Abstracts
Several oral nonsteroidal anti-inflammatory drugs (NSAIDs) are effective to treat migraine attacks. Lysine clonixinate (LC) is a NSAID derived from nicotinic acid that has proven to be effective in various pain syndromes such as renal colic and muscular pain. The aim of this double-blind, placebo-controlled study was to evaluate the efficacy of oral LC compared to placebo in the acute treatment of migraine. Sixty four patients with the diagnosis of migraine, according to the IHS criteria, were studied prospectively. Patients received LC or placebo once the headache reached moderate or severe intensity for 6 consecutive attacks. With regard to the moderate attacks, LC was superior than placebo after 1, 2 and 4 hours. The consumption of other rescue medications after 4 hours was significantly higher in the placebo group. With regard to the severe attacks, there was no difference between the active drug group and the placebo group concerning headache intensity and consumption of other rescue medications. We conclude that the NSAID lysine clonixinate is effective in treating moderately severe migraine attacks. It is not superior than placebo in treating severe migraine attacks.
migraine; acute treatment; lysine clonixinate; NSAIDs
Alguns antinflamatórios não esteroidais (AINEs) são eficazes para o tratamento de crises de migrânea. O clonixinato de lisina (CL) é um AINE derivado do ácido nicotínico comprovadamente eficaz no tratamento de várias síndromes dolorosas como a cólica renal e a dor muscular. O objetivo deste estudo duplo-cego placebo-controlado foi avaliar a eficácia do CL oral comparado ao placebo no tratamento agudo da migrânea. Sessenta e quatro pacientes com o diagnóstico de migrânea, de acordo com os critérios da Sociedade Internacional de Cefaléia (IHS), foram estudados prospectivamente. Os pacientes receberam CL ou placebo quando a cefaléia atingiu a intensidade moderada ou severa em 6 crises consecutivas. Para as crises moderadas, o CL foi superior ao placebo em 1, 2 e 4 horas. O consumo de outras medicações de resgate após 4 horas foi significativamente maior no grupo do placebo. Com relação às crises severas, não houve diferença entre os grupos da droga ativa e do placebo no tocante à intensidade da cefaléia e ao consumo de outras drogas de resgate após 4 horas. Concluímos que o AINE clonixinato de lisina é eficaz para o tratamento de crises moderadas de migrânea. Esta droga não é superior ao placebo no tratamento de episódios de cefaléia migranosa de intensidade severa.
migrânea; tratamento agudo; clonixinato de lisina
ORAL LYSINE CLONIXINATE IN THE ACUTE TREATMENT OF MIGRAINE
A double-blind placebo-controlled study
Abouch V. Krymchantowski11Headache Center of Rio; Headache Center of Rio; 2Oswaldo Cruz Institute, FIOCRUZ - Rio de Janeiro , Jackeline S. Barbosa11Headache Center of Rio; Headache Center of Rio; 2Oswaldo Cruz Institute, FIOCRUZ - Rio de Janeiro , Celia Cheim11Headache Center of Rio; Headache Center of Rio; 2Oswaldo Cruz Institute, FIOCRUZ - Rio de Janeiro , Luiz A. Alves21Headache Center of Rio; Headache Center of Rio; 2Oswaldo Cruz Institute, FIOCRUZ - Rio de Janeiro
ABSTRACT - Several oral nonsteroidal anti-inflammatory drugs (NSAIDs) are effective to treat migraine attacks. Lysine clonixinate (LC) is a NSAID derived from nicotinic acid that has proven to be effective in various pain syndromes such as renal colic and muscular pain. The aim of this double-blind, placebo-controlled study was to evaluate the efficacy of oral LC compared to placebo in the acute treatment of migraine. Sixty four patients with the diagnosis of migraine, according to the IHS criteria, were studied prospectively. Patients received LC or placebo once the headache reached moderate or severe intensity for 6 consecutive attacks. With regard to the moderate attacks, LC was superior than placebo after 1, 2 and 4 hours. The consumption of other rescue medications after 4 hours was significantly higher in the placebo group. With regard to the severe attacks, there was no difference between the active drug group and the placebo group concerning headache intensity and consumption of other rescue medications. We conclude that the NSAID lysine clonixinate is effective in treating moderately severe migraine attacks. It is not superior than placebo in treating severe migraine attacks.
KEY WORDS: migraine, acute treatment, lysine clonixinate, NSAIDs.
