Efectos de un extracto de hojas de Leucaena leucocephala en la actividad de xantina oxidasa y en los niveles séricos de oxipurinas en ratones
DOI:
https://doi.org/10.18633/biotecnia.v26.2155Palabras clave:
alopurinol, teoría de Chou-Talalay, sinergismo, hipoxantinaResumen
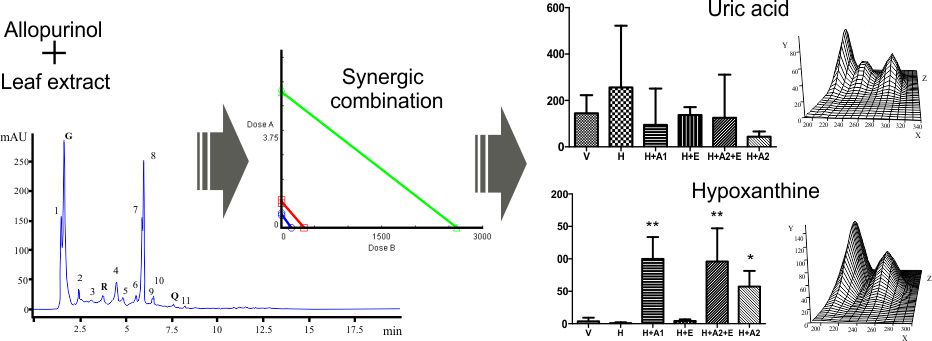
Hay una necesidad de nuevas alternativas al uso medicinal de alopurinol. En este sentido, el presente estudio obtuvo un extracto de hojas de L. leucocephala al cual se le determinó su composición química, acción inhibitoria contra xantina oxidasa (XO) in vitro, interacción inhibitoria entre el extracto y alopurinol, y acción inhibitoria contra XO in vivo empleando ratones bajo administración de hipoxantina y oxonato de potasio. Compuestos polifenoles y flavonoides fueron detectados y cuantificados en el extracto de hojas. Para el extracto solo, su valor IC50 y máximo de inhibición contra XO fueron de 334.60 µg/mL y 46.4 %. La combinación a una proporción 3:1 de alopurinol y extracto obtuvo valores IC50 y waDRI de 1.35 µg/mL, 1.13 (alopurinol) y 1015.72 (extracto) para inhibir a XO, resultando en una interacción sinérgica contra XO in vitro. Donde esta misma combinación incrementó el éxito terapéutico en el modelo murino al compararse con la administración de alopurinol solo. Nuestro estudio presenta la primera evidencia para el uso de una combinación de alopurinol y extracto de L. leucocephala a una proporción 3:1 como un sustituto para la administración de alopurinol solo.
Descargas
Citas
Al Shoyaib, A., Archie, S. R. and Karamyan, V. T. 2019. Intraperitoneal Route of Drug Administration: Should it Be Used in Experimental Animal Studies? Pharmaceutical Research. 37: 12. https://doi.org/10.1007/s11095-019-2745-x DOI: https://doi.org/10.1007/s11095-019-2745-x
Aragon-Martinez, O. H., Galicia, O., Isiordia-Espinoza, M. A. and Martinez-Morales, F. 2014. A novel method for measuring the ATP-related compounds in human erythrocytes. The Tohoku Journal of Experimental Medicine. 233: 205-214. https://doi.org/10.1620/tjem.233.205 DOI: https://doi.org/10.1620/tjem.233.205
Barthelmebs, L., Divies, C. and Cavin, J. F. 2000. Knockout of the p-coumarate decarboxylase gene from Lactobacillus plantarum reveals the existence of two other inducible enzymatic activities involved in phenolic acid metabolism. Applied and Environmental Microbiology. 66: 3368-3375. https://doi.org/10.1128/AEM.66.8.3368-3375.2000 DOI: https://doi.org/10.1128/AEM.66.8.3368-3375.2000
Bobulescu, I.A. and Moe, O.W. 2012. Renal transport of uric acid: evolving concepts and uncertainties. Advances in Chronic Kidney Disease. 19: 358-371. https://doi.org/10.1053/j.ackd.2012.07.009 DOI: https://doi.org/10.1053/j.ackd.2012.07.009
Cefali, L. C., Ataide, J. A., Fernandes, A. R., Sanchez-Lopez, E., Sousa, I. M. O., Figueiredo, M. C., Ruiz, A. L. T. G., Foglio, M. A., Mazzola, P. G. and Souto, E. B. 2019. Evaluation of in vitro solar protection factor (SPF), antioxidant activity, and cell viability of mixed vegetable extracts from Dirmophandra mollis Benth, Ginkgo biloba L., Ruta graveolens L., and Vitis vinífera L. Plants (Basel). 8: 453. https://doi.org/10.3390/plants8110453 DOI: https://doi.org/10.3390/plants8110453
