1. Introduction
Most characteristics of an organism relate to its body size, often in such regular ways that they can be described by simple power functions [1–4]. Therefore, a major focus of comparative biology is to explain these “size-scaling” (“allometric”) relationships [5–11] or apply them in various ways to understand better the variation of specific phenotypic traits among individuals, populations, or species [12, 13]. Traditionally, the scaling slopes of many kinds of allometric relationships, which are often different from 1 in log–log space for various rates/durations of various biological processes, have been explained in terms of physical constraints, such as surface area to volume (SA/V) ratios or the geometry and physics of internal resource-transport networks [5, 8, 14, 15]. However, during the last three decades, increasing attention has been given to ecological and evolutionary effects on the allometric scaling of the rates of metabolism, growth, reproduction, life span, and other life-history and demographic traits (e.g., [15–28]). At the same time, research has also been growing on how ecological interactions between species, and the structure/function of ecological communities, may depend on the body sizes of species in an ecosystem (e.g., [29–39]). However, relatively little is known about how and why the diversity and intensity of different kinds of species (biotic) interactions scale with body size and their consequences for the body-size scaling of other biological and ecological processes at the organismal, population, and ecosystem levels.
In this review, I argue that body-size scaling analyses of species interactions may reveal new insights into not only the structure/function of ecological communities but also the causes of many kinds of biological and ecological scaling relationships. I focus chiefly on predation, herbivory, and parasitism because they are extremely common species interactions that jeopardize the lives or health of countless organisms with important consequences for their demography, life histories, and adaptive evolution. I briefly consider other kinds of species (biotic) interactions in Section 4.3. “Predators” are consumers that kill their prey in the process of eating them, often as whole bodies [29, 40]. Predators are usually larger than their prey [29, 31, 41]. By contrast, “parasites” are consumers that live on or in their hosts, eating portions of their bodies (or resources that they provide) often in a nonlethal way [29, 40, 42, 43]. Parasites are usually smaller than their hosts [29, 31, 41]. As Elton [29] pithily remarked, predators live on the “capital” of their food, whereas parasites live on the “income” (p. 71). However, these categories intergrade in various ways: for example, some parasites (especially parasitoids and pathogens) may eventually kill their hosts [29, 40, 42–46]. “Herbivores” [42, 47] may act like “predators” or “parasites” depending on whether they consume their algal/plant prey/hosts as wholes or parts, either lethally or not. Although these categories of ecological (biotic) antagonism or contramensalism (one species benefits, the other is harmed [48, 49]) differ only in degree, they may have profoundly different consequences for biological scaling patterns, as will be seen.
My review is organized as follows. First, I describe examples showing that predators and parasites may significantly influence the intraspecific body-size scaling of metabolism, growth, and reproduction during ontogeny (Section 2). Second, I propose hypothetical schemes of how the diversity and body-size range of predators, herbivores, and parasites attacking a species should depend in different ways on its body size; and I test these schemes with available data (Section 3). Third, I review possible causes of these interspecific size-predation and size-parasitism (herbivory) spectra (Section 3.3). Fourth, I discuss possible consequences of these size spectra for the body-size scaling of various physiological, life-history, and demographic traits and other ecological properties at the population, community, and ecosystem levels (Section 4). In doing so, I promote a nascent “mortality theory of ecology” (MorTE) that focuses on how mortality (e.g., predation)-imposed time limits may drive the evolution of the size scaling of many kinds of biological rates/durations [21]. A MorTE may complement or even supersede, at least in part, the currently influential “metabolic theory of ecology” (MTE), which proposes that the rate of metabolism or energy use drives the scaling of many kinds of biological processes [4, 50]. Fifth, I advocate for further studies of how body-size effects on various kinds of biotic interactions, including not only contramensalism (a major focus of this review) but also competition, mutualism, commensalism, and amensalism, may affect various biological scaling relationships (Section 4.3). Sixth, I conclude by discussing general theoretical and practical implications of the body-size scaling of biotic interactions on biological scaling theory, community ecology, and our understanding of biological and ecological responses to environmental (e.g., climate) change (Sections 5 and 6).
2. Predators and parasites can affect intraspecific body-mass scaling of metabolism, growth, and reproduction during ontogeny
My review was inspired by several studies suggesting or providing evidence for significant effects of predation and parasitism on the rates of metabolism, growth, and reproduction and their intraspecific body-mass scaling during ontogeny. For example, two decades ago I suggested that the relatively steep intraspecific scaling of metabolic rate typically seen in pelagic (open-water) invertebrates (mean scaling exponent = 0.95 in log–log space), as compared to non-pelagic (bottom-dwelling) invertebrates (mean scaling exponent = 0.74), evolved as a result of (1) their relatively high exposure to predation that selected for relatively rapid rates of growth, reproduction, and supporting metabolism and associated steep ontogenetic scaling with body mass, thus ensuring early maturation and reproduction before they were eaten and (or) (2) relatively high sustained costs of locomotion at all stages of growth, required to prevent sinking in the water column [19, 20]. These hypothetical effects on the ontogenetic biological scaling of pelagic invertebrates remain to be rigorously tested.
Furthermore, motivated in part by the above comparative work, my colleagues and I employed a “natural experiment” approach that revealed significant effects of predators on the ontogenetic biological scaling of the freshwater amphipod species Gammarus minus [22, 51–53]. We showed that amphipods from freshwater springs with visually hunting fish predators (slimy sculpins: Cottus cognatus) exhibited shallower ontogenetic scaling of growth and supporting metabolism and gill surface area, but steeper scaling of reproductive investment (egg number and clutch mass), as compared to those from environmentally comparable springs without fish predators. We explained these results as being the result of fish predators selectively preying on large amphipods, which, in turn, favored by natural selection slower post-maturational growth and less visible, smaller mature sizes in these prey animals, but higher earlier reproductive effort to compensate for their higher probability of early death. More recently, my colleagues and I [54, 55] have also shown that fish-predator regime/odors elicit significant shifts in how temperature affects metabolic scaling in two amphipod species (the freshwater G. minus and the estuarine G. insensibilis).
