Abstract
Introduction: Sarcomatoid renal cell carcinoma is a rare and aggressive kidney tumor with poor prognosis and distinct microscopic features. Traditional modalities versus new targeted therapy are used in different patients with controversial but some promising results.
Method: A 73-year-old man was referred with right flank pain and hematuria. Computed tomography (CT) scan reported a solid mass measuring 62x100 mm in the posterior right kidney with perinephric infiltration. Multiple tumor nodules were present in perinephric fat. A metastatic mass measuring 21x33 mm in the right adrenal gland with a few metastatic lymph nodes measuring 18 mm in the paraaortic, renal hilum, and right retro crural space were also present.
Result: Radical nephrectomy was done. The cut section of the kidney revealed firm, homogeneous gray- yellowish huge mass measured 13.5 cm. The pathologist reported:" Renal cell carcinoma, Sarcomatoid type, High grade (4/4) invading perirenal fat with a greatest diameter of 13.5 cm and free surgical and vascular margin. IHC was done with CK, Vimentin, and CD10 positive and EMA, CK7, Desmin, Myogenin, and E-Cadherin negative". Unfortunately the patient passed away 25 days later in the background of lung and cardiac failure.
Conclusion: Pathologists and clinicians should be familiar by this rare, but aggressive, entity for sooner diagnosis and treatment. Different treatments should be assessed for better service to the patient.
Introduction
Renal cell carcinoma (RCC) is one of the most common malignancies of visceral organs in individuals aged 50 to 701, 2. Sarcomatoid RCC (sRCC) represents a transformation of RCC characterized by the presence of spindle-shaped cells, high cellularity, atypia, increased mesenchymal differentiation, and loss of epithelial features. It can occur in all subtypes of RCC. These features are most commonly observed in chromophobe RCC (8.7%) compared to clear cell RCC (5.2%) and papillary RCC (1.9%)1, 3, 4. Approximately 20% of sRCC cases are associated with metastases to sites such as the lymph nodes, lungs, and liver, significantly reducing the survival rate to less than one year5, 6. The most prevalent symptoms of sRCC include abdominal pain and hematuria7. sRCC is more commonly diagnosed in males (M/F ratio: 1.3-2/1)1, 2, 5. This article describes a significant case of sRCC with metastasis to the right adrenal gland, paraaortic lymph nodes, renal hilum, and right retrocrural space in a 73-year-old male.
Case Report
A 73-year-old man was referred on July 16, 2017, due to persistent right flank pain over two months and hematuria lasting two days. The hematuria was painful and contained blood clots. Symptoms also included nausea, urinary frequency, nocturia, and dribbling. He reported significant weight loss, anorexia, bone pain, dyspnea, and cough. His medical history noted chronic obstructive pulmonary disease (COPD) and cigarette smoking. Both of his nieces had undergone kidney transplants, as mentioned in his family history. During the physical examination, his vital signs were stable, with mild generalized wheezing and right costovertebral angle tenderness observed. A CT scan on July 3 reported moderate left pleural effusion with mild right pleural effusion and pericardial effusion. A solid mass measuring 62x100 mm in the posterior right kidney with perinephric infiltration and multiple tumor nodules in the perinephric fat were visible. Central necrosis in the masses and adhesion to the right superior psoas muscle were present. A metastatic mass measuring 21x33 mm in the right adrenal gland and several metastatic lymph nodes measuring 18 mm in the paraaortic, renal hilum, and right retrocrural space were noted. In the left liver lobe's internal segment, a mass measuring 56x80 mm with peripheral nodular enhancement suggested a cavernous hemangioma, but renal mass metastasis could not be excluded. MRI with contrast for the hepatic lesion was recommended. No venous thrombosis or bile duct dilation was observed. The spleen, left kidney, left adrenal, and pancreas were unremarkable.
