INTRODUCTION
Thoracic empyema is defined as a collection of pus in the pleural cavity, gram-positive, or pleural fluid culture1. Usually, it is associated with pneumonia, but can also develop after surgery or trauma. Every year in the USA and Great Britain, 65000 patients suffer from empyema, which leads to huge financial costs (US$500 million) and a 20–30% mortality rate2. Nearly one-third of the affected patients require additional surgery within the first year after developing an empyema.
The early prognostic assessment of empyema gives the possibility of differentiating the high-risk patients and a chance for a timely and adequate change of the therapeutic approach, which in turn can affect the unfavorable outcome. Various prognostic factors have been developed over the years, but none has shown the necessary qualities so far. Most are complex and difficult to calculate, require many clinical and laboratory parameters and can rarely be used outside intensive care units (ICUs).
In 2016, a working group created the current Sepsis-3 definitions and removed the concept of systemic inflammatory response syndrome (SIRS) from the definition of sepsis3. The same group introduced the quick-Sequential Organ Failure Assessment (qSOFA) as a prognostic score that can immediately identify which patients with suspected infection are likely to have a prolonged ICU stay or die in hospital. An important advantage of qSOFA is its easy calculation, without the need for any laboratory tests. Following the task force’s recommendations, many hospitals are implementing qSOFA in the rapid assessment of high-risk patients. There are studies that show the superiority of qSOFA over SIRS criteria in predicting the final outcome4,5. However, in surgical patients, there is evidence that qSOFA has a lack of sensitivity in predicting mortality6,7.
Based on the lack of sensitivity of qSOFA to predict an unfavorable outcome, we decided to add a fourth criterion to improve the prognostic performance of this scoring system. Our choice was serum lactate dehydrogenase (LDH) levels and we called this combination the LDH-qSOFA score. We chose LDH, because nowadays this biomarker has been used as an indicator of cellular injury and death in different clinical settings8-10, and its higher concentrations show promising diagnostic and prognostic qualities in various pulmonary diseases11-13.
The aim of this study was to demonstrate the prognostic superiority of our new score LDH-qSOFA compared to qSOFA alone, in patients with thoracic empyema.
METHODS
We conducted a single-center retrospective study in the Clinic of Thoracic Surgery (CTS) of the University Hospital ‘Prof. Dr. Stoyan Kirkovich’, at Stara Zagora. All adult patients who underwent emergency surgery due to thoracic empyema between January 2021 and October 2023 were included. Our exclusion criteria were those aged <18 years and who used immunosuppressive drugs; however, none of the patients met them, and finally 84 patients were included in this analysis. The diagnosis empyema was made based on collection of pus in the pleural cavity or pleural fluid culture1. No intra-pleural fibrinolytic therapy was used in the management of empyema.
Demographic information and clinical outcome were determined from the patients’ medical records at admission to CTS. All patients were divided into two groups according to outcome (in-hospital mortality) – survivors and non-survivors (Table 2).
SIRS criteria were defined as follows: heart rate (HR) > 90/min, respiratory rate (RR) > 20/min, temperature (T) <36°C or >38°C, and white blood cell count (WBC) <4×109/L or >12×109/L. A positive result was defined as ≥2 out of 4 possible signs14.
Renal, Age, Purulence, Infection source, and Dietary factors (RAPID) score15 was calculated based on five risk indicators (Table 1).
Table 1
RAPID score (0–7)
| Parameter | Score |
|---|---|
| Renal urea (mmol/L) | |
| <5 | 0 |
| 5–8 | 1 |
| >8 | 2 |
| Age (years) | |
| <50 | 0 |
| 50–70 | 1 |
| >70 | 2 |
| Purulence of pleural fluid | |
| Yes | 0 |
| No | 1 |
| Infection source | |
| Community | 0 |
| Hospital | 1 |
| Dietary factors albumin (g/L) | |
| ≥27 | 0 |
| <27 | 1 |
Table 2
Characteristics of patients in the study
The qSOFA was determined using the following criteria, each equal to 1 point: systolic blood pressure (SBP) ≤100 mmHg, respiratory rate (RR) ≥ 22/min, and Glasgow Coma Scale (GCS) <15. A score of ≥2 out of a possible 3 points was defined as positive3.
One point for LDH >752U/L was added to the qSOFA (0 to 3 points), which we established as an optimal cut-off level for prediction of fatal outcome in ROC curve analysis. We named this combination Lactate Dehydrogenase-quick-SOFA (LDH-qSOFA) score with a range of 0 to 4 points.
