中国组织工程研究 ›› 2024, Vol. 28 ›› Issue (1): 62-67.doi: 10.12307/2023.770
• 干细胞基础实验 basic experiments of stem cells • 上一篇 下一篇
血红素氧合酶1介导阿托伐他汀在巨噬细胞极化和胆固醇蓄积中的作用
邓 锐,黄科铭,罗 建 ,陈 功,冯 健 ,黄维义,魏 刚
- 西南医科大学附属医院心血管内科,四川省泸州市 646000
Effect of heme oxygenase-1-mediated atorvastatin on macrophage polarization and cholesterol accumulation
Deng Rui, Huang Keming, Luo Jian, Chen Gong, Feng Jian, Huang Weiyi, Wei Gang
- Department of Cardiology, Affiliated Hospital of Southwest Medical University, Luzhou 646000, Sichuan Province, China
摘要:

文题释义:
血红素氧合酶1:也称为热休克蛋白32,是血红素分解代谢的限速酶,主要催化游离血红素降解生成胆绿素、一氧化碳和亚铁,具有抗炎、抗氧化应激等作用。巨噬细胞极化:巨噬细胞受到局部微环境中不同刺激的调节和诱导转变为不同的表型,分泌不同的细胞因子以行使各自的功能,主要分为促炎的M1型和抗炎的M2型。
目的:探讨阿托伐他汀通过诱导血红素氧合酶1表达对氧化修饰型低密度脂蛋白刺激的RAW264.7巨噬细胞极化、炎症状态及胆固醇水平的影响和相关机制。
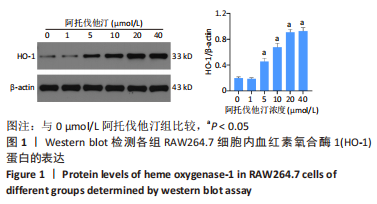
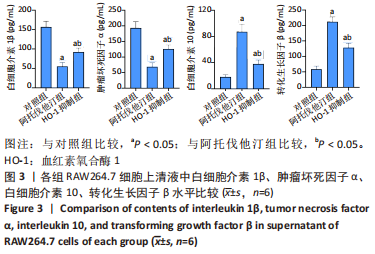
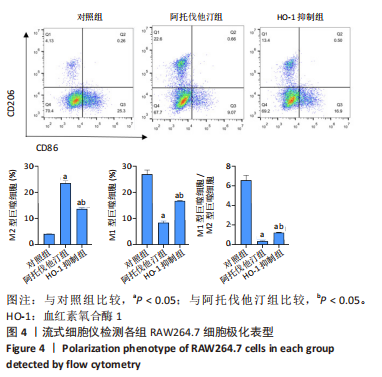
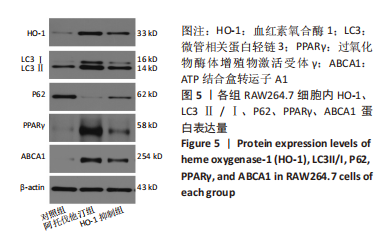
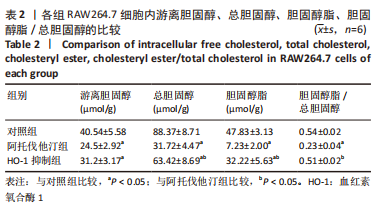
方法:先将体外培养RAW264.7细胞随机分为6组,分别给予不同浓度的阿托伐他汀孵育24 h,检测血红素氧合酶1蛋白表达量及细胞活性,摸索阿托伐他汀的最适剂量用于后续研究。另将RAW264.7细胞随机分为对照组、阿托伐他汀组、血红素氧合酶1抑制组,分别用纯培养基、阿托伐他汀20 μmol/L及阿托伐他汀20 μmol/L+锌原卟啉Ⅸ 10 μmol/L预先孵育细胞24 h,再加入50 mg/L的氧化修饰型低密度脂蛋白孵育48 h。流式细胞术检测巨噬细胞极化结果;ELISA法检测转化生长因子β、白细胞介素10、白细胞介素1β、肿瘤坏死因子α等炎症因子的分泌水平;Western blot检测血红素氧合酶1、LC3Ⅱ、LC3Ⅰ、P62、PPARγ、ABCA1的表达量;氧化酶法检测细胞内胆固醇含量并用油红O染色评价胞内脂滴的蓄积程度。
结果与结论:①阿托伐他汀可呈剂量依赖性诱导巨噬细胞血红素氧合酶1蛋白表达;②氧化修饰型低密度脂蛋白可诱导巨噬细胞主要向M1极化并分泌促炎因子,增加胞内胆固醇蓄积;③与对照组相比,阿托伐他汀干预后巨噬细胞血红素氧合酶1蛋白表达增加,细胞转向M2型极化且主要分泌转化生长因子β、白细胞介素10等抗炎因子,同时,PPARγ、ABCA1、LC3II/I等反映胆固醇外排及自噬的信号分子表达增加,胞内胆固醇及脂滴含量均显著减少(P﹤0.05);④同步加入锌原卟啉Ⅸ预处理的血红素氧合酶1抑制组则显著逆转了阿托伐他汀组的上述改变;⑤结果表明,阿托伐他汀可能通过上调血红素氧合酶1的表达促进氧化修饰型低密度脂蛋白刺激的巨噬细胞向M2型极化、抑制炎症,并通过上调PPARγ/ABCA1信号通路和增强自噬等增加胞内胆固醇流出,降低细胞内脂质蓄积。
https://orcid.org/0000-0003-4996-5412(邓锐)
中国组织工程研究杂志出版内容重点:干细胞;骨髓干细胞;造血干细胞;脂肪干细胞;肿瘤干细胞;胚胎干细胞;脐带脐血干细胞;干细胞诱导;干细胞分化;组织工程
中图分类号: