• •
李琢宇1( ), 金鹏1, 陈孝彦2, 赵泽玉1, 王庆宏1, 陈春茂1(
), 金鹏1, 陈孝彦2, 赵泽玉1, 王庆宏1, 陈春茂1( ), 詹亚力1
), 詹亚力1
收稿日期:2023-12-18
修回日期:2024-03-05
通讯作者:
陈春茂
作者简介:李琢宇(1992—),女,博士,讲师,Lizyhit@163.com
基金资助:
Zhuoyu LI1( ), Peng JIN1, Xiaoyan CHEN2, Zeyu ZHAO1, Qinghong WANG1, Chunmao CHEN1(
), Peng JIN1, Xiaoyan CHEN2, Zeyu ZHAO1, Qinghong WANG1, Chunmao CHEN1( ), Yali ZHAN1
), Yali ZHAN1
Received:2023-12-18
Revised:2024-03-05
Contact:
Chunmao CHEN
摘要:
双酚A(BPA)是含酚工业废水中代表性污染物,采用零价铁(ZVI)活化过氧乙酸(PAA)去除水中BPA,探究了ZVI和PAA投量、pH值以及工业废水中典型共存阴离子对PAA活化和BPA降解的影响,并通过探究反应活性物种和活性位点解析了ZVI活化PAA的反应机制。在投加50 mg/L ZVI, 1 mM PAA和初始pH为3.4的最优工艺条件下,ZVI/PAA体系反应30 min可去除水中99.24%的BPA;HCO3-和SO42-对BPA降解具有抑制作用,Cl-(0 - 20.0 mM)则加速BPA降解。反应中ZVI及其表面氧化层分别释放溶解性Fe(II)和Fe(III),溶出的Fe(II)活化PAA贡献26.46%的BPA降解,非均相ZVI活化PAA对BPA降解起主要作用;淬灭实验表明,ZVI/PAA体系存在CH3C(O)OO•、CH3C(O)O•、•OH和FeIVO2+,其中CH3C(O)OO•和FeIVO2+是降解BPA的主要活性物种。本研究为工业废水中双酚A的有效去除提供理论和数据支撑。
中图分类号:
李琢宇, 金鹏, 陈孝彦, 赵泽玉, 王庆宏, 陈春茂, 詹亚力. 零价铁活化过氧乙酸降解水中双酚A的效果与机制[J]. 化工学报, DOI: 10.11949/0438-1157.20231341.
Zhuoyu LI, Peng JIN, Xiaoyan CHEN, Zeyu ZHAO, Qinghong WANG, Chunmao CHEN, Yali ZHAN. Effect and mechanism on the degradation of aqueous bisphenol A by zero valent iron activated peroxyacetic acid system[J]. CIESC Journal, DOI: 10.11949/0438-1157.20231341.
| BPA | PAA | ||||
|---|---|---|---|---|---|
| kobs (min-1) | R2 | kobs (min-1) | R2 | ||
| ZVI浓度 (mg/L) | 0 | 0.0014 | 0.7915 | 0.0030 | 0.8333 |
| 25 | 0.0549 | 0.9994 | 0.0195 | 0.9835 | |
| 50 | 0.1658 | 0.9909 | 0.0776 | 0.8932 | |
| 75 | 0.2942 | 0.9722 | 0.2358 | 0.8677 | |
| 100 | 0.4630 | 0.9919 | 0.4978 | 0.8877 | |
| PAA浓度 (mM) | 0 | 0.0029 | 0.7982 | - | - |
| 0.10 | 0.0226 | 0.9227 | 0.0443 | 0.9950 | |
| 0.25 | 0.0653 | 0.9711 | 0.0907 | 0.9864 | |
| 1.00 | 0.1658 | 0.9909 | 0.0776 | 0.8932 | |
| 2.00 | 0.1168 | 0.9967 | 0.0305 | 0.9981 | |
| pH值 | 3.0 | 0.1104 | 0.9167 | 0.3233 | 0.7890 |
| 4.0 | 0.1171 | 0.9364 | 0.3908 | 0.9155 | |
| 5.0 | 0.0353 | 0.9813 | 0.0471 | 0.9684 | |
| 6.0 | 0.0094 | 0.919 | 0.0076 | 0.9836 | |
| 7.0 | 0.0029 | 0.6745 | 0.0117 | 0.9408 | |
| 8.0 | 0.0021 | 0.7382 | 0.0104 | 0.9782 | |
| Cl-浓度 (mM) | 0.0 | 0.1658 | 0.9909 | 0.0776 | 0.8932 |
| 0.2 | 0.1725 | 0.9986 | 0.0767 | 0.9727 | |
| 0.5 | 0.2866 | 0.9961 | 0.2192 | 0.8798 | |
| 1.0 | 0.2662 | 0.974 | 0.2388 | 0.9131 | |
| 2.0 | 0.2678 | 0.9787 | 0.2942 | 0.9267 | |
| 5.0 | 0.2795 | 0.9832 | 0.2134 | 0.8857 | |
| 10.0 | 0.3197 | 0.9954 | 0.2605 | 0.8823 | |
| 20.0 | 0.3465 | 0.978 | 0.2987 | 0.9484 | |
| HCO3-浓度 (mM) | 0.0 | 0.1658 | 0.9909 | 0.0776 | 0.8932 |
| 0.2 | 0.0658 | 0.9953 | 0.0272 | 0.9815 | |
| 0.5 | 0.0364 | 0.9910 | 0.0168 | 0.9889 | |
| 1.0 | 0.0082 | 0.8913 | 0.0087 | 0.8600 | |