Clonixinato de lisina oral para o tratamento agudo da migrânea: estudo duplo-cego e placebo-controlado
RESUMO - Alguns antinflamatórios não esteroidais (AINEs) são eficazes para o tratamento de crises de migrânea. O clonixinato de lisina (CL) é um AINE derivado do ácido nicotínico comprovadamente eficaz no tratamento de várias síndromes dolorosas como a cólica renal e a dor muscular. O objetivo deste estudo duplo-cego placebo-controlado foi avaliar a eficácia do CL oral comparado ao placebo no tratamento agudo da migrânea. Sessenta e quatro pacientes com o diagnóstico de migrânea, de acordo com os critérios da Sociedade Internacional de Cefaléia (IHS), foram estudados prospectivamente. Os pacientes receberam CL ou placebo quando a cefaléia atingiu a intensidade moderada ou severa em 6 crises consecutivas. Para as crises moderadas, o CL foi superior ao placebo em 1, 2 e 4 horas. O consumo de outras medicações de resgate após 4 horas foi significativamente maior no grupo do placebo. Com relação às crises severas, não houve diferença entre os grupos da droga ativa e do placebo no tocante à intensidade da cefaléia e ao consumo de outras drogas de resgate após 4 horas. Concluímos que o AINE clonixinato de lisina é eficaz para o tratamento de crises moderadas de migrânea. Esta droga não é superior ao placebo no tratamento de episódios de cefaléia migranosa de intensidade severa.
PALAVRAS-CHAVE: migrânea, tratamento agudo, clonixinato de lisina.
Migraines are highly prevalent primary headaches that can start in childhood or adolescence 1,2. It affects mostly women and manifests clinically as moderate to severe headache attacks, frequently of unilateral fronto-temporal locations. The pain quality is pulsating and/or pressure-type, incapacitating and worsens with physical activities, usually associated with nausea (may also be accompanied by vomiting), intolerance to light and/or noises and/or smells. Headache may last from 4 to 72 hours when it is not treated or is ineffectively treated. Frequency of pain occurrence is variable and some patients can have it on a weekly basis, while others will have it less than once a month.
There are several drug options for the treatment of acute migraine attacks. The choice of one or another will depend on pain intensity, its frequency and associated symptoms, co-morbidity, progression profile and individual patients response3. Several patients find pain relief using simple analgesics such as acetyl salicylic acid, acetaminophen, dipirone, mefenamic acid or some combination of these. In fact, a recent multicenter study found that the association of aspirin, acetaminophen and caffeine was efficient to treat most of the acute migraine attacks4. Nonsteroidal anti-inflammatory drugs (NSAIDs) like diclofenac 5, naproxen 6 and ibuprofen7 also proved to be efficacious in the acute treatment of migraine. Lysine clonixinate (LC) is a NSAID which belongs to the family of the non-salicylates and to the subgroup of anthranilic derivatives. It has a chemical structure resembling the flufenamic acid, although it is derived from nicotinic acid. Its efficacy in the treatment of acute migraine attacks was suggested by previous non-controlled studies, both in oral 8 as well as in parenteral administration 9. Lysine's structural formula as 2-(3-chloro-o-toluidine)piridino-3-carboxilate allows a fast absorption10 and its inhibiting effect on the enzyme cyclooxygenase, important in prostaglandin synthesis, is reversible. It bonds to plasma proteins in up to 96-98% and its metabolism takes place in the liver, four different inactive metabolites being derived. Seventy four percent of its excretion is renal and 25%, fecal.
The aim of this study was to evaluate the efficacy of oral lysine clonixinate compared to placebo in the treatment of acute migraine.
METHOD
Study group
We studied 64 patients with migraine, prospectively diagnosed according to the criteria of the International Headache Society (IHS) 11. Fifty five were women and 9 were men, aged from 18 to 53 years. Patients were randomized and divided into two groups (A and B), which received placebo or CL. Patients were instructed to take 2 tablets of medication (CL 125 mg/tablet or placebo) once the pain reached moderate or severe intensity in 6 consecutive attacks. Regardless of pain intensity, another tablet of LC or placebo had to be taken 40 minutes later by all patients. Patients were instructed to record in a diary their pain intensity just prior to taking the medication, and 1, 2 and 4 hours later. The patients also recorded the occurrence of vomiting after the drug ingestion and the consumption of other rescue medications after 4 hours. All patients in this study were permitted to take prophylactic drugs but not NSAIDs.
Statistical analysis
The data were analyzed by GraphPad software ( GraphPad Instat, V2.0.5a, 1994), using the Chi-square Test. A two-tailed P value < 0.05 was considered significant.