Ontogenetic scaling of growth may not only be affected by predators, but also conversely affect the behavioral responses and susceptibility of developing animals to predation. For example, the size of red drum (Sciaenops ocellatus) larvae during development importantly relates to their susceptibility and behavioral responses to predation by larger predatory fishes [56]. Indeed, predatory mortality rate generally scales negatively with fish larval size within and among fish species [56–59]. In many kinds of animals, predatory mortality is age- and size-specific with consequences for various life-history traits, including the rates and timing of growth, maturation, and reproduction (e.g., [22, 51, 60–64]).
Parasites may also significantly affect ontogenetic biological scaling. For example, infection by a trematode parasite reduces the metabolic rate of relatively large adult snails (Lymnaea stagnalis) more than that of smaller young snails, thus significantly altering the body-mass scaling of metabolic rate [65]. In addition, effects of trematode parasites on the growth and reproduction of snails are age- and size-specific [66]. The above-described studies showing effects of predators and parasites on intraspecific biological scaling stimulated me to consider their potential effects on interspecific biological scaling patterns, as well, as considered below.
3. Species diversity of predators and parasites in relation to prey/host body size
3.1. Hypothetical relationships
Based on the reasonable assumption that smaller organisms are physically more vulnerable to a wider range of predators of varying body sizes that can overpower and ingest them than larger organisms, I hypothesize that the diversity and body-size range of predators feeding on a species should negatively correlate with its body size (Figure 1). By contrast, based on the assumption that larger organisms represent larger physical targets, “habitat” spaces, and energy (resource) reservoirs better fostering the colonization and persistence of a wider range of parasites of varying body sizes, as compared to smaller organisms, I hypothesize that the diversity and body-size range of parasites feeding on a species should positively correlate with its body size (Figure 1). More rationale for these hypotheses is provided in Section 3.3.
3.2. Empirical relationships
In this section, I review available empirical data (taken from numerous relevant articles and books, facilitated by an extensive Google Scholar search) that bear on the hypotheses proposed in Section 3.1. The data concern broad “bacteria to whales” size spectra, as well as narrower size spectra within specific taxa and ecosystems.
Size-predation spectra
In general, small organisms are consumed by a greater range of organisms of varying body sizes than large organisms. This is well illustrated by a comparison of bacteria and whales, which are consumed by organisms ranging in body length by over eight orders of magnitude (from approximately 100 nm to 30 m for bacteriophages to blue whales) versus less than one-third of an order of magnitude (approximately 4–8 m for great white sharks to killer whales), respectively (Figure 2a). Zooplankton and fishes are consumed by predators with an intermediate range of body lengths (Figure 2a). Similar patterns of declining body-mass range of predators with increasing prey species size are shown in specific intertidal and lake food webs (see Figure 16.2 in Ref. [33]). In addition, within specific taxa and ecosystems, the species richness of predators tends to decrease with increasing prey size (Figure 3). For example, in African large mammal communities, larger ungulates are preyed on by fewer predator species than smaller ungulates (Figure 3a). Similar trends occur in marine animal and freshwater phytoplankton, zooplankton, and fish communities (Figure 3b). Other studies have also reported that the number of predator species (“vulnerability”) scales negatively with prey size in various food webs (e.g., [67–69]).
Figure 1
Hypothetical interspecific relationships between number of predator or parasite species and prey or host species body size.

Figure 2
Approximate ranges of body lengths of predators and parasites consuming bacteria, zooplankton, fishes, and whales. (a) Bacteria: bacteriophages (essentially “microscopic parasitoids”; 100 nm) to blue whales (30 m) [70–75]; zooplankton: chaetognaths/carnivorous copepods (3 mm) to blue whales (30 m) [76, 77]; fishes: ctenophores/cnidarians (10 cm) to killer whales (orcas: 8 m) [78–80]; whales (large cetaceans): great white sharks (4 m) to killer whales (8 m) [81–84]. (b) Bacteria: bacteriophages (lysogenic parasitic forms: 24–200 nm [70]; zooplankton: viruses (30 nm) to dinoflagellates/isopods/nematodes/cestode larvae (700 μm; [85–89]); fishes: viruses (30 nm) to tapeworms (1 m) [90, 91]; and whales: viruses (30 nm) to tapeworms (30 m [43, 92, 93]).
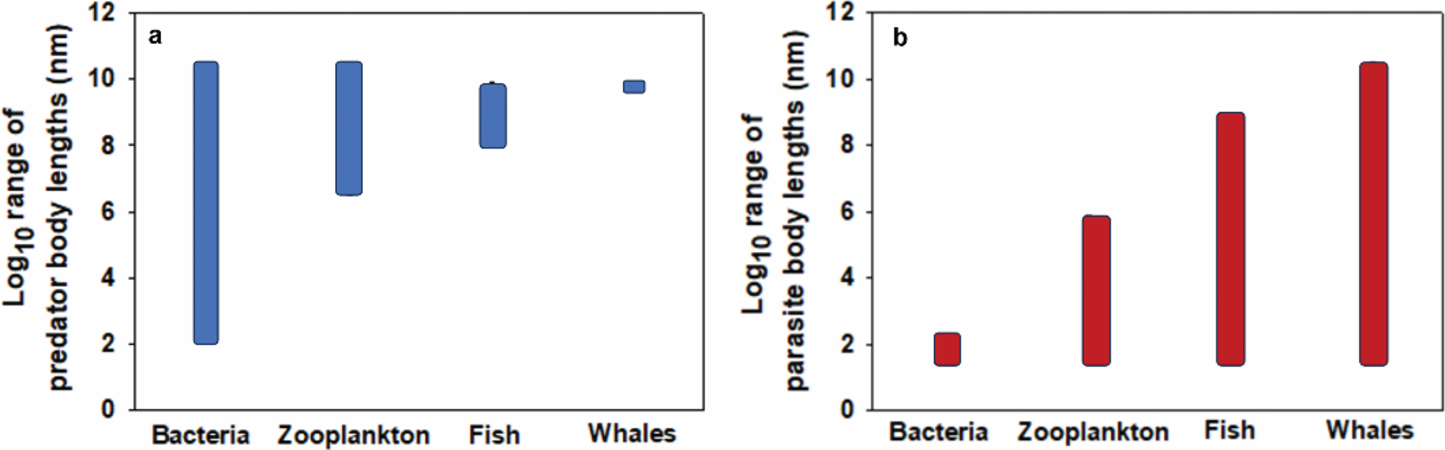
Figure 3
Species richness of predators versus prey species body mass (semi-log linear regression equations and statistics in parentheses) in large mammal communities of (a) the Serengeti (East Africa [94]; 9.589 – 2.907(X); r = 20; 0.973; N = 20; P < 0.001) and Kruger National Park (South Africa [95]: Y = 8.901 – 2.565(X); r = 0.882; N = 6; P = 0.020) and (b) Panamanian rocky intertidal (fishes and invertebrates [96]; Y = 4.779 – 0.764(X); r = 0.598; N = 12; P = 0.040) and Tuesday Lake (pelagic phytoplankton, zooplankton, and fishes [97]; phytoplankton: Y = –16.314 – 3.936(X); r = 0.796; N = 19; P < 0.001; zooplankton: Y = 0.810 – 0.776(X); r = 0.649; N = 17; P = 0.005) communities. The linear regression equation for all the organisms assessed in the food web for Tuesday Lake is Y= –2.669 – 1.681(X) (r = 0.666; N = 37; P < 0.001).