Checkmahomed, L., Padey, B., Pizzorno, A., Terrier, O., Rosa-Calatrava, M., Abed, Y., Baz, M. and Boivin, G. 2020. In Vitro Combinations of Baloxavir Acid and Other Inhibitors against Seasonal In-fluenza A Viruses. Viruses. 12: 1139. https://doi.org/10.3390/v12101139 DOI: https://doi.org/10.3390/v12101139
Chen, Y., Li, C., Duan, S., Yuan, X., Liang, J. and Hou, S. 2019. Curcumin attenuates potassium oxon-ate-induced hyperuricemia and kidney inflammation in mice. Biomedicine & Pharmacotherapy. 118: 109195. https://doi.org/10.1016/j.biopha.2019.109195 DOI: https://doi.org/10.1016/j.biopha.2019.109195
Chew, Y. L., Chan, E. W., Tan, P. L., Lim, Y. Y., Stanslas, J. and Goh, J. K. 2011. Assessment of phy-tochemical content, polyphenolic composition, antioxidant and antibacterial activities of Leguminosae medicinal plants in Peninsular Malaysia. BMC Complementary Medicine and Therapies. 11: 12. https://doi.org/10.1186/1472-6882-11-12 DOI: https://doi.org/10.1186/1472-6882-11-12
Chou, T. C. 2010. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Research. 70: 440-446. https://doi.org/10.1158/0008-5472.CAN-09-1947 DOI: https://doi.org/10.1158/0008-5472.CAN-09-1947
Chou, T. C. 2006. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacological Reviews. 58: 621-681. https://doi.org/10.1124/pr.58.3.10 DOI: https://doi.org/10.1124/pr.58.3.10
Chung, H. H., Chen, M. K., Chang, Y. C., Yang, S. F., Lin, C. C. and Lin, C. W. 2017. Inhibitory effects of Leucaena leucocephala on the metastasis and invasion of human oral cancer cells. Environmental Toxicology. 32: 1765-1774. https://doi.org/10.1002/tox.22399 DOI: https://doi.org/10.1002/tox.22399
Córdova-Guerrero, I., Aragon-Martinez, O. H., Díaz-Rubio, L., Franco-Cabrera, S., Serafín-Higuera, N. A., Pozos-Guillén, A., Soto-Castro, T. A., Martinez-Morales, F. and Isiordia-Espinoza, M. 2016. Antibacterial and antifungal activity of Salvia apiana against clinically important microorganisms. Revista Argentina de Microbiología. 48: 217-221. https://doi.org/10.1016/j.ram.2016.05.007 DOI: https://doi.org/10.1016/j.ram.2016.05.007
Cui, H., Zhang, X., Zhou, H., Zhao, C. and Lin, L. 2015. Antimicrobial activity and mechanisms of Salvia sclarea essential oil. Botanical Studies. 56: 16. https://doi.org/10.1186/s40529-015-0096-4 DOI: https://doi.org/10.1186/s40529-015-0096-4
Diehl, K. H., Hull, R., Morton, D., Pfister, R., Rabemampianina, Y., Smith, D., Vidal, J. M., van de Vorstenbosch, C. and European Federation of Pharmaceutical Industries Association and European Centre for the Validation of Alternative Methods. 2001. A good practice guide to the administration of substances and removal of blood, including routes and volumes. Journal of Applied Toxicology. 21: 15-23. https://doi.org/10.1002/jat.727 DOI: https://doi.org/10.1002/jat.727
Farasat, M., Khavari-Nejad, R. A., Nabavi, S. M. and Namjooyan, F. 2014. Antioxidant Activity, Total Phenolics and Flavonoid Contents of some Edible Green Seaweeds from Northern Coasts of the Persian Gulf. Iranian Journal of Pharmaceutical Research. 13: 163-170.