| 2.0 | 0.0053 | 0.9704 | 0.0088 | 0.9176 | |
| 5.0 | 0.0023 | 0.9777 | 0.0072 | 0.8708 | |
| 10.0 | 0.0022 | 0.9104 | 0.0152 | 0.9843 | |
| 20.0 | 0.0006 | 0.3193 | 0.0239 | 0.9969 | |
| SO42-浓度 (mM) | 0.0 | 0.1658 | 0.9909 | 0.0776 | 0.8932 |
| 0.2 | 0.1205 | 0.9890 | 0.0363 | 0.9868 | |
| 0.5 | 0.1347 | 0.9939 | 0.0622 | 0.9560 | |
| 1.0 | 0.1195 | 0.9917 | 0.0417 | 0.9867 | |
| 2.0 | 0.0819 | 0.9961 | 0.0333 | 0.9958 | |
| 5.0 | 0.0730 | 0.9950 | 0.0305 | 0.9995 | |
| 10.0 | 0.0923 | 0.9946 | 0.0522 | 0.9883 | |
| 20.0 | 0.0803 | 0.9985 | 0.0512 | 0.9883 | |
表1 不同工况和不同阴离子浓度下BPA降解和PAA消耗的伪一级反应速率常数
Table 1 Pseudo first-order reaction rate constants for BPA degradation and PAA consumption under different operating conditions and anion concentrations
| BPA | PAA | ||||
|---|---|---|---|---|---|
| kobs (min-1) | R2 | kobs (min-1) | R2 | ||
| ZVI浓度 (mg/L) | 0 | 0.0014 | 0.7915 | 0.0030 | 0.8333 |
| 25 | 0.0549 | 0.9994 | 0.0195 | 0.9835 | |
| 50 | 0.1658 | 0.9909 | 0.0776 | 0.8932 | |
| 75 | 0.2942 | 0.9722 | 0.2358 | 0.8677 | |
| 100 | 0.4630 | 0.9919 | 0.4978 | 0.8877 | |
| PAA浓度 (mM) | 0 | 0.0029 | 0.7982 | - | - |
| 0.10 | 0.0226 | 0.9227 | 0.0443 | 0.9950 | |
| 0.25 | 0.0653 | 0.9711 | 0.0907 | 0.9864 | |
| 1.00 | 0.1658 | 0.9909 | 0.0776 | 0.8932 | |
| 2.00 | 0.1168 | 0.9967 | 0.0305 | 0.9981 | |
| pH值 | 3.0 | 0.1104 | 0.9167 | 0.3233 | 0.7890 |
| 4.0 | 0.1171 | 0.9364 | 0.3908 | 0.9155 | |
| 5.0 | 0.0353 | 0.9813 | 0.0471 | 0.9684 | |
| 6.0 | 0.0094 | 0.919 | 0.0076 | 0.9836 | |
| 7.0 | 0.0029 | 0.6745 | 0.0117 | 0.9408 | |
| 8.0 | 0.0021 | 0.7382 | 0.0104 | 0.9782 | |
| Cl-浓度 (mM) | 0.0 | 0.1658 | 0.9909 | 0.0776 | 0.8932 |
| 0.2 | 0.1725 | 0.9986 | 0.0767 | 0.9727 | |
| 0.5 | 0.2866 | 0.9961 | 0.2192 | 0.8798 | |
| 1.0 | 0.2662 | 0.974 | 0.2388 | 0.9131 | |
| 2.0 | 0.2678 | 0.9787 | 0.2942 | 0.9267 | |
| 5.0 | 0.2795 | 0.9832 | 0.2134 | 0.8857 | |
| 10.0 | 0.3197 | 0.9954 | 0.2605 | 0.8823 | |
| 20.0 | 0.3465 | 0.978 | 0.2987 | 0.9484 | |
| HCO3-浓度 (mM) | 0.0 | 0.1658 | 0.9909 | 0.0776 | 0.8932 |
| 0.2 | 0.0658 | 0.9953 | 0.0272 | 0.9815 | |
| 0.5 | 0.0364 | 0.9910 | 0.0168 | 0.9889 | |
| 1.0 | 0.0082 | 0.8913 | 0.0087 | 0.8600 | |
| 2.0 | 0.0053 | 0.9704 | 0.0088 | 0.9176 | |
| 5.0 | 0.0023 | 0.9777 | 0.0072 | 0.8708 | |
| 10.0 | 0.0022 | 0.9104 | 0.0152 | 0.9843 | |
| 20.0 | 0.0006 | 0.3193 | 0.0239 | 0.9969 | |
| SO42-浓度 (mM) | 0.0 | 0.1658 | 0.9909 | 0.0776 | 0.8932 |
| 0.2 | 0.1205 | 0.9890 | 0.0363 | 0.9868 | |
| 0.5 | 0.1347 | 0.9939 | 0.0622 | 0.9560 | |
| 1.0 | 0.1195 | 0.9917 | 0.0417 | 0.9867 | |
| 2.0 | 0.0819 | 0.9961 | 0.0333 | 0.9958 | |
| 5.0 | 0.0730 | 0.9950 | 0.0305 | 0.9995 | |