Size-parasitism (herbivory) spectra
In general, large organisms are infected by a greater range of organisms of varying body sizes than small organisms. This is well illustrated by a comparison of bacteria and whales, which are infected by organisms ranging in body length by less than one order of magnitude (from approximately 24 to 200 nm for bacteriophages—note that lysogenic bacteriophages do not kill their hosts, at least immediately [98], and thus could be considered parasites) versus approximately nine orders of magnitude (approximately 30 nm to 30 m for viruses to tapeworms, respectively (Figure 2b). Zooplankton and fishes are consumed by parasites with an intermediate range of body lengths (Figure 2b). Furthermore, parasite species richness tends to increase with increasing host species size in several animal taxa, including marine fishes (Figure 4a), freshwater fishes [99, 100], anurans [101], birds [102], ungulate mammals [103], and terrestrial mammals (Figure 4b). In terrestrial plants, species richness of parasitic fungi and herbivorous insects also increases with host size, as indicated by comparisons of herbs, shrubs, and trees (Figure 4d; [42, 104, 105, 106]). Moreover, broad comparative meta-analyses have revealed positive correlations between parasite species richness and host size across many kinds of animal, plant, and fungal hosts [107–110]. However, weakly positive, negative, or absent relationships may be found when the ranges of host sizes/taxa and/or parasite taxa are relatively narrow, and/or phylogenetic adjustments are made (e.g., [111–115]). Note that variation in sampling effort can also obscure the detection of relationships between parasite species richness and host body size [116, 114, 117–119]: when corrections are made for variation in sampling effort, positive correlations may become more apparent (e.g., [101]).
Figure 4
Species richness of parasites versus host species body size (semi-log linear regression equations and statistics in parentheses) in (a) coastal marine teleost fishes (Brazil [120]; 0.045 + 9.597(X); r = 0.283; N = 50; P = 0.046), (b) terrestrial mammals ([116]: Y = 12.578 + 1.833(X); r = 0.294; N = 70; P = 0.013), and (c) plants grouped as herbs, shrubs, and trees (the mean number of fungal and insect herbivorous parasites in North America and Britain, respectively [104]).

Size-predation and size-parasitism spectra in the same food webs
In a saltmarsh food web, the number of predator species per prey species decreases with trophic level, whereas the number of parasite species per host species increases (Figure 5). Since larger animals tend to occur at higher trophic levels (as confirmed by data in Ref. [121]), these patterns conform to the size-predation and size-parasitism spectra proposed (Figure 1) and documented by other studies reviewed in Sections 3.2.1 and 3.2.2. This is an especially noteworthy study because both size spectra are apparent in the same food web. Similar patterns appear to exist in other species-rich food webs (e.g., kelp forest [122]), although less clearly in relatively species-poor food webs in harsh environments (e.g., pelagic zone of a subarctic lake [123]).
Figure 5
Species richness of predators/parasites versus trophic level of host/prey species in a saltmarsh food web (Carpinteria, California, USA) (figure modified from [124]). Since larger animals tend to occur at higher trophic levels, the number of predators tends to decrease with prey size (indicated qualitatively by a blue line), whereas the number of parasites tends to increase (indicated qualitatively by a red line), as predicted in Figure 1.

3.3. Possible causes of size-predation/parasitism spectra
Size-predation spectra
Increased body size appears to confer greater protection against predation and other environmental hazards, as shown by the negative scaling of mortality rate with body size that is commonly observed (e.g., [50, 57, 59, 125–127]). Large size by itself is a mode of defense against predation: the largest animals (e.g., elephant, rhinoceros, hippopotamus, giraffe, blue whale, great white shark, whale shark, etc.) are too big to overcome and eat without difficulty, and thus have few or no native predators [83, 94, 128–130]. Various potentially protective features associated with large size include enhanced muscular/structural strength or locomotor speed, thick/tough body surfaces, sophisticated behavior/intelligence, and high mobility, allowing escape to locations with relatively low predation risk [1, 3, 131–139]. High volume-to-surface-area ratios are also generally protective because most of the body’s interior is relatively remote from external environmental hazards [21].
Size-parasitism (herbivory) spectra
Larger bodies are larger targets for “colonization” by parasites (including parasitic herbivores); and they constitute larger (more heterogeneous) “habitat islands” supplying more resources that can sustain a higher diversity of herbivores/parasites [42, 120, 105, 106, 108, 118, 140, 141]. Large organisms may also be structurally more complex, thus providing a greater range of resources/microhabitats for herbivores/parasites [42, 43, 108]. In addition, bigger habitat (body) spaces allow a wider range of parasites with varying body sizes to survive there (as shown in Figure 2b).
However, body size is associated with many kinds of intrinsic/extrinsic factors that make it difficult to distinguish the relative effects of specific mechanisms on size-parasitism spectra. For example, larger hosts are longer-lived, thus providing more time for parasites to colonize them [99, 108, 109, 142–144]. Studies are needed that separate out the effects of host age and size. When this has been done, either host body size or longevity (or neither) has been shown to be more positively related to parasite species richness per host [99, 103, 143–146]. In addition, larger animal hosts tend to have larger home ranges [133], which could increase encounters with parasites/pathogens (but see [147]). However, conversely, smaller hosts tend to live at higher population densities, thus increasing “contagion” or the probability of transmission of parasites/pathogens between hosts [116, 146, 148]. Multiple influences that vary in different, sometimes opposing ways, with host body size (e.g., target size, habitat space/heterogeneity, local population density, home/geographical range size, etc.) may help explain why relationships between parasite species richness and host body size may not be as strong as those shown by size-predation spectra (compare Figure 3a and 3b with Figure 4a and 4b; also see [146, 148, 149]). Variation in sampling effort may also obscure positive relationships between parasite species richness and host body size [101].