RESULTS
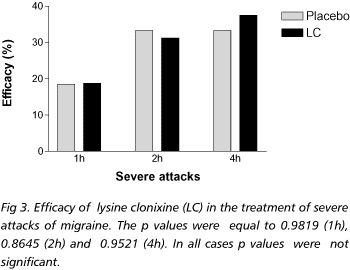
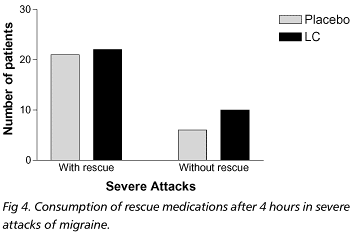
Group A included 31 patients, who had 152 moderate and 34 severe attacks, and were treated with placebo. Vomiting occurred in 11 of the moderate and 7 of the severe attacks. Thirty three patients in group B having 150 moderate and 48 severe attacks were treated with lysine clonixinate. Vomiting followed the ingestion of the medication in 8 moderate and in 16 severe attacks. With regard to the treatment of moderate attacks, 27.6% of group A and 65.4% of group B (p<0.0001) presented mild or no pain after 1h. After 2 hours, 29.8% of group A and 84.5% of group B (p<0.0001) had mild or no headache. After 4 hours, 34.7% of group A and 91.5% of group B (p<0.0001) had mild or no pain (Fig 1). Consumption of rescue medications after 4 hours was observed in 68% of group A and in 5.6% of group B (p<0.0001) (Fig 2). With regard to the severe attacks, after 1h, 18.5% of group A and 18.8% of group B (p=0.9819) had mild or no headache; after 2h, 33.4% of group A and 31.3% (p=0.8645) of group B indicated mild or no pain; after 4h, 33.4% of group A and 37.5% of group B (p=0.9521) reported mild or no pain) (Fig 3). Figure 4 shows that 77.8% patients of group A and 68.8% patients of group B consumed rescue medications after 4 hours.
DISCUSSION
Lysine clonixinate (2-(3-chloro-0-toluidine) piridin-3-lysine carboxilate) is a NSAID effective in the treatment of several pain syndromes such as renal colic, nerve entrapment pain, muscular pain and tooth ache10,12,13 . The efficacy of other NSAIDs in the acute treatment of migraine, as well as that of lysine clonixinate, have been demonstrated in previous studies5-9. This study confirms the efficacy of lysine clonixinate compared to placebo in reducing both intensity and duration of moderate migraine attacks, as well as the consumption of rescue medications after 4 hours. The speed of action of lysine clonixinate is favorable and can't be compared with that of ibuprofen 7, diclofenac 5, tolfenamic acid 14, NSAID also demonstrated as being efficient in the acute treatment of migraine, since the methodology and design of this study, when the patients took another capsule 40 minutes later, was unique and not done with the above mentioned NSAID.
In another hand, the efficacy of the triptans, selective 5-HT 1B/1D agonists and therefore specific drugs for the acute treatment of migraine, in reducing the intensity of migraine attacks is demonstrated at as early as 1 hour15,16,17,18. LC also has been proven effective after 1 hour in moderate attacks. However comparative postulations with regard to early efficacy of both drugs can't be accomplished due to different study designs, methods and patient selection.
Nonsteroidal anti-inflammatory drugs seem to act via inhibition of prostaglandin synthesis and platelet aggregation as well as the inhibition of serotonin release from platelets 19. Despite its efficacy and fast speed of action in many types of pain syndromes, it has been shown that lysine clonixinate also has a central pharmacological action, specifically in the thalamus and spinal cord 10,13. Sierralta12 have demonstrated an interaction between lysine clonixinate and the central serotoninergic system.
Lysine clonixinate was not superior to placebo in reducing the intensity and duration of severe headaches, nor the consumption of rescue medications after 4 hours. Which sites of action were important in obtaining the results achieved in moderate attacks remains uncertain. Remission of the pain probably resulted from association of all those mechanisms of action and the reason why it failed to control severe attacks is unknown. Based upon pharmacokinetics of triptans, efficient drugs employed in the treatment of the acute migraine attack, it is suggested that it is important to penetrate the blood-brain barrier in order to obtain a rapid and prolonged effect 16. That is observed with lysine clonixinate10,12,13. Headache recurrence, defined as the return of the headache within 24 hours after it has become mild or absent, may occur with triptans 17,20 and other headache drugs and should be studied with this NSAID. It is also necessary to evaluate the possibility of using lysine clonixinate in patients with ischaemic coronary artery disease and uncontrolled hypertension in whom triptans are contraindicated, as well as in patients with history of peptic disease in whom NSAIDs are contraindicated.
The authors have not observed any significant side effects using LC, although nausea, vomiting, allergic reactions, postural hypotension, vertigo and insomnia have been described in the literature 8,9,13.
This study indicates that oral administration of 250 mg lysine clonixinate followed by another 125 mg tablet 40 minutes later, is efficient and well tolerated in the treatment of moderate attacks of migraine. The authors would not recommend its use in severe attacks of migraine.