| 10.0 | 0.0923 | 0.9946 | 0.0522 | 0.9883 | |
| 20.0 | 0.0803 | 0.9985 | 0.0512 | 0.9883 | |
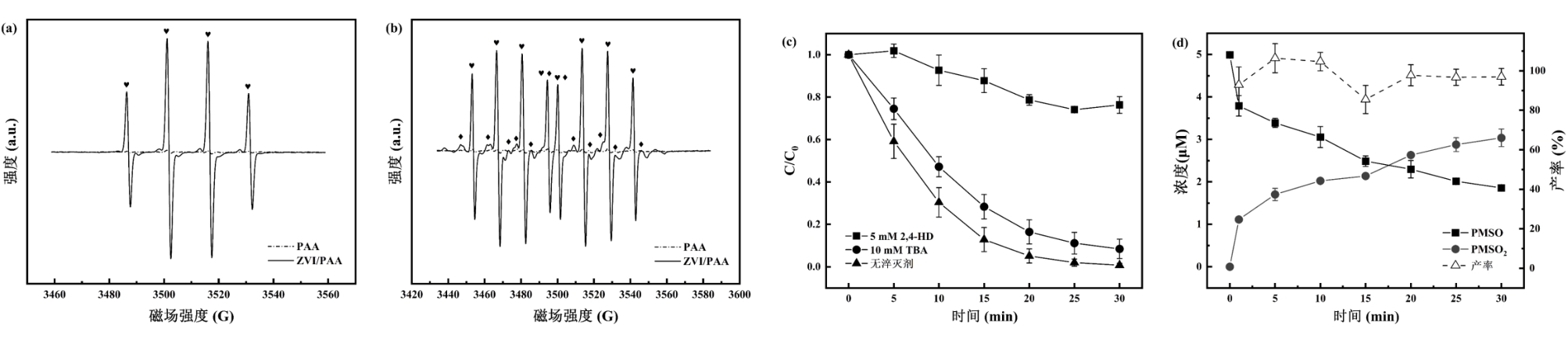
图4 (a)(b) ZVI/PAA体系的EPR谱图(反应条件:[PAA] = 2 mM,[ZVI] = 0.1 g/L,[DMPO] = [DIPPMPO] = 2 mM;(a)自旋捕获剂为DMPO,(b)自旋捕获剂为DIPPMPO,❤为-OH加合物,♦为-CH3加合物);(c) TBA和2,4-HD对ZVI/PAA体系降解BPA的影响;(d) ZVI/PAA体系降解BPA中甲基苯基亚砜氧化情况
Fig. 4 (a) (b) EPR spectra of the ZVI/PAA system. (Reaction conditions: [PAA]=2 mM, [ZVI]=0.1 g/L, [DMPO]=[DIPPMPO]=2 mM; (a) The spin capture agent is DMPO, and (b) the spin capture agent is DIPPMPO, ❤ for -OH adducts, ♦for -CH3 adducts); (c) Effect of TBA and 2,4-HD on the degradation of BPA in the ZVI/PAA system; (d) Oxidation of PMSO in BPA Degradation by ZVI/PAA System
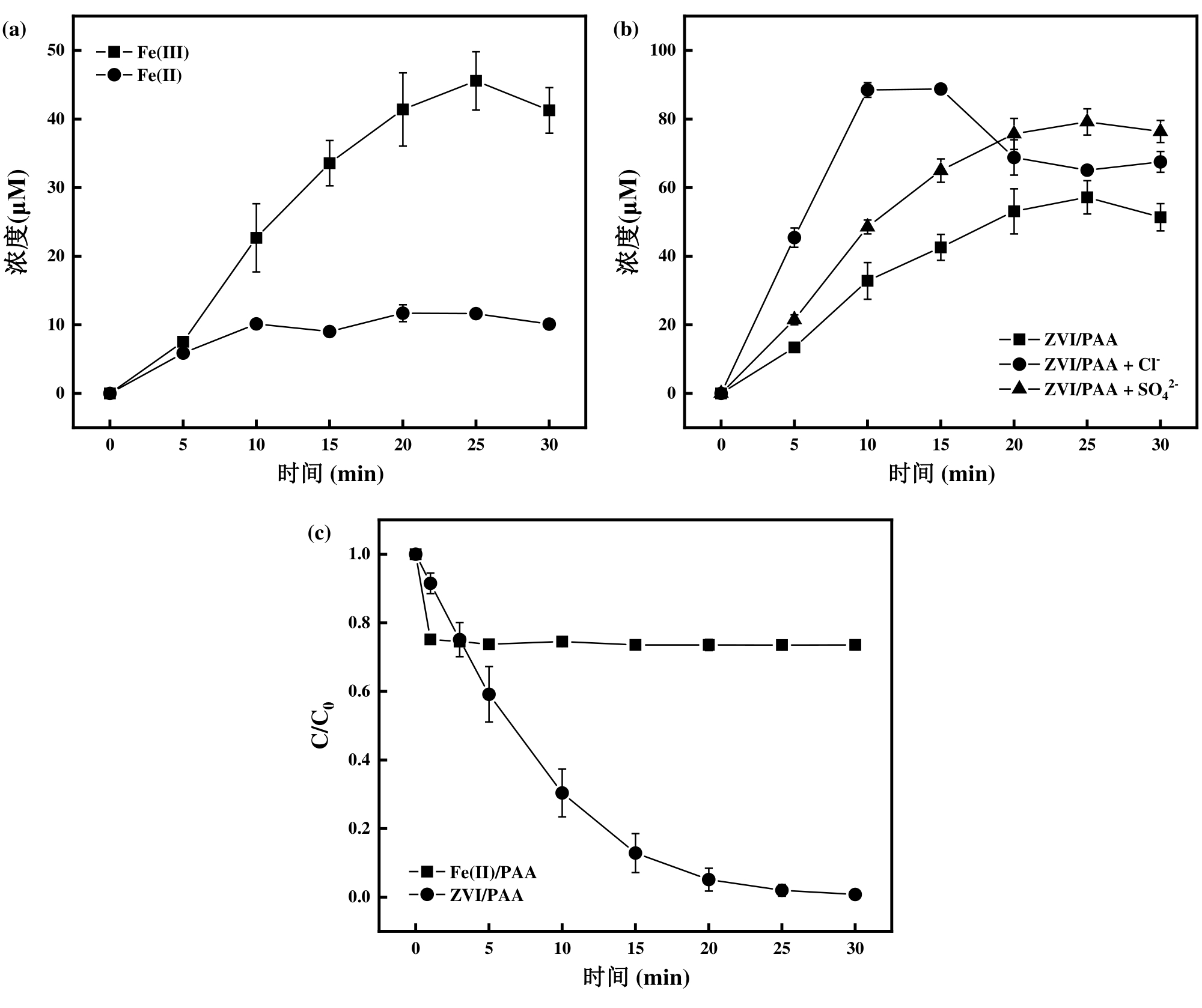
图5 (a) ZVI/PAA体系铁离子溶出量;(b) 不同阴离子作用下ZVI/PAA体系铁离子总溶出量,[Cl-] = [SO42-] = 10 mM;(c) Fe(II)/PAA与ZVI/PAA对BPA的降解效果
Fig. 5 (a) Iron ion dissolution in the ZVI/PAA system; (b) The total dissolution of iron ions in the ZVI/PAA system under different anionic interactions, [Cl-] = [SO42-] = 10 mM; (c) The degradation effect of Fe (II)/PAA and ZVI/PAA on BPA
| AOP的种类 | 反应条件 | BPA降解情况 | 活性物种 | 共存阴离子影响 | 参考文献 |
|---|---|---|---|---|---|
| VUV/H2O2 | [H2O2] = 25 mg/L,[BPA] = 100 mg/L,pH = 3.0 | 60 min降解97.6% | •OH | 100 mg/L HCO3-、Cl-、NO3-、SO42-对BPA的降解无明显影响 | [ |
| Fe(IV)/H2O2 | [Fe(VI)] = 250 µg/L,[H2O2] = 2.5 mg/L,[BPA] = 50 µg/L,pH = 8.0 | 60 min降解99.8% | Fe(V)、Fe(IV)、•OH、O•- | 350 µg/L Cl-、NO3-对BPA的降解无明显影响,PO43-(350 µg/L)显著抑制BPA的降解 | [ |
| CuO/还原氧化石墨烯泡沫(RGF)/PDS | [CuO/RGF] = 0.5 g/L,[PDS] = 0.1 g/L,[BPA] = 10 mg/L,pH = 7.0 | 120 min吸附48.97%,后120 min降解37.13% | 1O2、•OH、SO4•- | 12 mg/L HCO3-抑制BPA的降解,Cl-对BPA的降解无明显影响 | [ |
| 可见光(VL)/没食子酸(GA)/Fe3+/PI | [Fe3+] = 0.10 mM,[GA] = 0.10 mM,[PI] = 1 mM,[BPA] = 20 μM,pH = 7.0 | 30 min降解100% | 1O2、•OH、O2•-、•IO3、•IO4、Fe(IV) | 10 mM Cl-、NO3-、SO42-对BPA的降解无明显影响HCO3-抑制BPA的降解 | [ |
| 介质阻挡放电(DBD)/PAA | [输入功率] = 445 W,[液体流速] = 100 mL/min,[频率] = 3500 Hz,[PAA] = 3.0 mM,[BPA] = 40 µg/L,pH = 4.5 | 15 min降解93.4% | •OH、CH3C(O)OO•、1O2、O2•-、e- | 10 mM Cl-、SO42-对BPA的降解无明显影响,HCO3-显著抑制BPA的降解 | [ |
| ZIF-67/PAA | [HP] = 16.9 mM,[ZIF-67] = 0.1 g/L,[PAA] = 5 mM, [BPA] = 0.1 mM,pH = 3.4 | 30 min降解93.0% | R-O•、•OH、Co3+ | 10 mM Cl-略微促进BPA的降解,NO3-、SO42-对BPA的降解无明显影响,HCO3-显著抑制BPA的降解 | [ |
| CuCo2O4/PAA | [CuCo2O4] = 0.2 g/L,[PAA] = 400 µM,[BPA] = 0.088 mM,pH = 7.0 | 60 min降解92.3% | 1O2、R-O• | 2 mM HCO3-、CO32-、Cl-略微抑制BPA的降解,NO3-、SO42-对BPA的降解无明显影响 | [ |
| ZVI/PAA | [ZVI] = 50 mg/L,[PAA] = 1 mM,[BPA] = 10 mg/L,pH = 3.4 | 30 min降解99.24% | R-O•、•OH、Fe(IV) | 20 mM Cl-显著促进BPA的降解,HCO3-显著抑制BPA的降解,SO42-抑制BPA的降解 | 本实验 |
表2 与相关文献对BPA处理效果的比较