4. Possible consequences of species interactions for interspecific biological scaling relationships
In this section, I argue that the size-predation/parasitism spectra proposed, documented, and explained in Section 3 may have been important drivers of the body-mass scaling of many biological and ecological traits. In doing so, I assume that these spectra are not only generally applicable across the living world, but also usefully represent body-size-related variation in the intensity of predation and parasitism on prey/host species, both of which remain to be validated by analyses of more data sets in a greater variety of taxa and habitats. Some support for the latter assumption comes from studies documenting negative interspecific relationships between predation intensity and prey body mass [94, 68, 150–154] and positive relationships between parasite biomass or intensity (number per host) and host body mass [155–158]. However, a recent energetic study suggests that eukaryotic parasites may extract a larger fraction of a host’s energy budget in small versus large animal hosts [159]. In this section, I also speculate about the potential importance of other kinds of biotic interactions for biological scaling relationships, which I hope will stimulate further research.
4.1. Size-predation/parasitism spectra and the body-mass scaling of physiological, life-history, and demographic traits
Both size-predation and size-parasitism spectra may influence or be related to the body-size scaling of several kinds of physiological, life-history, and demographic traits, but in different ways. Different life-history scaling patterns may arise because (1) predator species richness tends to decrease with prey body size (Section 3.2.1), whereas parasite species richness tends to increase with host body size (Section 3.2.2) and (2) predators kill (abolish the fitness of) their prey, whereas parasites tend to harm (reduce the fitness of) their hosts without necessarily killing them [31]. As a result, one can reasonably infer that small organisms highly vulnerable to predation, but little attacked by parasites, should be favored by natural selection to have relatively rapid rates of growth and reproduction and to prioritize reproduction over survival, whereas large organisms highly vulnerable to parasitism, but well-protected against predators, should be favored to prioritize survival over reproduction, which entails increases in longevity, developmental time periods, anti-parasite defenses, and other protective somatic investments, and by association a reduction in the overall pace of life (Figure 6). In the next two sections, I provide specific hypothetical explanations for how size-predation and size-parasitism spectra may contribute to the body-size scaling of the “pace of death” (rates of mortality), the “pace of life” (including rates of growth and reproduction at the individual and population levels), and the relative allocation of resources to reproduction versus survival, all of which require further testing.
Potential effects of size-predation spectra
Smaller organisms are usually more vulnerable to predation than larger organisms (Section 3.2.1), a common pattern that appears to have contributed to the negative body-size scaling of mortality rate (the “pace of death”) observed in many kinds of organisms [50, 57, 59, 125–127, 160]. This is not surprising because predation is a major cause of mortality in most organisms, especially small ones (also see Section 5.4). The reduced vulnerability of large organisms to mortality (including predation) can be related to their greater physical protection, enhanced homeostatic regulatory systems, larger volume-to-surface-area ratios that distances much of the interior body from external environmental hazards, and other factors discussed in Section 3.3.1 (Figure 6; also see [21, 129, 161–163]).
Figure 6
Schematic comparison showing that small organisms (left) tend to have many predators, whereas large organisms (right) tend to have many parasites. In addition, large organisms tend to be more physically protected (including more developed body coverings) and often have more complex biochemical, physiological, and behavioral defenses against predators, parasites, and other enemies. Large surface area to volume (SA/V) ratios permit small organisms to have high resource uptakes, whereas small SA/V ratios in large organisms allow much of the interior body to be remote from environmental hazards. These collective differences contribute to the short, rapid lives of small organisms, which prioritize reproduction over survival, versus the long, slow lives of larger organisms, which prioritize survival over reproduction.

As a result of their higher mortality rates from predation and other causes, it seems reasonable to infer that relatively small organisms should have been favored by natural selection to evolve a relatively rapid “pace of life” to ensure that they mature and reproduce before they die (are eaten) in a relatively short period of time. In support, rates of growth and reproduction and supporting metabolism tend to scale negatively with increasing body size in a variety of organisms [1–4, 21, 50]. The maximal intrinsic rate of increase (rmax) of populations shows similar negative scaling with increasing body size [1, 2, 4, 50]. Furthermore, high rates of mortality (predation) tend to decrease populations of small organisms below the carrying capacity (K) of their environment, thus increasing resource availability per individual that can support rapid rates of growth and reproduction. The relatively small SA/V ratios of smaller organisms may also facilitate rapid resource uptake needed for sustaining a rapid pace of life (Figure 6). By contrast, low rates of mortality (predation) tend to cause populations of many large organisms to be near their K, thus increasing intraspecific competition for resources and thereby inhibiting rates of growth and reproduction (also see [21, 164]). In addition, life-history theory predicts that high mortality rates should favor increased prioritization of reproduction (fitness of the next generation) over survival (fitness of the current generation), as observed in smaller organisms (Figure 6; also see [51, 162, 164, 165]).
Evidence that increased protection from mortality by predation and other environmental hazards favors longer, slower paced lives is shown by such associations occurring in species with well-developed body coverings (e.g., armor, spines, shells) [166] or other protective adaptations (e.g., flight, arboreal lifestyle, or hibernation in refuges) [167–170] independently of their body size. In addition, on islands where predation is often absent or reduced, and thus intraspecific competition for food is relatively intense, many kinds of species have evolved increased longevity and slower rates of growth and reproduction (e.g., [171–185]). This so-called “island syndrome” [173, 177, 184–186] may also be associated with reduced rates of metabolism [187]. Conversely, species that are subject to high predation or occur in ephemeral habitats where life expectancy is short have relatively rapid rates of growth, maturation, and reproduction [162, 188–191].