Galano, A., Francisco-Márquez, M. and Alvarez-Idaboy, J. R. 2011. Mechanism and kinetics studies on the antioxidant activity of sinapinic acid. Physical Chemistry Chemical Physics. 13: 11199-11205. https://doi.org/10.1039/c1cp20722a DOI: https://doi.org/10.1039/c1cp20722a
Gao, J. 2020. P-values - a chronic conundrum. BMC Medical Research Methodology. 20: 167. https://doi.org/10.1186/s12874-020-01051-6 DOI: https://doi.org/10.1186/s12874-020-01051-6
Kim, O. K., Yun, J. M., Lee, M., Kim, D. and Lee, J. 2021. Hypouricemic Effects of Chrysanthemum indicum L. and Cornus officinalis on Hyperuricemia-Induced HepG2 Cells, Renal Cells, and Mice. Plants (Basel). 10: 1668. https://doi.org/10.3390/plants10081668 DOI: https://doi.org/10.3390/plants10081668
Kim, S., Chen, J., Cheng, T., Gindulyte, A., He, J., He, S., Li, Q., Shoemaker, B. A., Thiessen, P. A., Yu, B., Zaslavsky, L., Zhang, J. and Bolton, E. E. 2021. PubChem in 2021: new data content and improved web interfaces. Nucleic Acids Research. 49: D1388-D1395. https://doi.org/10.1093/nar/gkaa971 DOI: https://doi.org/10.1093/nar/gkaa971
Lemos Lima, R. de C., Ferrari, F. C., de Souza, M. R., de Sá Pereira, B. M., de Paula, C. A. and Saúde-Guimarães, D. A. 2015. Effects of extracts of leaves from Sparattosperma leucanthum on hyperuricemia and gouty arthritis. Journal of Ethnopharmacology. 161: 194-199. https://doi.org/10.1016/j.jep.2014.11.051 DOI: https://doi.org/10.1016/j.jep.2014.11.051
Liang, D., Yong, T., Chen, S., Xie, Y., Chen, D., Zhou, X., Li, D., Li, M., Su, L. and Zuo, D. 2018. Hypouricemic effect of 2,5-dihydroxyacetophenone, a computational screened bioactive compound from Ganoderma applanatum, on hyperuricemic mice. International Journal of Molecular Sciences. 19: 1394. https://doi.org/10.3390/ijms19051394 DOI: https://doi.org/10.3390/ijms19051394
Li, B., Zhang, W. and Ma, H. 2016. Physicochemical characterization of inclusion complex of catechin and glucosyl-β-cyclodextrin. Tropical Journal of Pharmaceutical Research. 15: 167-172. http://dx.doi.org/10.4314/tjpr.v15i1.23 DOI: https://doi.org/10.4314/tjpr.v15i1.23
Mabry, T. J., Markham, K. R. and Thomas, M. B. 1970. The Ultraviolet Spectra of Flavones and Flavonols. En The Systematic Identification of Flavonoids. T.J. Mabry, K.R. Markham y M.B. Thomas (ed.), pp. 41-164. Springer, Berlin. https://doi.org/10.1007/978-3-642-88458-0_5 DOI: https://doi.org/10.1007/978-3-642-88458-0_5
Malik, N., Dhiman, P. and Khatkar, A. 2019. In silico design and synthesis of targeted rutin derivatives as xanthine oxidase inhibitors. BMC Chemistry. 13: 71. https://doi.org/10.1186/s13065-019-0585-8 DOI: https://doi.org/10.1186/s13065-019-0585-8
Martinez-Morales, F., Zapata-Morales, J. R. and Aragon-Martinez, O. H. 2022. Evaluation of the antioxidant interaction between butylated hydroxytoluene and quercetin and their utility for beef patties preservation. Biotecnia. 24: 69-78. https://doi.org/10.18633/biotecnia.v24i1.1546 DOI: https://doi.org/10.18633/biotecnia.v24i1.1546
