According to the International Society for Heart and Lung Transplantation 2015 registry update, only 3.3% of adult heart transplants between 2009 and 2014 were for congenital heart disease (CHD).1 This may be due in part to a higher early 30-day mortality in CHD patients compared with non-CHD patients.2 The higher mortality in adults with CHD undergoing orthotopic heart transplantation (OHT) is the result of numerous factors, including surgical complexity and end-organ dysfunction, frailty and arrhythmias that present late in the disease process.
We report the case of a patient with complex CHD who underwent OHT at our centre and who had an extremely rare haemorrhagic complication. Permission was obtained from the patient’s family for the publication of this article.
Initial Medical History
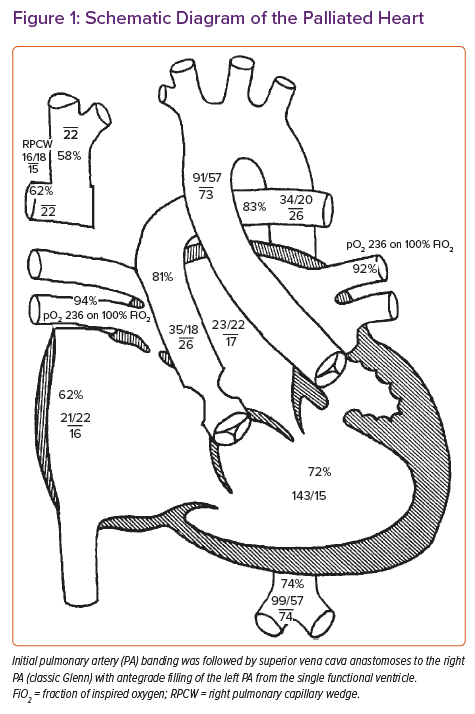
The patient was a 43-year-old woman with complex CHD and end-stage heart failure. With regard to the anatomy, the patient had a double-inlet left ventricle, with the aorta arising from the hypoplastic right ventricle. The patient was initially palliated with a pulmonary artery (PA) band, and later the superior vena cava (SVC) was anastomosed to the right PA (classic Glenn) with antegrade filling of the left PA from the single functional ventricle (Figure 1).
Details and an explanation of why the patient had never proceeded to total cavopulmonary connection (palliated as a Fontan) were not known. The patient had chronically elevated SVC pressures (20–24 mmHg), leading to decompression of the SVC to the inferior vena cava and pulmonary vasculature by the collateral veins, flow reversal in the azygous system, and pulmonary arteriovenous malformations. These venous collaterals, including a collateral vein traversing immediately posterior to the sternum, put the patient at prohibitive risk of bleeding during OHT, and she was ultimately deemed a non-candidate at other centres due to the surgical complexity despite the preserved end-organ function and favourable human leukocyte antigen antibody status (21% Class I panel reactive antibodies [PRA] and 0% Class II PRA; non-complement fixing). The patient was brought to our centre for full OHT evaluation.
Due to chronic hypoxia (baseline saturations 75–80%) the patient was polycythaemic (haemoglobin 24 g/dl). In addition, her saturations worsened with recurrent paroxysmal, but haemodynamically unstable, symptomatic atrial arrhythmias. Supraventricular tachycardia remained refractory to multiple cardioversions and treatment with amiodarone.