Table 2 Comparison of BPA treatment effects with relevant literature
| AOP的种类 | 反应条件 | BPA降解情况 | 活性物种 | 共存阴离子影响 | 参考文献 |
|---|---|---|---|---|---|
| VUV/H2O2 | [H2O2] = 25 mg/L,[BPA] = 100 mg/L,pH = 3.0 | 60 min降解97.6% | •OH | 100 mg/L HCO3-、Cl-、NO3-、SO42-对BPA的降解无明显影响 | [ |
| Fe(IV)/H2O2 | [Fe(VI)] = 250 µg/L,[H2O2] = 2.5 mg/L,[BPA] = 50 µg/L,pH = 8.0 | 60 min降解99.8% | Fe(V)、Fe(IV)、•OH、O•- | 350 µg/L Cl-、NO3-对BPA的降解无明显影响,PO43-(350 µg/L)显著抑制BPA的降解 | [ |
| CuO/还原氧化石墨烯泡沫(RGF)/PDS | [CuO/RGF] = 0.5 g/L,[PDS] = 0.1 g/L,[BPA] = 10 mg/L,pH = 7.0 | 120 min吸附48.97%,后120 min降解37.13% | 1O2、•OH、SO4•- | 12 mg/L HCO3-抑制BPA的降解,Cl-对BPA的降解无明显影响 | [ |
| 可见光(VL)/没食子酸(GA)/Fe3+/PI | [Fe3+] = 0.10 mM,[GA] = 0.10 mM,[PI] = 1 mM,[BPA] = 20 μM,pH = 7.0 | 30 min降解100% | 1O2、•OH、O2•-、•IO3、•IO4、Fe(IV) | 10 mM Cl-、NO3-、SO42-对BPA的降解无明显影响HCO3-抑制BPA的降解 | [ |
| 介质阻挡放电(DBD)/PAA | [输入功率] = 445 W,[液体流速] = 100 mL/min,[频率] = 3500 Hz,[PAA] = 3.0 mM,[BPA] = 40 µg/L,pH = 4.5 | 15 min降解93.4% | •OH、CH3C(O)OO•、1O2、O2•-、e- | 10 mM Cl-、SO42-对BPA的降解无明显影响,HCO3-显著抑制BPA的降解 | [ |
| ZIF-67/PAA | [HP] = 16.9 mM,[ZIF-67] = 0.1 g/L,[PAA] = 5 mM, [BPA] = 0.1 mM,pH = 3.4 | 30 min降解93.0% | R-O•、•OH、Co3+ | 10 mM Cl-略微促进BPA的降解,NO3-、SO42-对BPA的降解无明显影响,HCO3-显著抑制BPA的降解 | [ |
| CuCo2O4/PAA | [CuCo2O4] = 0.2 g/L,[PAA] = 400 µM,[BPA] = 0.088 mM,pH = 7.0 | 60 min降解92.3% | 1O2、R-O• | 2 mM HCO3-、CO32-、Cl-略微抑制BPA的降解,NO3-、SO42-对BPA的降解无明显影响 | [ |
| ZVI/PAA | [ZVI] = 50 mg/L,[PAA] = 1 mM,[BPA] = 10 mg/L,pH = 3.4 | 30 min降解99.24% | R-O•、•OH、Fe(IV) | 20 mM Cl-显著促进BPA的降解,HCO3-显著抑制BPA的降解,SO42-抑制BPA的降解 | 本实验 |
| 1 | Huang Y Q, Wong C K C, Zheng J S, et al. Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts[J]. Environment International, 2012, 42: 91-99. |
| 2 | 蒋俊, 李秀艳. 环境内分泌干扰物双酚A的降解研究进展[J]. 上海化工, 2009, 34(4): 25-30. |
| Jiang J, Li X Y. Advances in research of degradation of one of environmental endocrine disruptors bisphenol A[J]. Shanghai Chemical Industry, 2009, 34(4): 25-30. | |
| 3 | Bai X L, Acharya K. Removal of seven endocrine disrupting chemicals (EDCs) from municipal wastewater effluents by a freshwater green alga[J]. Environmental Pollution, 2019, 247: 534-540. |
| 4 | 宋新新, 刘杰, 林甲, 等. 碳中和时代下我国能量自给型污水处理厂发展方向及工程实践[J]. 环境科学学报, 2022, 42(4): 53-63. |
| Song X X, Liu J, Lin J, et al. The development direction and practice of energy self-sufficiency sewage treatment plants in China under Carbon Neutral Era[J]. Acta Scientiae Circumstantiae, 2022, 42(4): 53-63. | |
| 5 | 环境保护部, 国家质量监督检验检疫总局. 石油化学工业污染物排放标准: [S]. 北京: 中国环境科学出版社, 2015. |
| Ministry of Environmental Protection of the People's Republic of China, General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China. Emission standard of pollutants for petroleum chemistry industry: [S]. Beijing: China Environmental Science Press, 2015. | |
