摘要: 目的:通过建立大鼠下腔静脉血栓模型,并予氯吡格雷干预,探究其对静脉管壁血小板–单核细胞集合体水平及纤维化程度的影响。方法:将24只SD雄性大鼠随机分为假手术组、模型组、低剂量(10 mg/kg)干预组、高剂量(30 mg/kg)干预组,每组6只。对模型组和低、高剂量干预组建立下腔静脉血栓模型,造模成功后予以药物干预。假手术组和模型组予以生理盐水灌胃,低、高剂量干预组分别予以氯吡格雷10 mg/kg、30 mg/kg灌胃,每天1次,7天后取大鼠下腔静脉组织进行Masson染色、CD115免疫组化染色和CD41-CD115免疫荧光双染。结果:各组纤维化面积比例比较,差异有统计学意义(F = 132.0, P < 0.01),各组CD115荧光强度表达差异有显著性(F = 314.7, P < 0.01),各组CD41-CD115双阳率差异有显著性(F = 147.3, P < 0.01);模型组纤维化面积比例、CD115荧光强度表达、CD41-CD115双阳率明显高于假手术组(P < 0.01),氯吡格雷低、高剂量干预组三种指标水平均明显低于模型组(P < 0.01),氯吡格雷高剂量干预组三种指标水平均明显低于低剂量干预组(P < 0.05)。结论:深静脉血栓环境下,大鼠下腔静脉管壁血小板–单核细胞集合体水平、单核巨噬细胞浸润程度、纤维化程度升高,抗血小板药物氯吡格雷可能降低炎症、缓解管壁纤维化。
Abstract:
Objective: To establish a rat model of inferior vena cava thrombosis and intervene with clopidogrel to explore its effects on platelet monocyte aggregation levels and fibrosis degree in the venous wall. Method: 24 male SD rats were randomly divided into a sham surgery group, a model group, a low-dose (10 mg/kg) intervention group, and a high-dose (30 mg/kg) intervention group, with 6 rats in each group. Establish inferior vena cava thrombosis models for the model group and low-dose and high-dose intervention groups, and administer medication intervention after suc-cessful modeling. The sham surgery group and model group were given physiological saline by ga-vage, while the low-dose and high-dose intervention groups were given clopidogrel 10 mg/kg and 30 mg/kg respectively, once a day. After 7 days, the inferior vena cava tissue of rats was taken for Masson staining, CD115 immunohistochemistry staining, and CD41-CD115 immunofluorescence double staining. Result: The proportion of fibrotic area in each group was compared, and the dif-ference was statistically significant (F = 132.0, P < 0.01). There was a significant difference in the expression of CD115 fluorescence intensity in each group (F = 314.7, P < 0.01), and there was a sig-nificant difference in the double positive rate of CD41-CD115 in each group (F = 147.3, P < 0.01); the proportion of fibrosis area, CD115 fluorescence intensity expression, and CD41-CD115 double posi-tive rate in the model group were significantly higher than those in the sham surgery group (P < 0.01). The levels of three indicators in the low-dose and high-dose intervention groups of clopidogrel were significantly lower than those in the model group (P < 0.01), and the average levels of three indicators in the high-dose intervention group of clopidogrel were significantly lower than those in the low-dose intervention group (P < 0.05). Conclusion: In the environment of deep vein thrombosis, the levels of platelet monocyte aggregates, degree of monocyte-macrophage infiltration, and degree of fibrosis in the inferior vena cava wall of rats increase. The antiplatelet drug clopidogrel may reduce inflammation and alleviate wall fibrosis.