Evidence has also been accumulating that increasing body size is associated with a shift in population regulation from being primarily predator (top-down) controlled to primarily resource (bottom-up) controlled. Studies of African mammal communities have revealed this pattern especially well [94, 68, 150, 152, 154]. The proportion of mortality that is due to predation decreases with increasing size in African ungulate mammals, which results in populations of the largest species being primarily food-limited [150, 154]. This is why megaherbivores tend to have more impacts on vegetation than smaller herbivores [150, 154, 192–194]. Carroll [152, p. 146] regarded this body-size-related shift in the mode of population regulation as being so important for governing how ecosystems work that he exalted it as one of his six “Serengeti Rules”, namely “Animal body size is an important determinant of the mechanism of population regulation in food webs, with smaller animals regulated by predators (top-down regulation) and larger animals by food supply (bottom-up regulation)”. Studies of other animals have corroborated this rule. For example, large odontocete whales (e.g., killer whales, Orcinus orca) are relatively immune from predation and thus their populations appear to be primarily food-limited [195, 196]. In addition, larger (adult) copepods suffer less predation and are thus more food limited than smaller (juvenile) copepods [197–199]. The experimental work of Reznick and his colleagues [200] has also shown that the absence of fish predation causes guppies (Poecilia reticulata) to have more dense populations with more intense competition for food. The “island syndrome” also supports this pattern [173, 177, 181, 182, 185, 186].
This Serengeti Rule also has major consequences for the body-size scaling of life-history traits and the overall pace of life. Larger African ungulates, odontocete whales, and marine copepods all exhibit longer, slower lives than smaller related species [196, 201, 202]. Guppies subject to density-dependent population regulation in the absence of fish predators also exhibit slowed somatic growth and delayed maturity [200, 203]. This rule may even help to explain why small animals highly vulnerable to predation tend to have faster mass-specific metabolic rates than larger, more protected animals, because higher metabolic rates are needed to support more rapid rates of growth and reproduction in smaller animals. The rapid “pace of death” in small organisms favors a rapid “pace of life” by natural selection [11, 14, 15, 204–207].
Potential effects of size-parasitism spectra
A primary effect of size-parasitism spectra on biological scaling likely involves how anti-parasite defenses scale with body size across species. Since large hosts tend to have more parasite species than smaller hosts (Section 3.2.2), it is reasonable to infer that larger host species should have evolved more powerful, expansive defense systems that can thwart a greater diversity of parasites. Plants chiefly use chemical toxins against herbivorous parasites (as well as innate immune responses against pathogens and other kinds of parasites [208, 209]), whereas animals chiefly use a variety of immunological responses.
Investment in broad-spectrum chemical defenses (e.g., tannins) increases from herbs to shrubs to trees [210], as may be expected from their increasing body size and parasitic herbivore species richness (Figure 4; and as predicted by the “plant apparency” hypothesis [211]). During ontogeny, older, larger plants often (but not always) invest more in physical, chemical, and/or mutualistic defenses [212–215], but these patterns depend on taxon, life-history strategy, defense type, and age- or size-selective herbivory and parasitism [213–216]. It is also possible that older, larger hosts may have more parasites because of weakened defenses associated with aging. More research is required to understand how plant defenses vary across plant species of different sizes in response to size-dependent herbivory and parasitism.
Animals and plants have evolved immune defenses against parasites and pathogens (reviewed in [43, 217]). Since larger animals tend to have more parasite species (Section 3.2.2) and they have evolved to place a greater priority on survival over reproduction [162, 164, 165], it is expected that anti-parasite (pathogen) defenses should also have a higher priority for them compared to smaller animals. Pioneering work by Downs and her colleagues [147] appears to support this prediction. Larger birds and mammals appear to show greater immune competence and more powerful antibacterial defenses than smaller species [147, 218–220]. However, more research is needed in a variety of taxa to test the generality of these patterns (e.g., reptiles show isometric scaling of immune cell concentrations [221], in contrast to the hypermetric scaling exhibited by birds and mammals). In any case, it is noteworthy that, although many kinds of animals and plants have “innate immune systems”, only vertebrate animals, which are at the large end of the size spectrum for animals, have additional, more complex “adaptive immune systems” that expand their range of defenses against parasites and pathogens [222].
Since anti-parasite/herbivore defenses have energy costs that may entail reduction in resource investment to other vital processes such as growth and reproduction (e.g., [217, 223–229]), it is also reasonable to infer that they may have contributed to the lower rates of growth and reproduction observed in larger, more defended plants and animals, a hypothesis requiring testing. Applications of the growing field of ecological immunology [147, 223, 230, 231] to the study of biological scaling could be very rewarding.
4.2. Size-predation/parasitism spectra and body-size scaling of ecological properties at the population and ecosystem levels
In this section, I preview possible applications of size-predation and size-parasitism spectra to understanding effects of body size on populations, communities, and ecosystems, a topic that I shall cover in more detail elsewhere. At the population and community levels, recent studies have shown that predation can significantly alter size-abundance or size-population-biomass relationships [232–238]. At the ecosystem level, production/biomass ratios and efficiency of energy transfer between trophic levels may also depend importantly on body size and associated resistance to predation [160, 239]. Effects of predator body size and prey body size relative to predator size on “trophic cascades” (including positive effects of predators on the abundance of food organisms of their prey) have been examined (e.g., [240, 241]). As prey size decreases relative to predator size, and thus the vulnerability of prey to predation increases (following the size-predation spectrum: Section 3.2.1), the strength of trophic cascade effects should increase [193, 242, 243], but this has not always been observed (see references cited in [240]). Although yet unexplored, host-size-related effects of parasitism on organismal and population production rates may influence body-size-related flow of energy through ecosystems, as well.
4.3. Size spectra for other kinds of species (biotic) interactions, and their possible effects on biological scaling relationships
Little is known about how biotic interactions involving competition, mutualism, commensalism, and amensalism relate to the body sizes of the species involved, but they may reveal additional insights into the structure/function of ecological communities and the mechanisms underlying various biological and ecological scaling relationships. Here I speculate about how each of these types of biotic interactions may scale with body size, and their potential significance, with the hope of stimulating further research.
Size-competition spectra
Interspecific competition involves negative effects on the fitness of each species exploiting shared resources. Since competition is reciprocal, the diversity of competitors should scale zerometrically (scaling slope of 0) with the body size of each species involved. Furthermore, if competition is symmetric (i.e., each species has an equal effect on the other), I predict that size-competition spectra should have no significant effect on biological scaling patterns. However, if competition is asymmetric in a size-related way (i.e., competitive dominance relates to body size), then size-competition spectra may impact biological scaling patterns. For example, if larger competitors tend to dominate smaller competitors, as is commonly observed (e.g., [244, 245]), then size-competition spectra could reinforce the effects of size-predation spectra, thus causing greater negative effects (including increased mortality) on smaller species. Alternatively, if predation removes larger dominant competitors, smaller species may benefit (e.g., [244]).