| 6 | Priyadarshini M, Das I, Ghangrekar M M, et al. Advanced oxidation processes: performance, advantages, and scale-up of emerging technologies[J]. Journal of Environmental Management, 2022, 316: 115295. |
| 7 | Gürtekin E, Çelik A, Aydin E. Degradation and mineralization of tetracycline and oxytetracycline by Fenton process: effect of inorganic anions[J]. Desalination and Water Treatment, 2022, 261: 299-307. |
| 8 | 王阳毅, 高强, 刘赛, 等. 阴离子对Fenton氧化降解聚乙烯醇的影响[J]. 化工环保, 2017, 37(6): 644-647. |
| Wang Y Y, Gao Q, Liu S, et al. Effects of ions on oxidative degradation of polyvinyl alcohol by Fenton method[J]. Environmental Protection of Chemical Industry, 2017, 37(6): 644-647. | |
| 9 | Wang X C, Jing J N, Zhou M H, et al. Recent advances in H2O2-based advanced oxidation processes for removal of antibiotics from wastewater[J]. Chinese Chemical Letters, 2023, 34(3): 107621. |
| 10 | Kim J, Zhang T Q, Liu W, et al. Advanced oxidation process with peracetic acid and Fe(II) for contaminant degradation[J]. Environmental Science & Technology, 2019, 53(22): 13312-13322. |
| 11 | Kim C, Ahn J Y, Kim T Y, et al. Activation of persulfate by nanosized zero-valent iron (NZVI): mechanisms and transformation products of NZVI[J]. Environmental Science & Technology, 2018, 52(6): 3625-3633. |
| 12 | Pan Y W, Zhang Y, Zhou M H, et al. Enhanced removal of antibiotics from secondary wastewater effluents by novel UV/pre-magnetized Fe0/H2O2 process[J]. Water Research, 2019, 153: 144-159. |
| 13 | Lu B Z, Fang Z Q, Tsang P E, et al. Effect and mechanism of norfloxacin removal by guava leaf extract in the ZVI/H2O2 system[J]. Chemosphere, 2023, 316: 137801. |
| 14 | Dong S Y, Zhai X X, Pi R B, et al. Efficient degradation of naproxen by persulfate activated with zero-valent iron: performance, kinetic and degradation pathways[J]. Water Science and Technology, 2020, 81(10): 2078-2091. |
| 15 | Donadelli J A, Carlos L, Arques A, et al. Kinetic and mechanistic analysis of azo dyes decolorization by ZVI-assisted Fenton systems: pH-dependent shift in the contributions of reductive and oxidative transformation pathways[J]. Applied Catalysis B: Environmental, 2018, 231: 51-61. |
| 16 | Yousefi M, Ghanbari F, Ali Zazouli M, et al. Brilliant Blue FCF degradation by persulfate/zero valent iron: the effects of influencing parameters and anions[J]. Desalination and Water Treatment, 2017, 70: 364-371. |
| 17 | Zhang P Y, Zhang X F, Zhao X D, et al. Activation of peracetic acid with zero-valent iron for tetracycline abatement: the role of Fe(II) complexation with tetracycline[J]. Journal of Hazardous Materials, 2022, 424(Pt D): 127653. |
