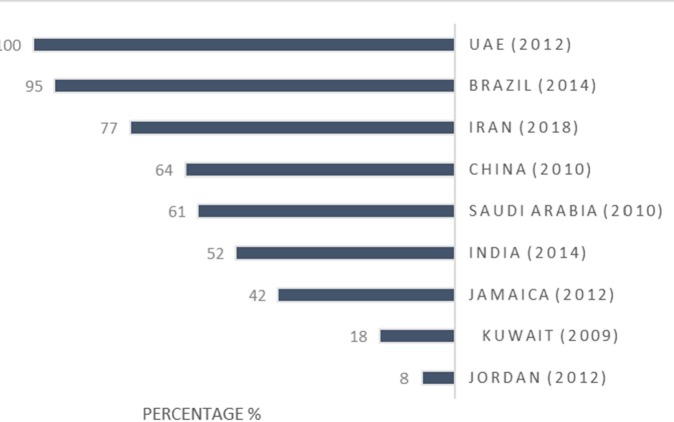
The percentage of medicines prescribed by generic name is another indicator showing rational use of medicines. Prescribing and using generic medicines which have as much efficacy as brand ones is more favorable for governments because it contributes to saving more money in public resources. However, pharmaceutical companies′ advertisements and families′ raise of income push physicians and people to prescribe and consume brand medicines. Figure 5 shows percentage of medicines prescribed by generic name in surveyed countries. These data are compared with that of other countries, including United Arab Emirates (36), Brazil (40), China (39), Saudi Arabia (37), India (35), Jamaica (22), Kuwait (34), and Jordan (23).
Although in UAE the public sectors are required to prescribe medicines with their generic names (36), according to survey conducted in private centers there, prescription indicator for generic medicines amounted to 7% (44).
Conclusion and Recommendation
This study intended to evaluate Iran pharmaceutical situation in terms of some indicators such as availability, affordability, quality of medicines and rational use of medicines indicators. This research revealed that Iran was in a good condition regarding availability and affordability of key medicines both in public and private sectors. Likewise, by inspecting whether there were any expired medicines and whether they were kept and stored in suitable conditions in pharmacies, the quality of medicines and pharmacy practices was approved. However, the results of this study showed that Iran is not in a favorable condition regarding rational use of medicines indicators, such as the average number of medicines, antibiotics, and injectable medicines per prescription. To improve the condition, it is recommended that some incentive and punitive measures for physicians to prescribe generic medicines, and pharmacists be encouraged to generic substitution. Also, physicians be obliged to follow the clinical guidelines. In addition, the electronic systems which monitor prescription behavior, and public education are recommended. It must be noted that insurance organizations, as the service purchasers, play a key role in directing physicians and promoting rational use of medicines indicators. Hence, to promote the pharmaceutical system′s performance, the followings are recommended.
In order to more efficient management of access, it is recommended that the list of essential medicines for different circumstances be classified and prescriptions directed to this list.
Although affordability for different types of medicines in Iran is mainly better than that in other countries, more incentive policies can be considered for generic medicine may improve rational use of medicines and lead to more efficient use of resource.
Due to the necessity of continuous and accurate observation of drug utilization, developing “personal electronic health record” can be an effective measure to promote rational use of medicines indicators.
Limitations
Every study has limitations. In this study, the relatively small sample size in each facility may decreases the reliability of the inter-facility comparison. In addition, the methodological pitfall regarding the wage of the lowest paid unskilled government worker is considered for unemployed persons, who have a daily wage less than the afore-mentioned amount, and who have more than one patient in a family.
Acknowledgements
References
-
1.
Buse K, Mays N, Walt G. Making health policy. 1. McGraw-Hill Education (UK); 2012. p. 1-10.
-
2.
Lu Y, Hernandez P, Abegunde D, Edejer T. The world medicines situation 2011. Geneva: Medicine expenditures World Health Organization; 2011. p. 35-8.
-
3.
Hogerzeil HV. Essential medicines and human rights: what can they learn from each other? Bull. World Health Organ. 2006;84:371-5.
