Mitral regurgitation (MR) is the most common type of valve disease in developed nations and affects more than 2 million people in the US.1 It is seen in approximately one-third of patients with chronic heart failure, and contributes to the progression of disease and a worsening prognosis.2 In patients aged ≥50 years, yearly mortality rates for moderate to severe MR range from 3% to 6%.3
MR can be caused by an abnormality in one or more components of the mitral valve apparatus, or from left atrial or left ventricular dysfunction. There are two main types of MR: primary (or degenerative) and secondary (or functional). Primary MR is a disease of the mitral valve apparatus, which includes the leaflets, chordae tendineae, and papillary muscles. Secondary MR is a disease of the left ventricle or atrium.4
In this article, we discuss secondary MR by reviewing its pathophysiology and diagnosis. We cover definitions of severe secondary MR on multimodality imaging, and finally, we discuss advances in surgical and transcatheter options for the management of secondary MR.
Pathophysiology and Classification
One of the most common causes of secondary MR is left ventricular (LV) ischemia and MI. Regional or global LV dysfunction and adverse LV remodeling post-MI lead to impaired leaflet motion and failure of leaflet overlap. Ischemic MR is a frequent complication of coronary artery disease and seen in approximately one-fifth of patients after an MI.5 Another cause of secondary MR is dilated cardiomyopathy, or non-ischemic causes of LV remodeling. Additionally, annular enlargement secondary to left atrial or ventricular dilatation and remodeling leads to impaired closing of the mitral leaflets and, in turn, regurgitation.6
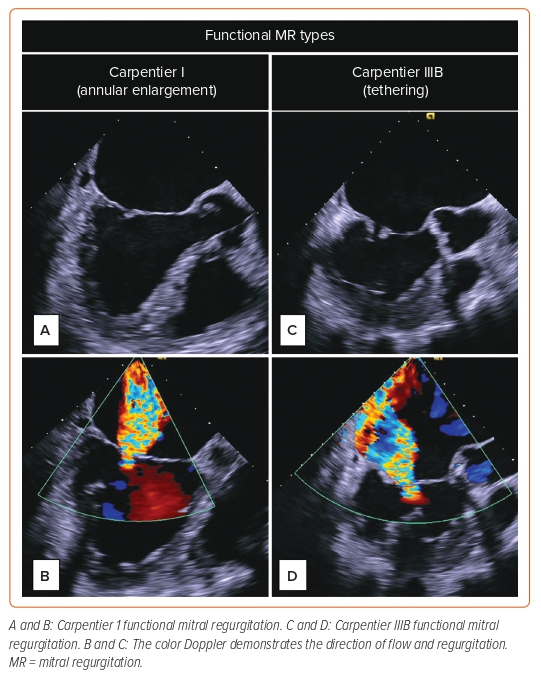
The Carpentier classification stratifies MR based on leaflet motion. Type I is defined by normal leaflet motion (leaflet perforation), while type II is excessive leaflet motion, and type III is characterized by restricted leaflet motion. This article focuses on type III-restricted leaflet motion, and more specifically, type IIIB, which is restricted motion during systole in patients with ischemic or dilated cardiomyopathy. The disease processes seen with this type are LV dilatation, chordae tethering, and papillary muscle displacement (Figure 1).7
The ACC/AHA guidelines classify secondary MR into four stages based on severity of disease. Stage A is defined as at risk for MR in a patient with coronary artery disease or cardiomyopathy. Patients may have symptoms related to coronary artery disease or heart failure that respond to revascularization or medical therapy. Stage B is progressive MR with mild tethering of mitral leaflets, annular dilation, and the same symptoms as stage A, which include manifestations related to coronary artery disease or heart failure that respond to revascularization or medical therapy. Stage C is asymptomatic severe MR with severe tethering of mitral leaflets and the same symptoms as stage A and B, as above. Stage D is symptomatic severe MR with similar severe tethering of mitral leaflets (Table 1).8
Diagnosis and Grading
Clinical manifestations of MR are correlated with the severity and progression of the valve disease, as well as the underlying cardiac conditions. Common symptoms include weakness, fatigue, and exercise intolerance. Patients with severe MR may present with dyspnea, pulmonary edema, arrhythmias, and other heart failure symptoms. However, as these patients have pre-existing cardiac disease, it is difficult to determine if these symptoms are a result of their primary LV dysfunction or from the added effects of secondary MR. On physical examination, S1 is usually diminished due to inadequate bridging of the leaflets, and a holosystolic murmur is normally heard best over the apex.9
The diagnosis of MR is typically made via echocardiography and particularly with Doppler echocardiography. Transthoracic echocardiography (TTE), the most commonly used method of identifying MR, provides anatomic and functional information on the mitral valve, and can help identify the severity and mechanism of the disease. A meticulous assessment with 2D imaging and Doppler interrogation should be performed to assess the effect of the MR on the cardiac chambers. Transesophageal echocardiography provides a clearer picture of the left heart, and is typically used when the TTE study is suboptimal.10 Additionally, it provides insight into determining the optimal treatment modality.