1. 引言
在全球范围内,深静脉血栓(deep vein thrombosis, DVT)已经成为危害人类健康的一大心血管疾病 [1] 。若不及时干预,随着病情进展,接近一半的DVT患者会罹患晚期并发症,即深静脉血栓形成后综合征(post-thrombotic syndrome, PTS) [2] ,这可能暗示了在发展为血栓后综合征的过程中持续存在的静脉血栓引发的静脉壁变化,引起血管重塑 [3] 。相关实验和形态学研究表明,相较于对照组,下腔静脉狭窄大鼠血栓重量、白细胞浸润及白细胞分泌促炎细胞因子水平均明显升高成 [4] 。血栓刺激下血小板活化并改变形态,其活性代表因子如血小板因子4 (platelet factor 4, PF4/CXCL4)分泌增加 [5] 。本实验通过建立下腔静脉血栓模型,探究血栓病理环境中静脉管壁炎症反应程度,同时试验性引入抗血小板药物氯吡格雷来评估其对管壁炎症反应的影响。
2. 材料
2.1. 实验动物
清洁级Sprague-Dawley雄性大鼠(8周龄,280.0 ± 13.0 g)大鼠24只,购自北京华阜康实验动物技术有限公司,合格证号:SCXK (京) 2019-0008。饲养于青岛大学SPF级动物实验室。
2.2. 主要试剂
氯吡格雷(波立维,赛诺菲制药有限公司)、CD41单抗(ab134131,Abcam公司)、巨噬细胞集落刺激因子受体抗体(即CD115抗体) (ab183316,Abcam公司)、Masson三色染色改良试剂盒(北京博奥森生物技术有限公司)。
2.3. 主要仪器
显微图像分析系统(TE2000-U,日本奥林巴斯公司),全自动组织切片机(KH-Q330,湖北阔海医疗科技公司)。
2.4. 伦理声明
所有实验均由青岛大学伦理委员会根据《国家卫生研究院实验动物护理与使用指南》批准,批件编号:No. 20220908SD7220221031109。
3. 实验方法
3.1. 动物模型建立与分组
将大鼠饲养于青岛大学SPF级动物实验室,适应性喂养7天后,采用随机数表法将动物随机分为A:假手术组,B:模型组,C:低剂量氯吡格雷(10 mg/kg/d)干预模型组,D:高剂量氯吡格雷(30 mg/kg/d)干预模型组,每组6只。参考Ponomaryov等 [6] [7] 通过结扎下腔静脉诱导大鼠DVT,用6 g/L的戊巴比妥钠(35 mg/kg)腹腔注射麻醉,仰卧位固定于鼠板,备皮后消毒,经大鼠腹部正中切口开腹,将小肠牵向术野左侧,用温生理盐水浸湿的纱布包裹,显露下腔静脉,打开后腹膜,选择在左肾静脉下方2 mm处结扎下腔静脉,在显微镜辅助下用显微器械仔细游离下腔静脉,用3-0爱惜康缝线逐一显露和结扎左肾静脉以下的下腔静脉分支至髂静脉水平。将1根直径0.25 mm超滑导丝与下腔静脉用7-0聚丙烯缝线一并绑绕,再移除导丝,无齿镊顺序自结扎线向下钳夹下腔静脉6次。假手术组手术方法同上,但不作结扎,假手术组旨在消除手术相关的创伤影响。手术中有下腔静脉出血或任何损伤的小鼠被排除在进一步分析之外。造模后,假手术组和模型组予以生理盐水灌胃,低、高剂量干预组分别予以氯吡格雷10 mg/kg、30 mg/kg灌胃,每天1次,连续灌胃7天。
3.2. 标本采集
第7天取材,依次打开胸腹腔,首先由左心室置灌洗导管于升主动脉并结扎。开放右心耳后,以4℃的生理盐水进行循环冲洗,待灌洗液澄清后,于左肾静脉下结扎点取下腔静脉组织,置于40 g/L的多聚甲醛固定液中,4℃恒温环境中保存。
3.3. 标本处理
每例样本至少做3张切片,按照所提供说明书对切片进行Masson染色、CD115免疫组化染色、CD41-CD115免疫荧光双染,在光学显微镜下观察形态学特征,每张切片至少选择5个高倍镜视野(40×),以ImageJ软件进行图像定量分析,测管壁纤维化面积比例(Masson染色)、巨噬细胞表达(CD115免疫组化染色)、血小板–单核细胞集合体阳性率(CD41-CD115免疫荧光双染)。纤维化面积比例 = 静脉壁胶原沉积面积/总静脉壁面积。为保证数据近似正态分布,CD115平均荧光强度(mean fluorescence intensity, MFI)数据采用对数log10进行转换后分析。阳性率 = CD41-CD115双阳性细胞相对数量/CD41阳性细胞相对数量。
3.4. 统计学方法
应用GraphPad Prism 9.5统计学软件进行数据处理,计量资料结果以(
)形式表示,多组均数间的比较采用单因素方差分析,组间两两比较采用Tukey检验。以P < 0.05表示差异有统计学意义。
4. 结果
4.1. 各组纤维化面积比例比较
各组管壁纤维化面积比例比较,差异有统计学意义(F = 132.0, P < 0.01),模型组纤维化面积比例明显高于其他各组(P < 0.01),氯吡格雷高剂量组低于低剂量组(P < 0.01),见表1和图1。

Table 1. Comparison of fibrotic area proportions in each group (
, n = 24)
表1. 各组纤维化面积比例比较(
, n = 24)