Different size-competition spectra may also occur for inter- versus intraspecific competition. For example, the Serengeti Rule described in Section 4.1.1 predicts that intraspecific competition should increase with increasing body size, which could result in more severe resource limitation favoring slower rates of energy use and overall pace of life in larger organisms.
Size-mutualism spectra
Mutualism involves positive effects on the fitness of each interacting species. A plausible size-mutualism spectrum may involve an increased number of mutualistic species (beneficial symbionts) for species partners having larger body sizes, thus paralleling size-parasitism spectra. This would make sense because more kinds of symbionts should associate with larger partners supplying more beneficial resources and “habitat” space, as is the case for parasites (harmful symbionts). In addition, symbiont species richness should scale positively with partner body size because of the fitness advantage of developing a mutualism with larger, more long-lived, more protected species. If so, size-mutualism spectra may contribute to the positive scaling of survival/longevity with body mass. The elevation of a size-longevity relationship should also increase because mutualism should benefit partners of all sizes. Data are needed to test these plausible hypotheses. Interestingly, McCaffrey et al. [246] have shown that symbiont species richness (including mutualistic, commensal, and parasitic species) positively correlates with snail host size.
Size-commensalism spectra
In a binary commensalistic relationship, one species benefits, whereas the other is unaffected. Like size-parasitism and size-mutualism spectra (and for similar reasons), a plausible size-commensalism spectrum may involve an increased number of commensal species associated with larger host species. For example, it seems reasonable to assume that larger plants (e.g., trees) supply more kinds of refuges for various kinds of commensal species than do smaller plants (e.g., herbs). Large old trees are known to harbor a large proportion of the animals and epiphytic plants living in a forest [247, 248]. In addition, large burrows made by large animals should supply more space for refuge-seeking commensal species of varying sizes than smaller burrows made by smaller animals. Tokeshi [249] has hypothesized that the species richness of commensal nematodes should positively relate to host size because larger hosts are more protected against predation. Species practicing phoresy (using other organisms for transportation) should also prefer larger, more mobile hosts. These hypothesized patterns should be readily amenable to testing. Commensalism may increase the fitness (including reducing the mortality) of many small species living on large hosts or in their habitations that would otherwise be highly vulnerable to predation. Indeed, arboreal mammals tend to lead longer, slower lives compared to other terrestrial mammals [168, 169].
Size-amensalism spectra
In a binary amensalistic relationship, one species is negatively affected, whereas the other is unaffected [250]. One might hypothesize that, like size-predation spectra, small organisms are affected by more kinds of amensalistic organisms than larger organisms. For example, small terrestrial animals may be trampled by a wider range of animals of varying sizes than larger animals. If so, size-amensalism spectra may reinforce the effects of size-predation spectra on the body-mass scaling of longevity, rates of growth and reproduction, and other life-history traits (see Section 4.1.1).
5. Theoretical and practical implications of recognizing the size-scaling of species interactions
5.1. Theoretical implications of size spectra for species interactions
Knowledge of size-predation and size-parasitism spectra (as well as other size spectra concerning other species interactions) may aid the development of theory explaining biological scaling relationships, the evolution of life histories, variation in the structure/function of ecological communities along environmental gradients, and other biological and ecological patterns.
Biological scaling theory
A major take-home message of this review is that a comprehensive theory explaining various biological scaling relationships requires that they be viewed in a proper ecological context. Traditionally, internal geometric and physical constraints have been emphasized. However, the relevant scope of influence has expanded to include phenotypically plastic effects of biological regulation (e.g., [11, 15, 53]) and evolutionary genotypic effects of life-history optimization (e.g., [14, 18, 22, 24, 26, 53]). Concurrently, there has been an increasing appreciation that biological scaling relationships depend not just on intrinsic physical and biological properties of organisms—a “skin-in view”, but also on extrinsic factors in their natural environments—a “skin-out view” (terminology of Bates [44]; also see [14, 15, 19, 20, 22]). Increasing/decreasing body size has not only internal geometric, physical, and biological consequences that are relatively easily measured and thus given much attention, but also external ecological repercussions that are equally fundamental, but more difficult to measure and thus insufficiently appreciated. Indeed, the sensitivity (vulnerability) of organisms to biotic and other environmental hazards depends largely on their body size. As emphasized in this review, evolving smaller/larger body size significantly alters the ecological network in which an organism/species is embedded, and ultimately its prospects for survival and reproductive success. Accordingly, the more rapid pace of life shown by small versus large organisms is best understood when both intrinsic and extrinsic factors are considered acting together.
For example, small organisms tend to have relatively large SA/V ratios that favor a rapid pace of life, for both intrinsic and extrinsic reasons. Intrinsically, a large SA/V ratio facilitates rapid resource uptake per metabolically demanding body volume or mass that in turn permits a rapid pace of life. Extrinsically, a large SA/V ratio contributes to making small organisms highly vulnerable to environmental hazards because much of their interior body is near their external environment. As a result (in part), small organisms have a high mortality rate that favors (by natural selection) rapid rates of growth, maturation, and reproduction. Small physical size not only facilitates internal resource transport by involving shorter travel distances, but also makes an organism more vulnerable to being eaten by a wider range of predators, both of which would favor a more rapid pace of life. In addition, higher mortality rates in small organisms favor a more rapid pace of life not only evolutionarily via natural selection but also ecologically via making more environmental resources available per individual because of decimation of populations below their carrying capacity.
By contrast, large organisms are less vulnerable to environmental hazards because they have fewer predators that can eat them; they are often protected by various kinds of resistant body coverings (e.g., bark, spines, shells, scales, fur, feathers, and other exoskeletal structures); they have sophisticated homeostatic regulatory systems; and their large V relative to SA means that much of their interior body is relatively remote from the harmful influences of their external environment. The lower mortality rate of larger organisms also results in their populations being more often near their environmental carrying capacity, thus usually causing more intense intraspecific competition and lower resource supply per individual than that of smaller organisms. All these effects are expected to be associated with increased longevity but slower rates of growth, maturation, and reproduction. The interplay between intrinsic and extrinsic factors in causing slower rates of living in larger and/or more long-lived organisms may have included the evolution of enhanced anti-predator/herbivore body coverings that reduced body surface area for oxygen uptake [129], thus causing slower rates of energy use. Larger, more long-lived plants and animals are also bigger and more long-lasting physical targets for the colonization of herbivores, parasites, and/or pathogens, thus causing them often to be afflicted by more of these harmful species than smaller, more short-lived organisms. Since herbivores/parasites/pathogens often harm, but do not kill their prey/hosts, large species most afflicted by them have evolved elaborate defense systems, including protective toxic chemicals and immunological defenses that contribute to their longevity, but at a cost to their reproduction. The non-discrete dichotomy between the lethal effects of predators and often nonlethal effects of herbivores/parasites/pathogens helps further explain why small organisms that are highly vulnerable to predators tend to have shorter lives and more rapid paces of life than larger organisms attacked by a more diverse range of herbivores/parasites/pathogens (Figure 6).