| 18 | Ghanbari F, Giannakis S, Lin K Y A, et al. Acetaminophen degradation by a synergistic peracetic acid/UVC-LED/Fe(II) advanced oxidation process: Kinetic assessment, process feasibility and mechanistic considerations[J]. Chemosphere, 2021, 263: 128119. |
| 19 | Bokare A D, Choi W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes[J]. Journal of Hazardous Materials, 2014, 275: 121-135. |
| 20 | Georgi A, Schierz A, Trommler U, et al. Humic acid modified Fenton reagent for enhancement of the working pH range[J]. Applied Catalysis B: Environmental, 2007, 72(1/2): 26-36. |
| 21 | Feng G D, Cheng P, Yan W F, et al. Accelerated crystallization of zeolites via hydroxyl free radicals[J]. Science, 2016, 351(6278): 1188-1191. |
| 22 | Kiejza D, Kotowska U, Polińska W, et al. Peracids-New oxidants in advanced oxidation processes: the use of peracetic acid, peroxymonosulfate, and persulfate salts in the removal of organic micropollutants of emerging concern-A review[J]. Science of the Total Environment, 2021, 790: 148195. |
| 23 | Wang Z, Qiu W, Pang S Y, et al. Relative contribution of ferryl ion species (Fe(IV)) and sulfate radical formed in nanoscale zero valent iron activated peroxydisulfate and peroxymonosulfate processes[J]. Water Research, 2020, 172: 115504. |
| 24 | Liang S, Zhu L Y, Hua J, et al. Fe2+/HClO reaction produces FeIVO2+: An enhanced advanced oxidation process[J]. Environmental Science & Technology, 2020, 54(10): 6406-6414. |
| 25 | Wang Z R, Shi H L, Wang S X, et al. Degradation of diclofenac by Fe(II)-activated peracetic acid[J]. Environmental Technology, 2021, 42(27): 4333-4341. |
| 26 | Lu Z, Continetti R E. Dynamics of the acetyloxyl radical studied by dissociative photodetachment of the acetate anion[J]. The Journal of Physical Chemistry A, 2004, 108(45): 9962-9969. |
| 27 | Chen Y F, Miller C J, Xie J L, et al. Challenges relating to the quantification of ferryl(IV) ion and hydroxyl radical generation rates using methyl phenyl sulfoxide (PMSO), phthalhydrazide, and benzoic acid as probe compounds in the homogeneous fenton reaction[J]. Environmental Science & Technology, 2023, 57(47): 18617-18625. |
| 28 | Wang L, Yang J, Li Y M, et al. Removal of chlorpheniramine in a nanoscale zero-valent iron induced heterogeneous Fenton system: influencing factors and degradation intermediates[J]. Chemical Engineering Journal, 2016, 284: 1058-1067. |
| 29 | 林光辉, 吴锦华, 李平, 等. 零价铁与双氧水异相Fenton降解活性艳橙X-GN[J]. 环境工程学报, 2013, 7(3): 913-917. |