All clinical and laboratory parameters necessary were extracted from each patient’s data at admission to the clinic. The parameters WBC, SBP, HR, RR, GCS, SIRS and qSOFA were determined for all 84 patients, while serum C-reactive protein levels for 81 patients (missing data in 3 medical records), RAPID for 74 patients (missing data in 10 medical records), neutrophil count (Neu) for 68 patients (missing data in 16 medical records), and LDH and LDH-qSOFA for 63 patients (missing data in 21 medical records).
Statistical analysis
Sensitivity, specificity, and areas under the curves (AUROC) for predicting mortality were calculated for each scoring system. Categorical variables are presented as frequency (%) and were analyzed by Pearson χ2 test or Fisher’s exact test. Continuous variables are presented as mean with standard deviation (SD) and compared by Student’s t-test or median with interquartile range (IQR) and compared by Mann-Whitney U test. A p<0.05 was considered statistically significant. For statistical analysis, we used statistical software SPSS (IBM, Chicago, Illinois, USA).
RESULTS
Patients’ characteristics
Of the 84 patients included, nine (10.7%) died during the hospital stay. Their median age was higher than the survivor group, but was not statistically significant (68 vs 61 years, p=0.186). No ability to discriminate non-survivors from survivors was found also for sex (p=0.194), blood type (p=0.092), length of hospitalization (p=0.302), location of infection (0.298) and comorbidity (p=1.00). In contrast, the type of surgical approach showed significant association with outcome, whereas patients who required video-assisted thoracoscopic surgery (VATS) had a higher chance of survival (p=0.031) (Table 2).
Laboratory findings, clinical parameters, and prognostic scores
No significant differences were observed in serum levels of WBC, Neu and CRP at admission between patients who died and those who survived p = 0.452, 0.18 and 0.663 respectively. However, non-survivors had significantly higher median concentrations of LDH than survivors (808 vs 395 U/L, p<0.0001). Poor outcome was associated with higher RR (p=0.011) and lower GCS (p=0.001), while better chance of survival had participants with higher median SBP values (p=0.019). Other prognostic factors like T (p=0.26), HR (p=0.202) and SIRS (p=0.16) could not discriminate patients according to outcome (Table 3).
Table 3
Laboratory findings, clinical parameters, and prognostic scores of study patients
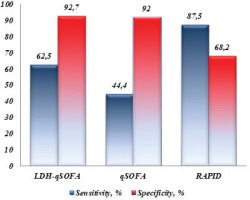
Both qSOFA and LDH-qSOFA demonstrated significant prognostic qualities, whereas higher median scores were indicative for fatal outcome (1 vs 0 points, p=0.003 for qSOFA; and 2 vs 0 points, p<0.0001 for LDH-qSOFA). Positive qSOFA ≥2 and LDH-qSOFA ≥2 were observed more frequently in non-survivors than survivors (44.4% vs 8%, p=0.01 for qSOFA; and 62.5% vs 7.3%, p=0.001 for LDH-qSOFA) (Table 3).
Sensitivity, specificity and AUROCs
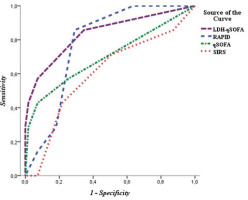
In prediction of mortality, qSOFA showed superiority to SIRS (AUROC=0.747; 95% CI: 0.546–0.947 vs AUROC=0.676; 95% CI: 0.481–0.871). Positive qSOFA demonstrated excellent specificity of 92% at the expense of low sensitivity (44.4%). The new score LDH-qSOFA fulfilled the idea of its creation by showing the best prognostic ability (AUROC=0.851; 95% CI: 0.687–1.000) (Figure 1). While maintaining the excellent specificity of qSOFA, we found a significantly higher sensitivity of LDH-qSOFA (62.5%) (Table 4 and Figure 2).
DISCUSSION
Empyema thoracis is one of the oldest and most severe pleural diseases, about which Hippocrates wrote: ‘If the empyema does not break through, death ensues’. Despite the evolution in management including advanced imaging methods, antibiotics, fibrinolytic drugs, and minimally invasive surgery, the morbidity and mortality remain high16.
Early prognostic assessment of empyema is crucial to determine disease severity and aggressiveness of conservative therapy and surgical management. Multiple variables have been described that affect prognosis, including patient-related and pleural space characteristics, as well as experience of general/thoracic surgeons and resources of hospital. Many researchers are still trying to deal with these problems focusing on the predictive reliability of different scoring systems.