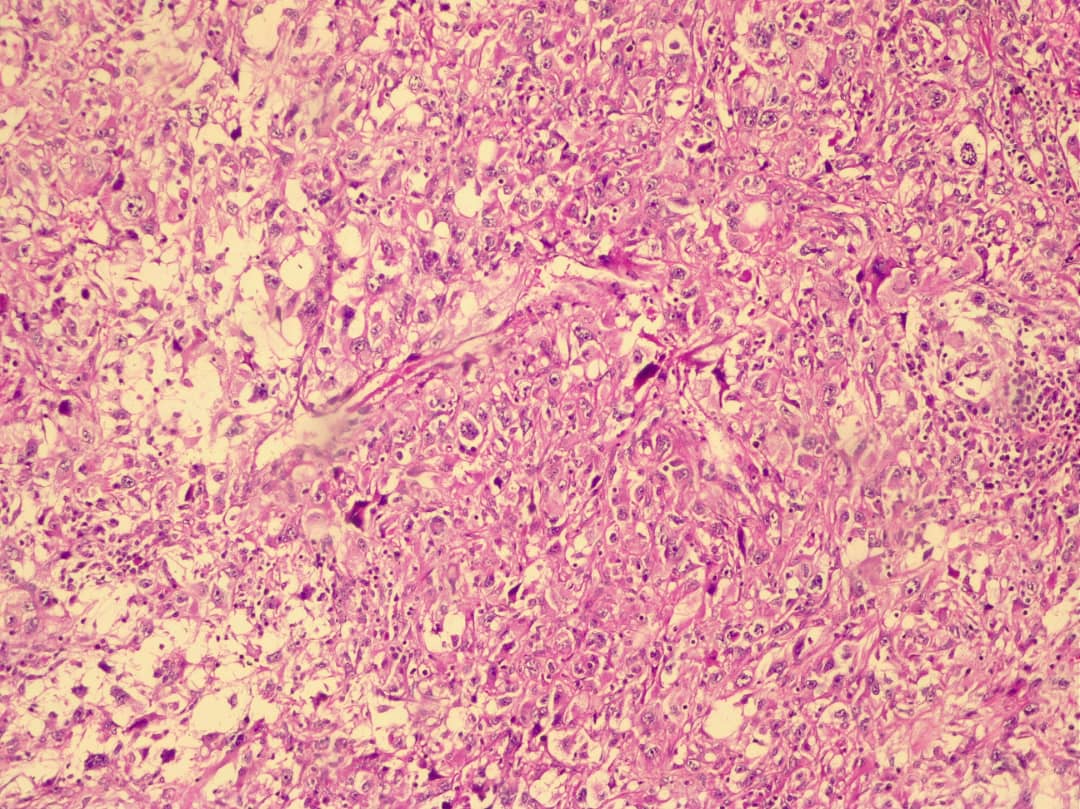
A right radical nephrectomy was performed on July 16 for a metastatic tumor involving the right kidney, aortic and vena cava lymph nodes, with adhesion and invasion into the abdominal wall muscle. The specimen sent to pathology consisted of a kidney tissue piece measuring 15.5x5 cm and several tan-brown separate pieces measuring 9x5x4 cm. The cut section of the kidney revealed a firm, homogeneous, gray-yellowish mass measuring 13.5 cm. The pathologist reported: "Renal cell carcinoma, Sarcomatoid type (Figure 1), high grade (4/4) invading perirenal fat with the largest diameter of 13.5 cm and clear surgical and vascular margins. Immunohistochemical (IHC) staining was positive for CK, Vimentin, and CD10 (Figure 2), and negative for EMA, CK7, Desmin, Myogenin, and E-Cadherin." The patient was assessed by a cardiologist as a case of congestive heart failure and managed accordingly. His ejection fraction was 35%. On the day of the operation, he experienced respiratory distress, and pleural effusion was suggested. His condition gradually deteriorated, and he was intubated. On July 30, 2017, a lung physician evaluated him for lung metastasis and pleural effusion, ordering a spiral lung CT scan. Bilateral pleural effusions with a loculated appearance on the left and passive lung collapse mainly on the right were noted, along with emphysematous changes in both lungs. Thinning of the right lower lobe bronchus and distal obstruction, as well as thickening of the interlobular septa in the left lingual lobe and right lower lobe, suggested carcinomatous lymphangiitis. On August 3, 2017, he was admitted to the ICU, and a bilateral chest tube was placed. The patient was unconscious with a Glasgow Coma Scale (GCS) score of 3-4/15. He required dialysis, but it was not feasible due to his shock state. On August 11, 2017, he suffered a cardiopulmonary arrest and despite resuscitation efforts, he expired 40 minutes later. Written consent for the case report was obtained from the family.
Discussion
Sarcomatoid renal cell carcinoma (sRCC) is a rare dedifferentiation of RCC observed across all subtypes, associated with poor prognosis and aggressive behavior6, 8. It exhibits specific histological features including spindle cells, sarcomatoid characteristics (compartments containing fibrous, leiomyomatous, rhabdoid, osteoid, or chondroid materials), pleomorphism, and high cellularity1, 5. sRCC often presents with coagulative necrosis (90%) and, less frequently, with microvascular invasion and rhabdoid features (15%)1. Macroscopically, sRCC is characterized by necrosis, hemorrhage, large size, and a firm texture with yellowish or gray solid lesions1, 2.