| Lin G H, Wu J H, Li P, et al. Effective degradation of reactive brilliant orange X-GN by heterogeneous Fenton reaction using zero-valent iron and H2O2 [J]. Chinese Journal of Environmental Engineering, 2013, 7(3): 913-917. | |
| 30 | Graat P C J, Somers M A J. Simultaneous determination of composition and thickness of thin iron-oxide films from XPS Fe 2p spectra[J]. Applied Surface Science, 1996, 100-101: 36-40. |
| 31 | 王贵. 高盐化工废水处理工艺分析[J]. 化工设计通讯, 2020, 46(4): 100-101. |
| Wang G. Process analysis of high salt chemical wastewater treatment[J]. Chemical Engineering Design Communications, 2020, 46(4): 100-101. | |
| 32 | Song Z, Zhang Y, Zhang X, et al. Kinetics study of chloride-activated peracetic acid for purifying bisphenol A: role of Cl2/HClO and carbon-centered radicals[J]. Water Research, 2023, 242: 120274. |
| 33 | Zhou F Y, Lu C, Yao Y Y, et al. Activated carbon fibers as an effective metal-free catalyst for peracetic acid activation: implications for the removal of organic pollutants[J]. Chemical Engineering Journal, 2015, 281: 953-960. |
| 34 | Kim H S, Ahn J Y, Kim C, et al. Effect of anions and humic acid on the performance of nanoscale zero-valent iron particles coated with polyacrylic acid[J]. Chemosphere, 2014, 113: 93-100. |
| 35 | Wang Z P, Wang J W, Xiong B, et al. Application of cobalt/peracetic acid to degrade sulfamethoxazole at neutral condition: efficiency and mechanisms[J]. Environmental Science & Technology, 2020, 54(1): 464-475. |
| 36 | Dai C M, Li S, Duan Y P, et al. Mechanisms and product toxicity of activated carbon/peracetic acid for degradation of sulfamethoxazole: implications for groundwater remediation[J]. Water Research, 2022, 216: 118347. |
| 37 | Chen S A, Cai M Q, Liu Y Z, et al. Effects of water matrices on the degradation of naproxen by reactive radicals in the UV/peracetic acid process[J]. Water Research, 2019, 150: 153-161. |
| 38 | Wang S X, Wang H B, Liu Y Q, et al. Effective degradation of sulfamethoxazole with Fe2+-zeolite/peracetic acid[J]. Separation and Purification Technology, 2020, 233: 115973. |
| 39 | Li T, Wang X, Chen Y, et al. Producing •OH, SO4 •- and •O2 - in heterogeneous Fenton reaction induced by Fe3O4-modified schwertmannite[J]. Chemical Engineering Journal, 2020, 393: 124735. |
| 40 | Moussavi G, Pourakbar M, Shekoohiyan S, et al. The photochemical decomposition and detoxification of bisphenol A in the VUV/H2O2 process: degradation, mineralization, and cytotoxicity assessment[J]. Chemical Engineering Journal, 2018, 331: 755-764. |
| 41 | Widhiastuti F, Fan L, Paz-Ferreiro J, et al. Oxidative treatment of bisphenol A by Fe(VI) and Fe(VI)/H2O2 and identification of the degradation products[J]. Environmental Technology & Innovation, 2022, 28: 102643. |