Common findings on echocardiography in patients with secondary MR are a dilated left atrium and increasingly dilated LV size, and decreased systolic function as the disease progresses. Doppler echocardiography allows for both qualitative and quantitative assessments of MR severity, including measurements of regurgitant volume (RVOL), regurgitant fraction, and effective regurgitant orifice area (EROA).11 On a TTE, an EROA area of at least 40 mm2 and an RVOL of at least 60 ml/beat is defined as severe (grade 4+) MR. Moderate MR (grade 2+/3+) is defined as an EROA 20–39 mm2 and RVOL of 30–59 ml/beat.12 Data have shown that a larger EROA and high RVOL are independently associated with increased mortality in both heart failure and ischemic MR patients (Table 1).13,14
Although the EROA cutoff for severe secondary MR was set back to 40 mm2 in the latest American College of Cardiology/American Heart Association guidelines (EROA cutoff for severe secondary MR was 20 mm2 in 2017 American College of Cardiology/American Heart Association guidelines), it is essential to note that EROA of 20 mm2 has been associated with poor clinical outcomes.8
Cardiac MRI (CMR) can be used if the measures of MR severity or LV function are not appropriately assessed by echocardiography. CMR provides detailed information on LV volumes and ejection fraction, and has been shown to be similar to transesophageal echocardiography in predicting outcomes.15 Moreover, as per Uretesky et al., CMR is more accurate in predicting the severity of MR than TTE, and can help to better guide decisions in management.16 The outcome data of MR has been derived from echocardiography, and CMR will likely become a reliable surrogate in predicting outcomes in the future.
Cardiac CT angiography (CTA) is not used for the evaluation of MR severity. However, it provides clear, detailed pictures of the mitral valve and helps identify discrepancies in anatomy. It is also essential in measuring the left ventricle outflow tract (LVOT) dimensions, which is particularly valuable for planning transcatheter mitral valve repair.17 Some studies have shown that CTA has better accuracy and reliability than TTE in diagnosing mitral valve prolapse.18 The importance of CTA evaluation of the mitral valve is essential when evaluating patients for transcatheter mitral valve replacement.
Finally, MR severity assessment can be complex and challenging, especially with MR being very dynamic and dependent on loading conditions with resting or exercise states. Hence, a multiparametric approach should be implemented in complex cases.
Transcatheter Management of Secondary Mitral Regurgitation
Transcatheter Mitral Valve Repair
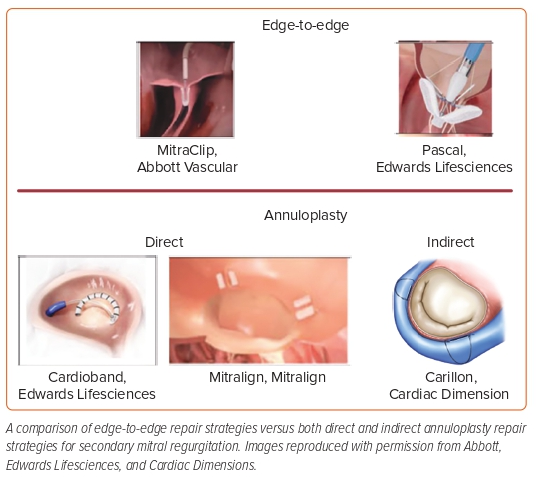
Catheter-based mitral valve repair interventions are geared toward targeting different pathologies of the leaflets, the annulus, and the chordae (chordal intervention devices specifically treat degenerative mitral valve regurgitation and are outside the scope of this review; Figure 2).