The symptoms of sRCC vary at presentation. Common clinical signs include flank pain (51%), hematuria (22-34.7%), weight loss (18-22.6%), fever (6-10.6%), fatigue (15%), night sweats (6-12.6%), and cough or dyspnea (6%). In this patient, symptoms included right flank pain, hematuria, weight loss, nocturia, and bone pain1, 5.
The epithelial-mesenchymal transition (EMT) in sRCC, a morphological shift between mesenchymal and epithelial cells, facilitates proliferation and metastasis, often resulting in high-stage diagnoses at presentation4, 5. Common metastasis sites include the lungs, bones, lymph nodes, and brain1. In this case, metastases involved the right adrenal gland, paraaortic lymph nodes, renal hilum, right retrocrural space, and carcinomatous lymphangitis in the lungs. It is noteworthy that the WHO and the International Society of Urologic Pathology (ISUP) classify sRCC as a high-grade tumor (grade 4) in the TNM classification system8. The high-grade classification of sRCC is often attributed to the absence or interaction of factors like ARID1A and BAP19. These characteristics contribute to a median survival time of less than one year at every stage, compared to non-sarcomatoid RCC1, 9.
sRCC's incidence varies by gender, being more prevalent in males. Possible reasons for this gender differentiation include smoking habits, the effects of sex hormones, and occupational exposure1, 5.
Immunohistochemical markers often found positive in sRCC include AE1/AE4, epithelial membrane antigen, and vimentin, while markers like CK7, actin, desmin, and S-100 are typically negative2, 7. In this patient, IHC was positive for CK, vimentin, and CD10, and negative for EMA, CK7, desmin, myogenin, and E-cadherin.
Mutations commonly observed in sRCC include TP53 (42%), VHL (35%), CDKN2A (27%), and NF2 (19%)3.
Therapeutic approaches for sRCC vary. Identification via biopsy and radiography aids in selecting appropriate treatments9. However, the absence of specific clinical features or limited sarcomatoid areas may render these diagnostic methods less effective9. The recommended treatments include surgery and systemic therapy9. In cases with metastasis or where surgery is not available, systemic therapy using targeted agents and immune checkpoint inhibitors is beneficial9. These treatments can restrict tumor growth, with agents like VEGF inhibitors being particularly effective against metastatic RCC9. Combining VEGF with Trebananib, an Ang/Tie-2 pathway inhibitor, can enhance the effectiveness of blocking vessel formation10. Other effective treatments include combinations of sunitinib with gemcitabine or gemcitabine with doxorubicin3.
Immunotherapy, particularly using interferon-alpha (IFN-α) and interleukin-2 (IL-2), or targeting biomarkers like PD-L1, is another recommended treatment approach8, 11. Cytoreductive nephrectomy can be attempted, but outcomes are not always favorable8. A nephrectomy was performed in our case, but unfortunately, it did not save the patient's life.
Conclusion
Sarcomatoid RCC (sRCC) represents a transformation of RCC characterized by spindle-shaped cells, high cellularity, atypia, increasing mesenchymal differentiation, and loss of epithelial features. sRCC is a rare and aggressive kidney tumor associated with a poor prognosis. Metastases to organs such as the lungs and liver are common, resulting in a median survival time of less than a year after diagnosis. Pathologists and clinicians should be familiar with this rare but aggressive entity to enable earlier diagnosis and treatment. Various treatments should be evaluated to enhance patient care.
Abbreviations
AE1/AE4: Pan-cytokeratin markers, ARID1A: AT-rich interaction domain 1 A, BAP1 : BRCA1 associated protein-1, CDKN2A: Cyclin-Dependent Kinase Inhibitor 2A, CK: Cytokeratin, CK7: Cytokeratin 7, COPD: Chronic Obstructive Pulmonary Disease, CT: Computed Tomography, EMA: Epithelial Membrane Antigen, GCS: Glasgow Coma Scale, ICU: Intensive Care Unit, IFN-α: Interferon-alpha, IL-2: Interleukin-2, IHC: Immunohistochemical, ISUP: International Society of Urologic Pathology, MRI: Magnetic Resonance Imaging, NF2: Neurofibromin 2, PD-L1: Programmed Death-Ligand 1, RCC: Renal Cell Carcinoma, sRCC: Sarcomatoid Renal Cell Carcinoma, TNM: Tumor, Node, Metastasis (a staging system), TP53: Tumor Protein P53, VEGF: Vascular Endothelial Growth Factor, VHL: Von Hippel-Lindau gene, WHO: World Health Organization
Acknowledgments
We thank the Clinical Research Development Center of Imam Reza Hospital for Consultation.