Mehmood, A., Zhao, L., Ishaq, M., Xin, W., Zhao, L., Wang, C., Hossen, I., Zhang, H., Lian, Y. and Xu, M. 2020. Anti-hyperuricemic potential of stevia (Stevia rebaudiana Bertoni) residue extract in hyperu-ricemic mice. Food & Function. 11: 6387-6406. https://doi.org/10.1039/c9fo02246e DOI: https://doi.org/10.1039/C9FO02246E
Mohos, V., Pánovics, A., Fliszár-Nyúl, E., Schilli, G., Hetényi, C., Mladěnka, P., Needs, P. W., Kroon, P. A., Pethő, G. and Poór, M. 2019. Inhibitory Effects of Quercetin and Its Human and Microbial Me-tabolites on Xanthine Oxidase Enzyme. International Journal of Molecular Sciences. 20: 2681. https://doi.org/10.3390/ijms20112681 DOI: https://doi.org/10.3390/ijms20112681
Musci, M. and Yao, S. 2017. Optimization and validation of Folin-Ciocalteu method for the determination of total polyphenol content of Pu-erh tea. International Journal of Food Sciences and Nutrition. 68: 913-918. https://doi.org/10.1080/09637486.2017.1311844 DOI: https://doi.org/10.1080/09637486.2017.1311844
National Research Council US. 2011. Committee for the update of the guide for the care and use of laboratory animals, guide for the care and use of laboratory animals. National Academies Press (US). Washington, D.C.
Nguyen, M. T., Awale, S., Tezuka, Y., Tran, Q. L., Watanabe, H. and Kadota, S. 2004. Xanthine oxidase inhibitory activity of vietnamese medicinal plants. Biological and Pharmaceutical Bulletin. 27: 1414-1421. https://doi.org/10.1248/bpb.27.1414 DOI: https://doi.org/10.1248/bpb.27.1414
Oh, D. R., Kim, J. R., Choi, C. Y., Choi, C. H., Na, C. S., Kang, B. Y., Kim, S. J. and Kim, Y. R. 2019. Effects of chondroT on potassium oxonate-induced hyperuricemic mice: downregulation of xanthine oxidase and urate transporter 1. BMC Complementary Medicine and Therapies. 19: 10. https://doi.org/10.1186/s12906-018-2415-2 DOI: https://doi.org/10.1186/s12906-018-2415-2
Park, J. H., Jo, Y. I. and Lee, J. H. 2020. Renal effects of uric acid: hyperuricemia and hypouricemia. The Korean Journal of Internal Medicine. 35: 1291-1304. https://doi.org/10.3904/kjim.2020.410 DOI: https://doi.org/10.3904/kjim.2020.410
Pleskacova, A., Brejcha, S., Pacal, L., Kankova, K. and Tomandl, J. 2017. Simultaneous determination of uric acid, xanthine and hypoxanthine in human plasma and serum by HPLC–UV: uric acid metabolism tracking. Chromatographia. 80: 529-536. https://doi.org/10.1007/s10337-016-3208-8 DOI: https://doi.org/10.1007/s10337-016-3208-8
Seal, T. 2016. Quantitative HPLC analysis of phenolic acids, flavonoids and ascorbic acid in four different solvent extracts of two wild edible leaves, Sonchus arvensis and Oenanthe linearis of North-Eastern region in India. Journal of Applied Pharmaceutical Science. 6: 157-166. https://doi.org/10.7324/JAPS.2016.60225 DOI: https://doi.org/10.7324/JAPS.2016.60225
Sebaugh, J. L. 2011. Guidelines for accurate EC50/IC50 estimation. Pharmaceutical Statistics. 10: 128-134. https://doi.org/10.1002/pst.426 DOI: https://doi.org/10.1002/pst.426