Transcatheter Edge-to-edge Repair
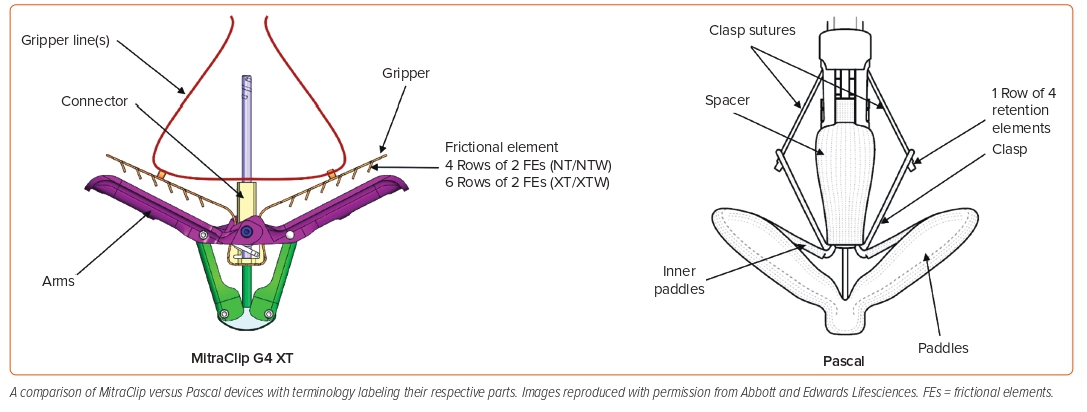
Leaflet targeted transcatheter intervention for functional MR is the only strategy that has gained Food and Drug Administration (FDA) approval for secondary MR so far. This strategy mimics the surgical edge-to-edge repair technique or the Alfieri stitch. The MitraClip system (Abbott Vascular) was approved for treatment of secondary MR based on the results of the COAPT trial. In this trial, 614 patients with heart failure and symptomatic secondary MR despite treatment with guideline-directed medical therapy were enrolled, of which 302 underwent transcatheter edge-to-edge repair (TEER) using the MitraClip system, and the remainder were treated with medical management alone. Exclusion criteria included vertical coaptation length <2 mm in valves with leaflet tethering, evidence of calcification in the grasping area of the A2 or P2 scallops, presence of a significant cleft of A2 or P2 scallops, and lack of both primary and secondary chordal support.8 The primary outcome was heart failure hospitalization over 24 months, while all-cause mortality was a secondary outcome. Patients randomized to the TEER arm were found to have lower rates of hospitalization within 2 years of follow-up and lower all-cause mortality than the patients who had medical therapy alone.19
However, another trial, MITRA-FR, randomized 152 patients with severe secondary MR and symptomatic heart failure to either the TEER with medical therapy group or medical therapy alone group. It found no difference in rates of death or unplanned hospitalizations between the two cohorts at 1 year.20 The apparent difference in the outcomes of these two trials can be attributed to many factors. First, the COAPT trial mandated that patients be seen by a specialized heart failure team to optimize medical management for heart failure prior to enrollment into the trial. Second, left ventricular dimensions for patients enrolled in the COAPT trial were smaller than those enrolled in the MITRA-FR trial, emphasizing the notion of proportionate versus disproportionate MR severity relative to the cardiomyopathy severity.21 This emphasizes the point that the secondary MR patients represent a heterogeneous group that can be further classified based on left ventricular end-diastolic volume (LVEDV).