| 42 | Zhou Q W, Luo L J, Xia L H, et al. Persulfate enhanced removal of bisphenol A by copper oxide/reduced graphene oxide foam: influencing factors, mechanism and degradation pathway[J]. Chemosphere, 2023, 340: 139786. |
| 43 | Yuan Y L, Wang W Y, Nie M H, et al. Visible light-mediated activation of periodate for bisphenol A degradation in the presence of Fe3+ and gallic acid at neutral pH[J]. Chemical Engineering Journal, 2024, 479: 147541. |
| 44 | Su Y Y, Yang Y X, Jiang W X, et al. A novel strategy of peracetic acid activation by dielectric barrier discharge plasma for bisphenol A degradation: feasibility, mechanism and active species dominant to degradation pathway[J]. Chemical Engineering Journal, 2023, 476: 146469. |
| 45 | Tan Z X, Wang Z, Song Z, et al. Mechanistic insights into the efficient activation of peracetic acid by ZIF-67 for bisphenol A degradation[J]. Surfaces and Interfaces, 2024, 44: 103808. |
| 46 | Shen P, Hou K J, Chen F, et al. Ultra-rapid and long-lasting activation of peracetic acid by Cu-Co spinel oxides for eliminating organic contamination: Role of radical and non-radical catalytic oxidation[J]. Chemical Engineering Journal, 2023, 463: 142344. |
| [1] | 王承泽, 顾凯丽, 张晋华, 石建轩, 刘艺娓, 李锦祥. 硫化协同老化零价铁增效去除水中Cr(Ⅵ)的作用机制[J]. 化工学报, 2023, 74(5): 2197-2206. |
| [2] | 李瑞康, 何盈盈, 卢维鹏, 王园园, 丁皓东, 骆勇名. 电化学强化钴基阴极活化过一硫酸盐的研究[J]. 化工学报, 2023, 74(5): 2207-2216. |
| [3] | 杨庆云, 李青松, 陈泽铭, 邓靖, 李玉瑛, 杨帆, 陈国元, 李国新. UV/PMS、UV/PDS、UV/SPC工艺降解尼泊金甲酯[J]. 化工学报, 2023, 74(3): 1322-1331. |
| [4] | 靳文章, 张玉玲, 贾晓宇. 电化学高级氧化对HEDP的降解效能研究[J]. 化工学报, 2022, 73(9): 4062-4069. |
| [5] | 朱文会, 王夏晖, 杨欣桐, 王兴润, 何俊, 黄国鑫, 季国华. 海藻酸钙固定化零价铁抗团聚及堵塞的作用机制[J]. 化工学报, 2020, 71(5): 2344-2351. |
| [6] | 焦昭杰, 陈立功, 柳云骐, 张贤明, 龚海峰, 高旭. CuCe氧化物催化剂的制备及CWPO降解双酚A废水研究[J]. 化工学报, 2020, 71(4): 1646-1656. |
| [7] | 王柯晴, 徐劼, 沈芷璇, 陈家斌, 吴玮. LaCoO3钙钛矿活化过一硫酸盐降解萘普生[J]. 化工学报, 2020, 71(3): 1326-1334. |
| [8] | 岳敏,王璟,韩玉泽,张萍. 盐助溶液燃烧法制备MnFe2O4催化过一硫酸盐降解双酚A[J]. 化工学报, 2020, 71(12): 5589-5598. |
| [9] | 赵斌,刘念,王虹利,钱怡冉,张朝晖,王亮. 道南渗析-零价铁耦合工艺除砷效果研究[J]. 化工学报, 2020, 71(11): 5303-5308. |
| [10] | 李艳鹰, 李先春. 生物质活性炭负载零价铁纳米晶簇直接催化还原NO[J]. 化工学报, 2019, 70(3): 1111-1119. |
| [11] | 李德生, 张超, 邓时海, 胡智丰, 李金龙, 刘元辉. 基于铁基质高效催化还原污水中硝酸盐氮的实验研究[J]. 化工学报, 2019, 70(3): 1065-1074. |
| [12] | 尹飞, 王翠, 童少平. rGO-Fe3O4活化过硫酸盐处理酸性红73[J]. 化工学报, 2019, 70(1): 207-213. |
| [13] | 樊鹏, 陈杰, 关小红, 乔俊莲. 过硫酸盐强化零价铁还原去除硝基苯的实验研究[J]. 化工学报, 2018, 69(5): 2175-2182. |
| [14] | 陈莉荣, 成路姣, 谷振超, 樊健, 张凯, 郑春丽. 天然磁铁矿/UV/S2O82-对焦化废水中不同种类有机物的去除特性[J]. 化工学报, 2018, 69(12): 5292-5300. |
| [15] | 曹贝, 李锦祥, 关小红. 弱磁场强化零价铁对水中U(Ⅵ)去除效能[J]. 化工学报, 2017, 68(8): 3282-3290. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号