Structure and function of ecological communities in space and time
Predation is a powerful force structuring communities [185, 251–253], and effects of parasitism on community structure are also gaining recognition [254–258]. Therefore, the size-predation and size-parasitism spectra documented in my review should be considered when attempting to understand variation in the structure/function of ecological communities in both space and time. As heuristic hypothetical examples, I discuss how knowledge of these two size spectra allows one to generate testable predictions about how the frequency/intensity of predator-prey and parasite-host interactions in ecological communities should vary with (1) trophic level, (2) latitude (environmental temperature), and (3) ecological (successional) or (4) geological (evolutionary) time.
Since the mean body size of organisms often tends to increase with increasing trophic level in many aquatic communities [29, 30, 121, 259–262], knowledge of size-predation and size-parasitism spectra enables one to predict that the number of predator species attacking an organism should decrease with increasing trophic level, whereas the number of parasites infecting an organism should increase. As predicted, these patterns have been observed in two species-rich marine food webs for which the required data were available (see Section 3.2.3).
Since body size tends to increase with increasing latitude or decreasing habitat temperature in diverse groups of organisms (called Bergmann’s Rule or the temperature-size rule; see e.g., [263–273]), one may predict that the frequency/intensity of predator-prey interactions should decrease with increasing latitude, whereas the frequency/intensity of parasite-host interactions should increase. As predicted, a recent study has shown just that in terrestrial ecosystems: the intensity of predation decreases from the equator to the poles, whereas the intensity of parasitism increases [274]. Kamiya et al. [109] have also shown that parasite species richness increases significantly toward the poles for metazoan hosts, though non-significantly for other kinds of hosts. A similar poleward increase in parasite species richness was found for mammalian carnivore hosts [275]. In addition, Ashton et al. [276] have shown that predator control in marine communities increases with temperature (as expected from a combination of the temperature-size rule [266] and the size-predation spectra documented in Section 3.2.1). Although these opposing latitudinal trends in predation and parasitism intensity may not be entirely body-size dependent (see e.g., [119, 277, 278]), I recommend that future studies of these trends should, at the very least, control for possible body-size effects.
Since the body size of plants tends to increase during ecological succession in forest biomes, one can predict that the diversity of herbivores, parasites, and other symbionts living on them should also increase. These successional trends are likely, given that the species richness of herbivorous insects and parasitic fungi on host plants tends to increase from herbs to shrubs to trees (Figure 4c; [42, 104, 105, 106]), but it remains to be rigorously tested in specific successional communities.
Since body size has often increased in many evolutionary lineages over geological time (called Cope’s Rule [132, 135]) and maximum body size has increased since the origin of life [139, 279], one may predict that ecological communities should have shown expanded and possibly otherwise altered size-predation and size-parasitism spectra over time with potentially significant effects on their structure/function and the body-size scaling of various life-history and demographic traits. The appearance of larger predators may have exerted more predation on smaller organisms by expanding the range in size of predators feeding on them, thus increasing their mortality rates and consequent selection for a faster pace of life, whereas the appearance of larger structural organisms (e.g., trees) may have provided more refuges for smaller organisms to use to avoid predation, thus decreasing their mortality rates and associated pace of life [21]. The evolution of larger herbivores largely immune from predation, and thus subject to high intraspecific competition and food limitation (following the Serengeti Rule described in Section 4.1.1), may have exerted more negative impacts on vegetation and associated coexisting species. Protection from predation may have thus enabled megaherbivores to become major “ecosystem engineers” [192–194]. The evolution of larger organisms should also have expanded the diversity of parasites and other symbionts living on them. All these hypothetical patterns are plausible when viewed in the light of size-predation and size-parasitism spectra, but they require testing with empirical data.
Toward a mortality theory of ecology
The rates of various biological and ecological processes and how they scale with body size are commonly believed to be driven by rates of metabolism and energy use (e.g. [50, 206, 280]; and references therein). This is understandable because all biological processes are energized by metabolism, and their rates often scale with body size with similar slopes as metabolic rates [1, 4, 8, 50]. Indeed, the “metabolic theory of ecology” (MTE) proposes that effects of body size and temperature on metabolic rates can explain much of the variation of the rates and durations of diverse biological and ecological processes seen across the living world [4, 50]. This highly influential theory has provided some useful insights into the structure/function of ecological communities and various patterns of biological scaling. However, the general applicability of several assumptions and predictions of the MTE have been questioned, including that (1) the geometry and physics of resource-transport networks explains metabolic scaling via body-size-related limits on resource supply (this assumption only applies to a small proportion of living species that have closed vascular networks [11, 281], and furthermore, both resource supply and demand—not just resource supply—appear to be involved in causing metabolic scaling [11, 14, 15, 282–284]), (2) the scaling slope for metabolic rate should be 3/4 or nearly so (there are hundreds of exceptions to this prediction [15, 19, 20, 282, 283]), (3) metabolic rate is a universal “pacemaker” for other biological and ecological processes (reverse causation can occur, and metabolic rate can be dissociated from the rates of growth, development and other biological processes [21, 206]), and (4) the body-size scaling of various biological processes should parallel that for metabolic rate (many examples of discordant allometry exist: see references cited in [21, 206]).