The Gorlin hydraulic orifice equation allows an estimated EROA quantification of the MR based on the LVEDV and ejection fraction, and assuming a regurgitant fraction of 50%. The MITRA-FR trial enrolled patients who had MR that was proportionate to the degree of LV dilatation, whereas the COAPT trial had a disproportionately higher EROA of 30% higher while having LV volumes that were 30% smaller. Mean EROA were 31 ± 10 mm2 versus 41 ± 15 mm2, respectively, for MITRA-FR and COAPT. Conversely, LVEDV was 135 ± 35 ml/m2 versus 101 ± 34 ml/m2, respectively. The disproportionate MR noted in the latter trial showed a decrease in LVEDV with MitraClip therapy, which was ultimately associated with a reduced risk of death and hospitalization for heart failure, highlighting the importance of patient selection.22,23
Another TEER device that is now being investigated for secondary MR treatment is the Pascal (Edwards Lifesciences) device. Compared with the MitraClip system, it has wider paddles, which, in theory, can grasp more leaflet tissue in addition to a spacer between the two paddles for better coaptation. The Pascal device was investigated in the CLASP safety and feasibility study. The CLASP study, which was a prospective single-arm study of safety and efficacy of the Pascal device, enrolled 109 patients with symptomatic grade ≥3 MR (67% with secondary MR). At 1 year, survival was 92% (89% for functional MR [FMR]) with 88% freedom from heart failure hospitalization (80% for FMR), and MR was ≤1+ in 82% of patients (79% in FMR) and ≤2+ in 100% of patients.24 The CLASP IIF randomized trial is currently investigating a head-to-head comparison between the Pascal and MitraClip systems in patients with secondary MR (Figure 3).25 Of note, the Pascal device is now commercially available and also FDA approved for degenerative (primary) MR.
Procedural failure is defined as an inadequate resolution of regurgitation or the development of mitral stenosis with a mitral valve area (MVA) of <1.5 cm2 or transmitral mean pressure gradient >5 mmHg. However, a recent study showed that transmitral mean pressure gradient >5 mmHg does not carry any predictive value for adverse clinical outcomes for patients with secondary MR. This is in stark contrast to those with degenerative MR, where transmitral mean pressure gradient >5 mmHg was predictive of worse clinical outcomes.26 A possible explanation is that gradients in patients with secondary MR may not necessarily indicate smaller MVAs, but instead better LV function. This suggests that higher gradients may not denote functional failure in patients with secondary MR.27 Residual MR is the degree of MR that continues to occur after the repair of baseline MR. In several studies, patients with grade 3+ or 4+ residual MR have been found to have worse outcomes with higher rates of heart failure exacerbations and all-cause death than patients with grade 0/1+ or 2+ residual MR.28,29 Moreover, both Kar et al. and Kaneoko et al. suggest that grade 2+ residual MR does not portend worse outcomes compared with grade 1+ residual MR, except in patients with certain risk factors, including worse LV function, renal dysfunction, and heart failure.30