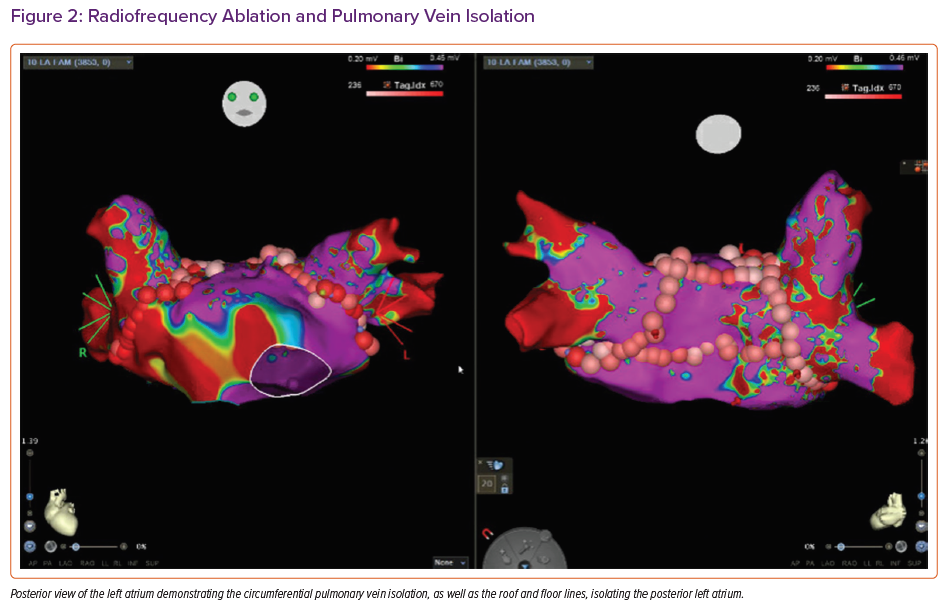
The best course of action to stabilise the patient was deemed to be radiofrequency ablation (RFA). The procedure involved left atrial (LA) pulmonary vein isolation, posterior LA isolation, cavotricuspid isthmus ablation and ablation along the septal atriotomy for atrial arrhythmias (Figure 2). Despite the RFA, the patient continued to have frequent arrhythmias, severe shortness of breath and hypoxaemia. It was the decision of a multidisciplinary team from multiple institutions that the best course of action would be to move forward with urgent OHT at our institution.
Pre-transplant Hospital Course
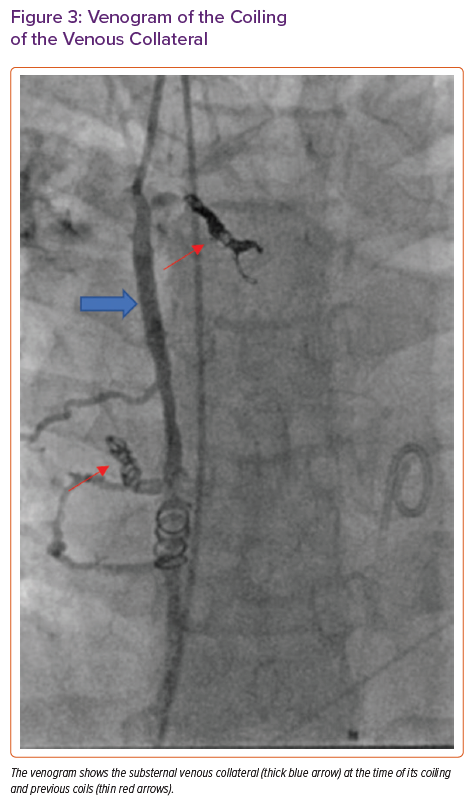
Six days after the RFA, the patient was admitted to our centre and underwent successful coiling of the large vessel immediately posterior to the sternum (Figure 3) in the paediatrics cardiac catheterisation lab to reduce her risk of bleeding on surgical entry for OHT.
Cardiac catheterisation demonstrated elevated SVC pressure (22 mmHg), a mean right PA pressure of 22 mmHg and a mean left PA pressure of 26 mmHg, with a calculated cardiac output (Qs) of 3.51 l/min (cardiac index, 2.31 l/min/m2; Figure 1).
Chest CT angiography showed severe stenosis just distal to the pulmonic valve with post-stenotic dilatation, a dilated inferior vena cava draining into the right atrium and a venous collateral immediately posterior to the sternum. The patient was subsequently listed for OHT.
Post-transplant Hospital Course
Twenty-eight days after ablation and 7 days after the listing for transplantation, the patient underwent OHT with a sex-matched donor allograft with bicaval anastomosis, with the recipient’s LA cuff including pulmonary veins anastomosed to the donor’s LA. No abnormalities were noted of the LA posterior wall at the time of transplantation. The ischaemic time was 252 minutes. The patient did not require any packed red blood cell transfusions, and post-transplant trans-oesophageal echocardiography (TOE) confirmed normal biventricular function.