Given the limitations of the MTE, and the fact that variation in life-history traits appears to be more often related to mortality rate than metabolic rate (see references cited in [21]), I have recently proposed that a new, yet to be fully developed “mortality theory of ecology” (MorTE) should be considered as a possible alternative or complementary approach to understanding many biological scaling patterns (especially for the rates and durations of various biological processes) [21]. According to the MorTE, mortality-imposed time limits drive the body-size scaling of the rates of growth, maturation, and reproduction, and overall, the pace of life [21]. All organisms have an “expiration date”, and therefore they must complete fitness-enhancing growth and reproduction before that date. The penalty for not doing do so is failure to pass on one’s genes to the next generation, and thus a lethal loss of evolutionary fitness. Shorter time periods until expiration (death), as found in smaller, more vulnerable organisms, require quicker rates of living, as dictated by natural selection.
My review of the body-size scaling of various species interactions supports the MortE. Size-predation spectra, in particular, help explain why rates of mortality and living scale negatively with body size (see Sections 3.1, 3.2.1, and 3.3.1). This is because predation is a major cause of death in many organisms [29, 125, 160, 277, 285–287], especially small organisms (e.g., prokaryotes [71], zooplankton [126], insects [277], fish larvae [57, 58, 78], and small mammals [150, 151, 154]).
The MorTE deals with not only death but also resistance to death. Two fundamental ways to perpetuate life, despite inescapable death, include (1) produce new life to replace oneself and (2) sustain oneself as long as possible before death. According to the MorTE, these strategies shift in emphasis from 1 to 2 with growing body size [21]. Therefore, larger organisms, which are attacked by more kinds of parasites than smaller organisms, have evolved sophisticated anti-parasite defenses that ensure long life, but at the expense of slower rates of growth and reproduction (also see Section 4.1.2). Therefore, size-parasitism spectra can also help explain biological scaling patterns within the framework of the MorTE. Although parasites often do not kill their hosts immediately, they can afflict them in ways that can make them more susceptible to death by other killing factors, such as predators and stressful abiotic hazards [45, 288, 289]. Simply put, much death (and resistance to death) is due to being eaten from the outside or inside.
In short, studies of the body-size scaling of species interactions help shift our perspective on biological scaling theory from focusing only on internal body design to also including external environmental factors, such as other interacting species, a neglected topic. The development of an ecological theory of allometry that dovetails in important ways with a MortE is much needed. Measuring mortality in nature, including biotic interactions affecting mortality and other demographic/life-history traits, is difficult but could yield great rewards in advancing our understanding of how ecological communities work and why the pace of life scales so strongly with organismal size.
5.2. Practical applications of size spectra for species interactions
Knowledge of size spectra for species interactions may have important applications for understanding effects of climate change, nutrient enrichment, and size-selective exploitation on ecological systems. For example, global warming is causing shifts in body-size distributions toward smaller sizes in ecological communities [290–293] that may have corollary effects on the species interactions occurring in them. Smaller organisms in warmed environments should not only have altered rates of metabolism, growth, and reproduction, but also experience changes in the number/abundance of predators or parasites exploiting them, as expected from the size spectra for predation and parasitism documented in this review (see Section 3). Such spectra may even apply within species (see e.g., [155, 294–299]) and thus may be pertinent for intraspecific changes in body size caused by temperature changes. Furthermore, temperature-caused shifts in body size may change prey/host preferences by size-selective predators, herbivores, and parasites [60–64, 200, 214, 215, 300–303]. These body-size-related changes in species interactions should be considered in addition to direct effects of temperature on rates of species interactions [50, 303–306] and changes resulting from temperature-related shifts in the latitudinal distributions of predators, parasites, pathogens, and disease vectors [306–310]. In short, effects of climate change cannot be completely understood only with a perspective based on physiological energetics [311] but should also include the ecological context (also see [54, 55, 312]).
Nutrient enrichment and size-selective exploitation by humans may also cause changes in the body-size distributions of ecological communities [252, 313] that could have corollary effects on various species interactions. Increasing/decreasing body sizes associated with human-caused environmental changes should be associated with increases/decreases in parasite species richness but decreases/increases in predator species richness. These predictions are yet to be tested.
6. Conclusion
Examining the body-size scaling of species (biotic) interactions shows much potential for greatly improving our understanding of the structure/function of ecological communities, the mechanisms underlying biological scaling relationships, the evolution of life histories, and the effects of climate change and other human effects on ecosystems. I have focused on the causes and consequences of size spectra for predation and parasitism because considerable pertinent data are available, but size spectra for other biotic interactions should also be explored.
The ecological perspective promoted here represents an extension of recently advocated Darwinian and ecological approaches to explaining biological (metabolic) scaling [11, 14, 15, 18–20, 22, 24, 26, 53], by placing ecological interactions between species at the “high table” of general theory. Darwin [314] regarded species interactions as fundamentally important in evolution, a view that has been promoted by many biologists since then, as embodied in the terms/concepts of “coevolution”, “escalation”, “biological arms races”, the “Red Queen” hypothesis, and more generally the “evolutionary importance of the biotic environment” (e.g., [49, 315–321]). As evolutionary theory shifted from emphasizing physical deterministic causes to ecological contingent causes [322], so has the theory of biological (metabolic) scaling in a way that is currently unfolding [14–20, 22, 24]. Similarly, Terborgh and Estes [253] have discussed how the study of the structure/function of ecological communities has shifted from emphasizing physical to biotic effects and processes.
Naturally, early interpretations of biological scaling relationships focused on easily measured internal factors related to body shape, anatomy, and function. However, a major objective of my review has been to emphasize that growing bigger or smaller entails not only vital physical somatic changes, but also equally important ecological changes involving how a species relates to its abiotic and biotic environments that are much harder to see and quantify. Both kinds of changes—skin in and skin out—are fundamentally important regardless of their ease/difficulty of measurement. Ecological networks of species interactions matter just as much or perhaps even more than internal somatic networks of resource distribution in explaining biological scaling relationships. Biological scaling is not just about “engineering” or “ecology”, but interactions between both (as shown in Figure 6). Bridging the fields of community ecology and biological scaling could be a boon for the development of new synthetic theory about how nature works and has evolved.
In conclusion, a comprehensive understanding of the tempo, functioning, and evolution of life requires knowledge of not only the processes sustaining it (e.g., effective acquisition and use of energy and other resources, the major focus of the influential “metabolic theory of ecology” [50]), but also the processes terminating it (e.g., injury, aging, and various environmental hazards, the major focus of a newly proposed “mortality theory of ecology” [21]). Limits on both energy and time govern the pace of life and its scaling with body size, with important implications for understanding the structure/function of ecological communities and their responses to environmental change.