Independent predictors of procedural success include preprocedural Thrombolysis in MI myocardial perfusion grade and MVA, the presence of mitral annular calcification, and implantation of multiple clips. One study found that multiple clips were implanted in 40% of patients. However, although adequate reduction of MR may require multiple clips, there is a trade-off with the increased risk for mitral stenosis in these patients.31 In patients with a single clip implanted, another study found that a large preprocedural MVA predicted low postprocedural Thrombolysis in MI myocardial perfusion grade. After multiple clips, there was no longer an accurate correlation between the two.32 The MitraScore can be used to predict mortality in patients treated with TEER. It consists of eight clinical variables, including age, LV ejection fraction, anemia, kidney function, peripheral artery disease, high dose of diuretic, absence of therapy with renin–angiotensin system inhibitors, and chronic obstructive pulmonary disease, which are all well-known risk factors associated with mortality after TEER.33
In 2018, the original data from the COAPT trial with 614 patients showed that TEER in addition to maximally tolerated guideline-directed medical therapy in patients with moderate or severe MR was superior to guideline-directed medical therapy alone in reducing the rates of hospitalization for heart failure (HFH): 35.8% versus 67.9% (HR 0.53; 95% CI [0.40–0.70]) at 2 years, and more surprisingly, the mortality rate: 29.1% versus 46.1% (HR 0.62; 95% CI [0.46–0.82]). The 5-year data showed an annualized rate of HFH of 33.1% versus 57.2% (HR 0.53; 95% CI [0.41–0.68]) and death rate of 57.3% versus 67.2% (HR 0.72; 95% CI [0.58–0.89]) in the MitraClip versus the control group (Abbott), respectively. The gap is narrowing, as approximately 45% of surviving control group patients (n=67) crossed over to TEER after 2 years. The combined endpoint, defined as death or HFH within 5 years, reached 91.5% in the control group versus 73.6% in the MitraClip group (HR 0.53; 95% CI [0.44–0.64]), signifying how critically ill these patients were, and the importance of identifying and treating them as early as possible. Interestingly, the event curves of the crossover group were similar to those of patients treated with TEER at the index study. Also demonstrated was the improvement of the heart failure class and durability of MR reduction. Among the 57 device-treated patients alive at 5 years, 20% had grade 1+ or no residual MR. With the proper screening and stringent echocardiographic assessments, the ‘right’ patients can be identified and offered definitive life-saving transcatheter therapies.19,34
Annuloplasty Devices
The purpose of these devices is to reduce the annular circumference to obtain better coaptation of the mitral valve leaflets. The indirect annuloplasty device uses the proximity of the coronary sinus to the mitral annulus. The Carillon device (Cardiac Dimensions) has gained CE mark approval in Europe based on the TITAN trial. The REDUCE FMR study, which demonstrated significant MR and LV volume reduction in symptomatic patients with secondary MR receiving optimal medial therapy at 1-year follow-up in 73 of the 87 (84%) device-implanted patients, resulted in the device’s inclusion in European Society of Cardiology guidelines. The Carillon device is currently being investigated in the EMPOWER study (NCT03142152), a blinded randomized trial comparing the Carillon device with a sham-controlled group in patients with symptomatic HF with at least mild functional MR.