Acknowledgements - The authors thank Dr. Alan Rapoport for reviewing the manuscript and emphasize that this study did not receive any financial support.
Received 2 August 2000, received in final form 21 September 2000. Accepted 26 September 2000.
Dr. Abouch Valenty Krymchantowski - Rua Siqueira Campos 43 / 1002 - 22031-070 Rio de Janeiro RJ - Brasil. FAX 55 21 235 2688. E-mail: abouchkrym@openlink.com.br (www.dordecabeca.com.br).
- 1. Stewart WF, Shechter A, Lipton RB. Migraine heterogeneity, disability, pain intensity, and attack frequency and duration. Neurology 1994;44(Suppl4):S24-S39.
- 2. Rasmussen BK. Epidemiology of headache. Cephalalgia 1995;15:45-68.
- 3. Tfelt-Hansen P, Lipton RB. Prioritizing treatment. In Olesen J, Tfelt-Hansen P, Welch KMA (eds) The headaches. New York: Raven Press, 1993:359-362.
- 4. Lipton RB, Stewart WF, Ryan RE, Saper J, Silberstein S, Sheftell F. Efficacy and safety of the nonprescription combination of acetaminophen, aspirin, and caffeine in alleviating headache pain of an acute migraine attack: three double-blind, randomized, placebo-controlled trials. Arch Neurol 1998;55:210-217.
- 5. Dahlof C, Bjorkman R. Diclofenac-K (50 and 100mg) and placebo in the acute treatment of migraine. Cephalalgia 1993;13:117-123.
- 6. Nestvold K, Kloster R, Partinen M, Sulkava R. Treatment of acute migraine attack: naproxen and placebo compared. Cephalalgia 1985;5:115-119.
- 7. Kloster R, Nestvold K, Vilming S. A double-blind study of ibuprofen versus placebo in the tratment of acute migraine attacks. Cephalalgia 1992;12:169-171.
- 8. Mesquita CT, Caçăo JC, Villas Boas P, Mattos LF. Clonixinato de lisina no tratamento de enxaquecas. RBM 1996;53:1128-1131.
- 9. Krymchantowski A, Barbosa J. Clonixinato de lisina injetável intravenoso para o tratamento agudo das enxaquecas: um estudo piloto aberto. Arq Neuropsiquiatr 1999;57:606-609.
- 10. Pereyra Quintana JR. Comparacion del efecto analgesico del clonixinato de lisina com un hipno-analgesico. EDM 1978;50:6-23.
- 11. Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8(Suppl 7): 1-96.
- 12. Sierralta F. Clonixinato de lisina. Interacciones com el sistema serotoninérgico y naloxona. Proceedings of the 9th Latinoamerican Congress of Pharmacology and Therapeutics. Santiago, 1982:64.
- 13. Paredes H, Solis R, Palacios J, et al. The use of intravenous lysine clonixinate for rapid pain relief: an open clinical study. Curr Therap Res 1996;40:86-91.
- 14. Hakkarainen H, Vapaatalo H, Gothoni G, Parantainen J. Tolfenamic acid is as effective as ergotamine during migraine attacks. Lancet 1979;2:326-328.
- 15. Visser WH, de Vriend RH, Jaspers MW, Ferrari MD. Sumatritpan in clinical practice: a 2-year review of 453 migraine patients. Neurology 1996;47:46-51.
- 16. Visser WH, Klein KB, Cox RC, Jones D, Ferrari MD. 311C90, a new central and peripherally acting 5-HT1D receptor agonist in the acute treatment of migraine: a double-blind, placebo-controlled, dose-range finding study. Neurology 1996;46:522-526
- 17. International 311C90 Long-term Study Group. The Long-term tolerability and efficacy of oral zolmitriptan (Zomig 311C90) in the acute treatment of migraine: an international study. Headache 1998;38:173-183.
- 18. Dechant KL, Clissold SP. Sumatriptan: a review of its pharmacokinetic properties, and therapeutic efficacy in the acute treatment of migraine and cluster headache. Drugs 1992;43:776.
- 19. Pradalier A, Rancurel G, Dordain G, Verdure L, Rascol A, Dry J. Acute migraine attack therapy: comparison of naproxen sodium and an ergotamine tartrate compound. Cephalalgia 1985;5:107-113.
- 20. Ferrari MD, James MH, Bates D, et al. Oral sumatriptan: effect of a second dose, and incidence and treatment of headache recurrences. Cephalalgia 1994;14:330-338.
Publication Dates
-
Publication in this collection
06 Apr 2001 -
Date of issue
Mar 2001
History
-
Accepted
26 Sept 2000 -
Reviewed
21 Sept 2000 -
Received
02 Aug 2000