-
4.
WHO Medicines strategy 2004-2007: countries at the core. World Health Organization. 2004:13-6.
-
5.
Treasury HM, Revenue I. Increasing access to essential medicines in the developing world. UK Government policy and plans. 2004:32-9.
-
6.
Precious M, German V. WHO Operational package for assessing, monitoring and evaluating country pharmaceutical situations. Geneva. 2007:1-147.
-
7.
World Health Organization. Quality assurance of pharmaceuticals: A compendium of guidelines and related materials. Good manufacturing practices and inspection. 2007;2:20-7.
-
8.
Hogerzeil H. Promoting rational prescribing. Med. Educ. 1995;39:1-6.
-
9.
World Health Organization. Promoting rational use of medicines saves lives and money. WHO experts say. 2004:5-9.
-
10.
The Rational Use of Drugs: Report of the Conference of Experts Nairobi, World Health Organization. Geneva. 1987:4-5.
-
11.
Cheraghali AM, Nikfar S, Behmanesh Y, Rahimi V, Habibipour F, Tirdad R. Evaluation of availability, accessibility and prescribing pattern of medicines in the Islamic Republic of Iran. East. Mediterr. Heal. J. 2004;10:406-15.
-
12.
Prices, availability, affordability and price components of medicines to treat non-communicable diseases in Iran. 2014. p. 1-10.
-
13.
Niëns LM, Brouwer WBF. Measuring the affordability of medicines: importance and challenges. 112. New. York: Health Policy; 2013. p. 45-52.
-
14.
Hogerzeil H, Reed T. Measuring medicine prices, availability, affordability and price components. 2008. p. 1-7.
-
15.
Julie E. International drug Price indicator Guide. Management Science for Health. 2014:1-393.
-
16.
Ewen M, Zweekhorst M, Regeer B, Laing R. Baseline assessment of WHO’s target for both availability and affordability of essential medicines to treat non-communicable diseases. PLoS One. 2017;12:1-7.
-
17.
Wong SL, Ibrahim S, Kadir NA, Salleh SM. Access and Affordability of Medicines in Malaysia: Need for a National Pricing Policy. Appl. Health Econ. Health Policy. 2019:1-14. [PubMed ID: 30143995].
-
18.
Karam R. Prices, availability, affordability and price components of medicines to treat non-communicable diseasesn in lebanon. 2013. p. 1-11.
-
19.
Tetyana Dumenko. Medicine prices, availability, affordability and price components in Ukraine. 2012. p. 1-8.
-
20.
Kotwani A. Where are we now: assessing the price, availability and affordability of essential medicines in Delhi as India plans free medicine for all. BMC Health Serv. Res. 2013;13:1-14. [PubMed ID: 23286781].
-
21.
Xi X, Li W, Li J, Zhu X, Fu C, Wei X. A survey of the availability, prices and affordability of essential medicines in Jiangsu Province, China. BMC Health Serv. Res. 2015;15:1-7. [PubMed ID: 25603697].
-
22.
Graham CL. Pharmaceutical Situation in Jamaica. 2012. p. 1-124.
-
23.
Naffa S. Pharmaceutical Situation Assessment in Jordan (level II). 2012. p. 1-57.
-
24.
Saeed A, Saeed H, Saleem Z, Fang Y, Babar Z-U-D. Evaluation of prices, availability and affordability of essential medicines in Lahore Division, Pakistan: A cross-sectional survey using WHO/HAI methodology. PLoS One. 2019;14:1-16.
-
25.
Younis NA-M. Medicine prices, availability, affordability and price components in United Arab Emirates. 2006. p. 1-6.
-
26.
Helfer AP, Camargo AL, Tavares NUL, Kanavos P, Bertoldi AD. Affordability and availability of drugs for treatment of chronic diseases in the public health care system. Pan American Journal of Public Health. 2012;31:1-5. [PubMed ID: 22427158].
-
27.
Alefan Q, Amairi R, Tawalbeh S. Availability, prices and affordability of selected essential medicines in Jordan: a national survey. BMC Health Serv. Res. 2018;18:787.