In contrast, the direct annuloplasty devices use anchors that directly attach to the mitral valve annulus. Compared with the indirect devices, these devices usually carry higher risk and are more challenging to place given the higher complexity of navigating the left atrium. Cardioband (Edwards Lifesciences) is a transfemoral–transseptal system. In the CE Mark Cardioband Trial, 61 patients with secondary MR were enrolled and followed for 2 years. The survival rate was 79%, with 96% of patients having an MR grade of ≤2+ at 2-year follow-up. There was also a 9% reduction in mitral annular area, and 83% of patients were New York Heart Association (NYHA) class I or II, which translates to improved 6-minute walk tests at 2-year follow-up.35
Transcatheter Mitral Valve Replacement
There are no FDA-approved transcatheter mitral valve replacement (TMVR) systems. At this time, 10 different platforms are being evaluated in clinical trials for safety and efficacy (Table 2). Seven of these devices have been developed into fully percutaneous transfemoral–transseptal platforms. The main issue with TMVR is the risk of developing LVOT obstruction (LVOTO). The interactions of many factors influence the potential development of LVOTO, including the aorto-mitral angle, septal hypertrophy, the size and shape of the left ventricle, and anterior mitral valve leaflet size. Additionally, each device has specific angulation and positioning that require careful preprocedural simulation to estimate the risk of LVOTO development.
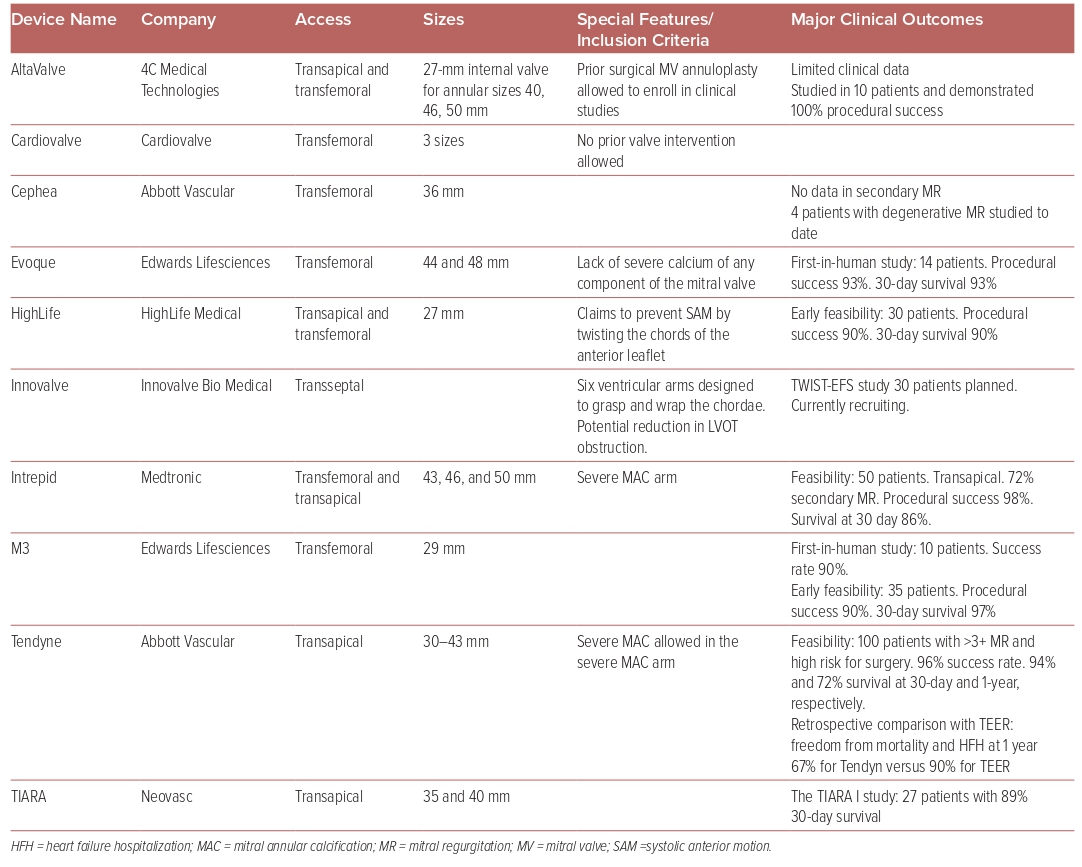
A gated cardiac CTA is essential in the evaluation of the mitral valve anatomy and measurement of the neo-LVOT to predict the degree of LVOTO. The simulated neo-LVOT (which is formed by the interventricular septum anteriorly and the displaced anterior mitral valve leaflet posteriorly) is used as a surrogate to estimate the LVOTO risk. A neo-LVOT area of 1.7–1.9 cm2 at end systole has been largely used as a cutoff to identify those cases in which LVOTO risk is low when using closed cell bioprosthesis (i.e. Tendyn and S3), but the optimal cutoff is not yet well understood for bioprosthesis with hybrid cell technology. Additionally, a mean measurement of the neo-LVOT area throughout the cardiac cycle may be useful, but has not been validated.36
Of the TMVR systems the most studied are the Tendyne and the Intrepid valve systems. The Tendyne mitral valve system (Abbott Medical) is a transapical TMVR system, which has been evaluated in severe MR patients of which 89% had secondary or mixed MR. All-cause mortality in 2 years was 39.0%. Moreover, 93% of the patients had no MR, and there were sustained improvements in the HFH rate, NYHA class, and LV ejection fraction.37
A retrospective analysis comparing TMVR with Tendyne valve to TEER with MitraClip have shown higher freedom from all-cause mortality and HFH at 1 year with TEER (TEER: n=45 [90%]; HR 11.26; 95% CI [10.59–11.93] versus TMVR: n=39 [67.4%]; 95% CI [10.09–11.33]; p=0.008).38 The ongoing SUMMIT trial (NCT03433274) is investigating the Tendyne system against TEER with the MitraClip system in a randomized fashion.