Over the years, various prognostic scores have been recommended, of which the Sequential Organ Failure Assessment (SOFA) score has proven to be the most accurate and has even been included in the new Sepsis-3 definitions3. SOFA showed better prognostic ability than qSOFA and SIRS in ICU patients with suspected infection17; however, it is complex and difficult to assess in a timely manner, especially outside an ICU, and its calculation requires many laboratory parameters. In such cases, the qSOFA, designed for rapid and easy recognition of sepsis and high risk of in-hospital mortality, can be used.
SIRS eventually gave way to qSOFA, as it failed as a prognostic tool in patients with empyema, as also indicated by the results of the present study where almost half of the survivors (49.3%) and more than three-quarters of the deceased (77.8%) had clinical evidence of systemic inflammation (p=0.16). ROC curve analysis confirmed these findings – we observed low AUROC value for SIRS as predictor of death (0.676) with lack of significance (p=0.085). No other study investigating prognostic ability of SIRS in empyema was found.
In predicting fatal outcome, qSOFA was found to be superior to SIRS criteria4,5. However, some authors reported a lack of sensitivity of qSOFA as mortality predictor in sepsis (37%)18, acute infectious diseases (38.1%)19, and complicated intra-abdominal infections (cIAIs): 46% by Jung et al.6, 32% by Dimitrov et al.20 and 37% by Tolonen et al.21.
Only one study (to the best of our knowledge) investigated prognostic performance of qSOFA score in thoracic empyema exclusively. In 2022, Asai et al.7 conducted a retrospective analysis evaluating qSOFA in 53 patients with acute empyema. The authors reported a sensitivity of 43%, specificity of 91%, and no prognostic ability (AUROC=0.563, p=0.539). The ROC curve analysis performed by us revealed a similar sensitivity of 44.4% and specificity of 92%; however, we found better prognostic performance (AUROC=0.747) with a significant value (p=0.016), which may be due to the larger sample size in our study.
Several researchers have already tried to improve the predictive ability of qSOFA by combining with it elevated serum lactate or CRP levels. Shetty et al.22 and Jung et al.6 added lactate to qSOFA in emergency department (ED) and cIAIs patients, respectively, to successfully achieve their goal (Lactate-qSOFA vs qSOFA: 65.5 % vs 47.6%, and 72% vs 46%, respectively). By adding CRP to the score, Kim et al.23 in ED patients and Dimitrov et al.24 in cIAIs patients, improved the expected sensitivity (qSOFA-CRP vs qSOFA: 76.9% vs 53.8%, and 60% vs 35%, respectively).
To improve the sensitivity of qSOFA, we studied several combinations of qSOFA and biomarkers, such as white blood cells, neutrophils, C-reactive protein, and lactate dehydrogenase. The analysis revealed that the optimal combination was qSOFA and LDH.
LDH is a cytoplasmic enzyme expressed in nearly all types of cells of the body and is released into blood when the cells experience injury or death. Many researchers suggested LDH as an important predictor of cellular injury and death10. First, Ewig et al.12 reported about the association between this biomarker and unfavorable outcome in community-acquired pneumonia (NS=399 U/L vs S=254 U/L). Mortality was also higher in sepsis patients with elevated serum LDH according to Lu et al.25 (NS=328.39 vs S=200.13 U/L), Zein et al.26 (NS=656 vs S=369 U/L) and Wang et al.27 (NS=300 vs S=258.5 U/L). Increased levels were associated with higher risk of death in patients with coronavirus disease28 and critically ill patients with acute kidney injury29.
In our study LDH discriminated non-survivors from survivors with highly significant value (p<0.0001) and death was more likely in patients with higher serum levels (808 vs 395 U/L). The identified threshold >752 U/L was observed in 87.5% of patients who died and in only 9.1% of those with favorable outcome. The new LDH-qSOFA score showed significantly higher sensitivity than qSOFA (62.5% vs 44%). In our study group, we found that LDH-qSOFA had a better prognostic ability than qSOFA (AUROC=0.851 vs 0.747).
Our new score also showed a better prognostic performance than the RAPID score (AUROC=0.851 vs 0.786), which was introduced and validated in patients with pleural infection15. We suggest that one of the reasons for the lower predictive power of RAPID is a consequence of the fact that it was designed to predict 3-month mortality, while we use LDH-qSOFA to assess in-hospital mortality, which in our study did not exceed 28 days.
This is the first study (to our knowledge) to evaluate the combination of qSOFA and LDH as a predictor of mortality. With four criteria and improved sensitivity and prognostic accuracy, the new score could differentiate patients at high risk of fatal outcome who were previously incorrectly defined as non-severe and treated as such.
Limitations
Limitations of the study are the single-center setting, the small sample size, and the retrospective nature of the analysis. Future larger prospective multicenter studies could overcome these limitations and provide a definitive answer regarding the utility of the LDH-qSOFA score in clinical practice.