After transplantation, the oxygenation saturations improved to above 90% and the patient was extubated on postoperative day (POD) 1. The patient was transferred out of the intensive care unit in a stable condition on POD 5. Pending discharge, the patient underwent a screening nasopharyngeal swab for COVID-19 on POD 10. After the swab, the patient experienced mild epistaxis that self-resolved.
The following morning the patient had large-volume haematemesis, leading to cardiopulmonary arrest with pulseless electrical activity. The initial aetiology of the massive haematemesis was believed to be swab-related trauma to chronically dilated (due to the single-ventricle physiology) venous structures in the posterior nasopharynx; however, the actual aetiology was confirmed later. The patient underwent emergency cannulation at the bedside for veno-arterial extracorporeal membrane oxygenation (VA-ECMO) and was taken to the operating theatre for LA venting along with exploration of persistent haematemesis. Interestingly, the bleeding resolved almost immediately once the LA was vented.
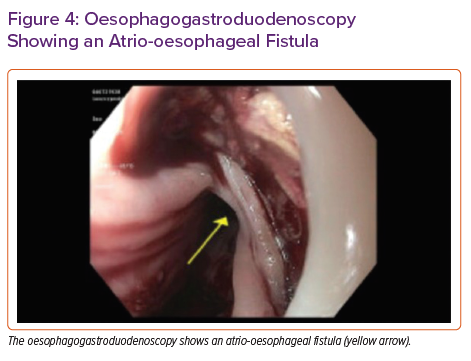
The otolaryngology team performed an emergency nasopharyngoscopy to rule out bleeding from the nasopharynx. The gastroenterology team performed an emergency oesophagogastroduodenoscopy (OGD) in the operating theatre. Immediately following oesophageal insufflation, air was noted in the arterial cannula of the VA-ECMO circuit with a drop in flow. This finding prompted an evaluation for a fistula capable of explaining air in the ECMO circuit due to insufflation. A large LA–oesophageal fistula was identified (Figure 4), which was repaired by oversewing the LA and then placing a 23 × 125 Wallflex oesophageal stent. Despite initial cardiac pulsatility, in the hours following, this worsened as it became evident that the patient had had an MI as a probable result of air in the coronaries. The patient was also noted to have had ischaemia on head CT. Ultimately, the patient’s condition was deemed unrecoverable. After discussion with her family, the patient’s care was withdrawn.
Discussion
We report a case of post-RFA atrio-oesophageal fistula, a rare but often fatal complication. The reported incidence of atrio-oesophageal fistula after RFA in the general population ranges from <0.01% to 0.25%, and it usually occurs at a median of 19.3 days after the procedure.3,4 Although post-RFA atrio-oesophageal fistula is well-described, this case is unique because the patient had RFA performed on her native heart and the complication occurred after OHT, with a seemingly ‘new’ heart.
However, it should be highlighted that in the present case the patient’s native atrial cuff was anastomosed to the donor heart during the most common bicaval anastomosis surgical technique of OHT. Hence, the cuff had undergone an ablation. As such, we hypothesise that the patient had experienced atrial and oesophageal tissue damage during the RFA procedure, which progressed to a fistula over the following 3 weeks. It is also possible that the already friable atrial cuff tissue of the native heart was further exacerbated during the transplant surgery, increasing the odds of such a fistula formation.
For patients in whom atrio-oesophageal fistula is suspected, any oesophageal manipulation should be avoided (TOE, nasogastric tube placement etc.), and in particular insufflation, given that it will lead to LA air and resultant stroke or coronary embolisation. Unfortunately, in the present case none of the workup prior to the OGD was suggestive of atrio-oesophageal fistula.
End-stage single-ventricle patients frequently require extensive atrial ablations for symptomatic and haemodynamically significant arrhythmias. The present patient’s unfortunate outcome raises the question of whether, prior to OHT, routine surveillance imaging such as chest CT or direct visualisation with OGD should be obtained in patients who have undergone RFA in the past month to rule out oesophageal trauma.
Furthermore, despite a well-planned and thorough ablation, the present patient still continued to have atrial arrhythmias, highlighting the challenge in the treatment of patients such as this. As such, it is of the utmost importance to recognise rhythm disturbances as a warning sign of the potential need of evaluation for advanced therapies.