The Intrepid TMVR system (Medtronic) system is also transapical, with a new generation that is delivered through the transfemoral–transseptal approach. The Intrepid Global Pilot Study reported experience in the first 50 patients; successful implantation was achieved in 48 patients (96%). The 30-day mortality was 14%, with no disabling strokes or repeat interventions.39 The investigational Intrepid TMVR system was also evaluated in prohibitive or high surgical risk with moderate-to-severe MR. Implants were successful in 14 out of the 15, and there were no deaths, strokes, or reinterventions at 30 days, with the majority of patients having trace or no valvular or paravalvular MR. However, 11 (73%) of the cases required closures for the iatrogenic atrial septal defect.40 The Apollo trial (NCT03242642) is randomizing patients eligible for surgery 1:1 to TMVR versus surgery. Those who are ineligible for surgery will be included in a non-randomized registry.41
Additionally, a recent study comparing medical therapy from the COAPT medical therapy arm with TMVR using a dedicated TMVR device from the CHOICE-MI trial showed better MR resolution with TMVR. At 1 and 2 years, residual MR was ≤1+ in all patients of the TMVR group compared with 6.9% and 7.7% in the medical therapy group, respectively. Furthermore, the 2-year rate of heart failure hospitalization was significantly lower in the TMVR group (32.8% versus 54.4%; HR 0.59; 95% CI [0.35–0.99]; p=0.04). However, 2-year mortality was similar in the two groups (TMVR versus medical therapy, 36.8% versus 40.8%; HR 1.01; 95% CI [0.62–1.64]; p=0.98).42
In a retrospective study, Simard et al. compared TMVR with redo surgical mitral valve replacement in patients with degenerated mitral prostheses. In the TMVR group, 87% were treated with valve-in-valve and 13% with valve-in-ring. The TMVR group was an older and sicker population. The 30-day mortality was lower, with TMVR versus surgical mitral valve replacement 2.4% versus 10.2% (OR 4.69; 95% CI [1.25–30.5]), but the 5-year mortality was 49.9% versus 34.0%. In patients with prosthetic mitral valve deterioration, TMVR might be preferred in the short term, especially with a sicker patient population, whereas surgical mitral valve replacement will provide durable results, especially in patients with a lower burden of comorbidities.43
The MITRAL trial is a prospective study evaluating SAPIEN XT/3 valves (Edwards Lifesciences) in three treatment arms: valve-in-mitral annular calcification, mitral valve-in-ring, and mitral valve-in-valve. The study included patients with severe mitral stenosis or regurgitation with NYHA class II or higher symptoms and high surgical risk. There was a dramatic benefit in terms of echocardiographic parameters, heart failure symptoms, and improved quality of life. Soberingly, the mitral valve-in-ring and valve-in-mitral annular calcification arms had a staggering mortality of approximately 40–50% versus 6.7% in the mitral valve-in-valve arm. Additionally, the valve-in-mitral annular calcification arm had the highest stroke rate at 10.7% compared with the other two arms (6.7% mitral valve-in-valve, 3.3% mitral valve-in-ring). These procedures show high early mortality and risk profile, as was seen in the early stages of the PARTNER and TEER trials, which had iterative improvement with each subsequent study and technical modification.44
Surgical Treatment for Functional Mitral Regurgitation
Currently, the evidence to support mitral valve surgery for FMR remains weak. There is no evidence that surgical correction of chronic severe secondary MR prolongs life. However, a substudy from the STITCH trial showed that it is beneficial to address the MR at the time of coronary artery bypass grafting.45 Furthermore, when surgical intervention is indicated for secondary MR, a randomized clinical trial comparing mitral valve repair with mitral valve replacement showed that the rate of recurrence of moderate or severe MR over 2 years was higher in the repair group than in the replacement group, leading to a higher incidence of heart failure and repeat hospitalization.46,47
Conclusion
Medical versus invasive? Transcatheter versus surgical? Repair versus replacement? Those are the questions that medical professionals face when evaluating patients with severe symptomatic functional MR. Unfortunately, there are no straight answers to these questions at this time.
Management of these patients remains complex, which highlights the importance of heart teams that are composed of highly skilled and experienced cardiac surgeons, interventional cardiologists, cardiac imagers, anesthesiologists, and now advanced heart failure specialists. The management of these complex patients starts on a bedrock of appropriately titrated goal-directed medical therapy.
Currently, TEER is the only device that shows improved survival when compared with guideline-directed medical therapy for carefully selected patients, while isolated surgery has the least supporting evidence. Retrospective analyses failed to show added survival benefit of TMVR when compared with TEER or guideline-directed medical therapy. Atrial functional MR is a more recently described subtype, which has gained a better understanding in the past few years. It stems from left atrial enlargement with AF and often with preserved LV function without leaflet degeneration. Newer data in this subtype show that MitraClip significantly improves the MR grade and symptoms, which is followed by reverse remodeling of the left atrium and mitral annulus.48
Finally, all the ongoing device trials emphasize the importance of guideline-directed medical therapy of the underlying heart failure.