Stamp, L. K. and Barclay, M. L. 2018. How to prevent allopurinol hypersensitivity reactions? Rheumatology (Oxford). 57: i35-i41. https://doi.org/10.1093/rheumatology/kex422 DOI: https://doi.org/10.1093/rheumatology/kex422
Tosovic, J. 2017. Spectroscopic features of caffeic acid: theoretical study. Kragujevac Journal of Science. 39: 99-108. https://doi.org/10.5937/KgJSci1739099T DOI: https://doi.org/10.5937/KgJSci1739099T
Tsikas, D., Wolf, A. and Frölich, J. C. 2004. Simplified HPLC method for urinary and circulating creatinine. Clinical Chemistry. 50: 201-203. https://doi.org/10.1373/clinchem.2003.024141 DOI: https://doi.org/10.1373/clinchem.2003.024141
Wishart, D. S., Feunang, Y. D., Guo, A. C., Lo, E. J., Marcu, A., Grant, J. R., Sajed, T., Johnson, D., Li, C., Sayeeda, Z., Assempour, N., Iynkkaran, I., Liu, Y., Maciejewski, A., Gale, N., Wilson, A., Chin, L., Cummings, R., Le, D., Pon, A., Knox, C. and Wilson, M. 2018. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Research. 46: D1074-D1082. https://doi.org/10.1093/nar/gkx1037 DOI: https://doi.org/10.1093/nar/gkx1037
Xu, Y., Tao, Z., Jin, Y., Yuan, Y., Dong, T. T. X., Tsim, K. W. K. and Zhou, Z. 2018. Flavonoids, a potential new insight of Leucaena leucocephala foliage in ruminant health. Journal of Agricultural and Food Chemistry. 66: 7616-7626. https://doi.org/10.1021/acs.jafc.8b02739 DOI: https://doi.org/10.1021/acs.jafc.8b02739
Yong, T., Li, D., Li, M., Liang, D., Diao, X., Deng, C., Chen, S., Xie, Y., Chen, D. and Zuo, D. 2018. Anti-Hyperuricemic effect of 2-hydroxy-4-methoxy-benzophenone-5-sulfonic acid in hyperuricemic mice through XOD. Molecules. 23: 2671. https://doi.org/10.3390/molecules23102671 DOI: https://doi.org/10.3390/molecules23102671
Yong, T., Zhang, M., Chen, D., Shuai, O., Chen, S., Su, J., Jiao, C., Feng, D. and Xie, Y. 2016. Actions of water extract from Cordyceps militaris in hyperuricemic mice induced by potassium oxonate combined with hypoxanthine. Journal of Ethnopharmacology. 194: 403-411. https://doi.org/10.1016/j.jep.2016.10.001 DOI: https://doi.org/10.1016/j.jep.2016.10.001
Zhang, D., Zhao, M., Li, Y., Zhang, D., Yang, Y. and Li, L. 2021. Natural xanthine oxidase inhibitor 5-O-caffeoylshikimic acid ameliorates kidney injury caused by hyperuricemia in mice. Molecules. 26: 7307. https://doi.org/10.3390/molecules26237307 DOI: https://doi.org/10.3390/molecules26237307
Zhang, L., Liu, Y. and Wang, Y. 2018. Deprotonation mechanism of methyl gallate: UV spectroscopic and computational studies. International Journal of Molecular Sciences. 19: 3111. https://doi.org/10.3390/ijms19103111 DOI: https://doi.org/10.3390/ijms19103111
Descargas
Archivos adicionales
Publicado
Cómo citar
Número
Sección
Licencia
Derechos de autor 2023

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
La revista Biotecnia se encuentra bajo la licencia Atribución-NoComercial-CompartirIgual 4.0 Internacional (CC BY-NC-SA 4.0)












_(2).jpg)