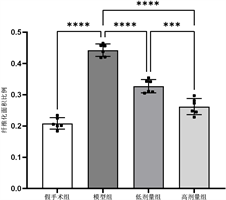
Figure 1. Comparison of fibrotic area proportions in each group
图1. 各组纤维化面积比例比较
4.2. 各组CD115平均荧光强度比较
各组静脉管壁CD115平均荧光强度表达差异有统计学意义(F = 314.7, P < 0.01),模型组荧光强度明显高于其他各组(P < 0.01),高剂量干预组低于低剂量干预组(P < 0.01),见表2和图2。

Table 2. Comparison of average fluorescence intensity of CD115 in each group (
, n = 24)
表2. 各组CD115平均荧光强度比较(
, n = 24)
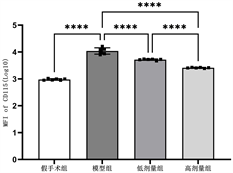
Figure 2. Comparison of average fluorescence intensity of CD115 in each group
图2. 各组CD115平均荧光强度比较
4.3. 各组CD41-CD115双染阳性率比较

Table 3. Comparison of CD41-CD115 double staining positive rates in each group (
, n = 24)
表3. 各组CD41-CD115双染阳性率比较(
, n = 24)
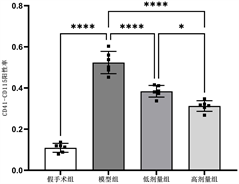
Figure 3. Comparison of CD41-CD115 double staining positive rates in each group
图3. 各组CD41-CD115双染阳性率比较
各组静脉管壁CD41-CD115双染阳性率差异有统计学意义(F = 147.3, P < 0.01),模型组双染阳性率明显高于其他各组(P < 0.01),高剂量干预组低于低剂量干预组(P < 0.01),见表3和图3。
5. 讨论
血栓形成后综合征(PTS)是深血栓形成或血栓溶解不完全而导致的长期后遗症 [8] ,20%~50%的DVT患者有罹患PTS的风险 [9] 。普遍认为,在血栓病理性状态下,炎症作用所导致的静脉管壁不可逆性损伤才是PTS的根源所在,在疾病中后期会使患者的生活质量严重下降 [10] 。
目前雄鼠模型是DVT模型常用实验动物,下腔静脉部位建模具有成栓率高的优势 [11] 。深静脉血栓从形成之初就伴随着管壁的慢性损伤,而损伤必定与炎症相伴,DVT的栓体和管壁中伴随炎症细胞的浸润和活化 [12] 。静脉血栓主要是由于血液在静脉血管中淤积,其成分和血液基本类似,既有血小板,又有红细胞、白细胞。在DVT中,血小板主要被招募为单个细胞,可以直接附着于白细胞形成小的异型聚集体 [13] ,如血小板–单核细胞集合体(platelet-monocyte aggregation, PMA),PMA以往被认为在急性冠状动脉综合征和动脉粥样硬化的发病机制中起作用 [14] 。Mandel等 [15] 学者研究内容提示沉积在血管损伤部位的血小板–白细胞聚集体的形成,是白细胞促进血栓形成和血小板促进炎症的重要机制。本研究结果显示造模后的大鼠管壁CD41-CD115双染阳性率远高于假手术组,即管壁血小板–单核细胞集合体水平明显升高。CD41系血小板抗原,巨噬细胞集落刺激因子受体又名CD115。CD115表达情况可以作为单核巨噬细胞浸润及慢性炎症程度 [16] ,实验结果显示造模后的大鼠管壁CD115荧光强度也远高于假手术组。
氯吡格雷可选择性不可逆地阻断二磷酸腺苷(adenosine diphosphate, ADP)和血小板P2Y12受体结合,达到抑制血小板聚集的作用 [17] ,是临床常用抗血小板药物。ADP受体是血小板活化时的主要受体,ADP受体拮抗剂(如氯吡格雷)能够抑制ADP诱导的血小板活化,从而减少血小板表面P-选择素和血小板膜糖蛋白特异性抗体(Glycoprotein IIb/IIIa, GPIIb/IIIa)的表达,使血小板与单核细胞之间的聚集明显减少 [18] 。本实验设置了氯吡格雷低、高剂量干预组,以了解不同剂量氯吡格雷干预下对实验指标的影响。本文研究结果也显示,氯吡格雷对大鼠血小板–单核细胞聚合有抑制作用,并且氯吡格雷干预显著减少了管壁纤维化面积,抑制了管壁重塑,高剂量干预组相比低剂量干预组效果更理想。前文所述,血小板可在血栓形成部位聚合单核巨噬细胞,从而形成PMA参与炎症反应,结合本实验结果,氯吡格雷干预模型组的静脉管壁CD41-CD115 (即血小板单核细胞集合体)水平和纤维化程度较模型组明显降低,本课题组推测抗血小板治疗对于静脉血栓管壁的益处来源于血小板介导下的单核巨噬细胞炎症的减轻。
综上所述,深静脉血栓伴随管壁的炎症浸润,抗血小板药物氯吡格雷可能抑制炎症、减轻纤维化。需要进一步研究以探索对静脉炎症的治疗靶点。
基金项目
青岛市科技计划项目(21-1-4-rkjk-13-nsh)。
NOTES
*第一作者。
#通讯作者。