-
28.
Jaffar SA. Medicine prices, availability, affordability and price components in Oman. 2007. p. 1-6.
-
29.
Xi X, Chen P, Yang F, Yang Y, Chen L, Mao N. Evaluating the accessibility of essential medicines in China. J. Med. Econ. 2018:784-92. [PubMed ID: 29741406].
-
30.
Thawani V. Rational use of medicines: Achievements and challenges. Indian J. Pharmacol. 2010;42:63-4. [PubMed ID: 20711366].
-
31.
How to investigate drug use in health facilities: selected drug use indicators. 1993. p. 1-5.
-
32.
Meskarpour-Amiri M, Dopeykar N, Mehdizadeh P, Ayoubian A, Motaghed Z. A Study on the Factors Affecting the Prescription of Injection Medicines in Iran: A Policy Making Approach. Glob. J. Health Sci. 2015;7:291-7. [PubMed ID: 25948462].
-
33.
Karimi A, Haerizadeh M, Soleymani F, Haerizadeh M, Taheri F. Evaluation of medicine prescription pattern using World Health Organization prescribing indicators in Iran: A cross-sectional study. J. Res. Pharm. Pract. 2014;3:39-45. [PubMed ID: 25114935].
-
34.
Awad A, Al-Saffar N. Evaluation of drug use practices at primary healthcare centers of Kuwait. Eur. J. Clin. Pharmacol. 2010;66:1247-55. [PubMed ID: 20669012].
-
35.
Parveen Z, Gupta S, Kumar D, Hussain S. Drug utilization pattern using WHO prescribing, patient care and health facility indicators in a primary and secondary health care facility. Natl. J. Physiol. Pharm. Pharmacol. 2016;6:182-90.
-
36.
Mahmood A, Elnour AA, Ali AAA, Hassan NAGM, Shehab A, Bhagavathula AS. Evaluation of rational use of medicines (RUM) in four government hospitals in UAE. Saudi Pharm. J. 2016;24:189-96. [PubMed ID: 27013912].
-
37.
El Mahalli AA. WHO/INRUD drug prescribing indicators at primary health care centres in Eastern province, Saudi Arabia. East. Mediterr. Heal. J. 2012;18:1091-6.
-
38.
Lima MG, Álvares J, Guerra Junior AA, Costa EA, Guibu IA, Soeiro OM. Indicators related to the rational use of medicines and its associated factors. Rev. Saude Publica. 2017;51:1-8. [PubMed ID: 28099550].
-
39.
Dong L, Yan H, Wang D. Drug prescribing indicators in village health clinics across 10 provinces of Western China. Fam. Pract. 2011;28:63-7. [PubMed ID: 20876222].
-
40.
Lima MG, Dutra KR, Martins UCM. Prescribing indicators in primary health care in Belo Horizonte, Brazil: associated factors. Int. J. Clin. Pharm. 2017;39:913-8. [PubMed ID: 28626850].
-
41.
Yousefi N, Rashidian A, Soleymani F, Kebriaeezade A. Relationship between the provision of injection services in ambulatory physician offices and prescribing injectable medicines. Iran. J. Pharm. Res. 2017;16:399-405. [PubMed ID: 28496493].
-
42.
Sadeghian G-H, Safaeian L, Mahdanian A-R, Salami S, Kebriaee-Zadeh J. Prescribing quality in medical specialists in Isfahan, Iran. Iran. J. Pharm. Res. 2013;12:235-42. [PubMed ID: 24250595].
-
43.
Holloway KA, Ivanovska V, Wagner AK, Vialle-Valentin C, Ross-Degnan D. Have we improved use of medicines in developing and transitional countries and do we know how to? Two decades of evidence. Trop. Med. Int. Heal. 2013;18:656-64.
-
44.
Sharif SI, Al-Shaqra M, Hajjar H, Shamout A, Wess L. Patterns of drug prescribing in a hospital in Dubai, United Arab Emirates. Libyan J. Med. 2009;3:10-2.