Author’s contributions
All authors significantly contributed to this work, read and approved the final manuscript.
Funding
None.
Availability of data and materials
Data and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The article is written according to the orders of ethics committee of KUMS.
Consent for publication
Written informed consent was obtained from the family of the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing interests.
References
-
Blum
K.A.,
Gupta
S.,
Tickoo
S.K.,
Chan
T.A.,
Russo
P.,
Motzer
R.J.,
Sarcomatoid renal cell carcinoma: biology, natural history and management. Nature Reviews. Urology.
2020;
17
(12)
:
659-78
.
View Article PubMed Google Scholar -
Neşiu
A.,
Neam\ctu
C.,
Nicolescu
C.M.,
Totolici
B.D.,
Mureşanu
H.D.,
Roşu
M.C.,
Sarcomatoid renal cell carcinoma with clear cells and eosinophilia: a case report and short review of the literature. Romanian Journal of Morphology and Embryology.
2021;
62
(3)
:
835-9
.
View Article PubMed Google Scholar -
Pichler
R.,
Compérat
E.,
Klatte
T.,
Pichler
M.,
Loidl
W.,
Lusuardi
L.,
Renal cell carcinoma with sarcomatoid features: finally new therapeutic hope?. Cancers (Basel).
2019;
11
(3)
:
422
.
View Article PubMed Google Scholar -
Miolo
G.,
Ash
A.,
Buonadonna
A.,
Re
G. Lo,
Torrisi
E.,
Cervo
S.,
Grade 4 unclassified renal cell carcinoma with sarcomatoid component expressing S-100 protein. A case report with peculiar diagnostic and therapeutic implications. Cancer Biology & Therapy.
2014;
15
(11)
:
1439-43
.
View Article PubMed Google Scholar -
Warli
S.M.,
Andy
A.,
Mariedina
C.T.,
Nasution
R.,
Kadar
D.D.,
A Rare Case of Sarcomatoid Renal Cell Carcinoma in a Young Adult Patient. Research and Reports in Urology.
2022;
14
:
241-5
.
View Article PubMed Google Scholar -
Liao
X.,
Abu-Farsakh
S.H.,
Zhang
D.,
Sarcomatoid renal cell carcinoma with unusual metastasis to the small intestine manifesting as perforated appendicitis. in vivo.
2019;
33
(6)
:
2225-8
.
-
Golshayan
A.R.,
George
S.,
Heng
D.Y.,
Elson
P.,
Wood
L.S.,
Mekhail
T.M.,
Metastatic sarcomatoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. Journal of Clinical Oncology.
2009;
27
(2)
:
235-41
.
View Article PubMed Google Scholar -
Oudad
F.,
Karam
R.,
Tawfiq
N.,
Jouhadi
H.,
Aboutaieb
R.,
Karkouri
M.,
Sarcomatoid Renal Cell Carcinoma, or Renal cell carcinoma with sarcomatoid dedifferentiation: review of literature. Translational Oncology and Therapeutics..
2023;
1
(1)
:
1-5
.
-
Liang
X.,
Liu
Y.,
Ran
P.,
Tang
M.,
Xu
C.,
Zhu
Y.,
Sarcomatoid renal cell carcinoma: a case report and literature review. BMC Nephrology.
2018;
19
(1)
:
84
.
View Article PubMed Google Scholar -
Makhov
P.,
Joshi
S.,
Ghatalia
P.,
Kutikov
A.,
Uzzo
R.G.,
Kolenko
V.M.,
Resistance to systemic therapies in clear cell renal cell carcinoma: mechanisms and management strategies. Molecular Cancer Therapeutics.
2018;
17
(7)
:
1355-64
.
View Article PubMed Google Scholar -
Barata
P.C.,
Rini
B.I.,
Treatment of renal cell carcinoma: current status and future directions. CA: a Cancer Journal for Clinicians.
2017;
67
(6)
:
507-24
.
View Article PubMed Google Scholar
Comments
Article Details
Volume & Issue : Vol 11 No 3 (2024)
Page No.: 6263-6267
Published on: 2024-03-31
Citations
Copyrights & License

This work is licensed under a Creative Commons Attribution 4.0 International License.
Search Panel
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Google Scholar
Pubmed
Search for this article in:
Google Scholar
Researchgate
- HTML viewed - 318 times
- PDF downloaded - 152 times
- XML downloaded - 17 times
 Biomedpress
Biomedpress



