Article Text
Abstract
Background The aim of the study was to evaluate how different parameters in the preoperative, perioperative, and postoperative period affect time to full enteral feeding (TFEF) in children undergoing pyloromyotomy.
Methods A retrospective study of all children operated for infantile hypertrophic pyloric stenosis between 2001 and 2017 was conducted. Parameters in demographics and in the preoperative and postoperative period were evaluated against TFEF (hours) using linear regression models.
Results In the whole cohort of 175 children, mean TFEF was 47 hours with Standard Deviation (SD) of ±35. In the multivariate model, TFEF decreased with age [beta (B): −0.62; 95% confidence interval (95% CI) −1.05 to −0.19; p=0.005) and increased with the presence of severe underlying disease (congenital heart defect or syndrome) (B: 26.5; 95% CI 3.3 to 49.7; p=0.026). Hence, for every day of age, the time to fully fed decreased by 0.6 hour, and the presence of an underlying disease increased the time to fully fed with over one day. TFEF did not seem to be affected by prematurity, weight loss, symptom duration, preoperative acid/base balance or electrolyte values, surgical method, or method of postoperative feeding.
Conclusions TFEF decreased with higher age and increased in children with a severe underlying disease. These results may be useful in providing adequate parental information regarding what affects TFEF and the length of hospital stay.
- pediatric surgery
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Summary box
What is already known about this subject?
Infantile hypertrophic pyloric stenosis is a common disease.
Time to full goals after surgery seem to be affected by the degree of preoperative electrolyte disturbances.
There is no study adjusting for confounding factors that has evaluated other parameters that may affect TFEF after surgery.
What are the new findings?
TFEF decreased with higher age and increased in children with a severe underlying disease.
No evidence was found for parameters reflecting preoperative emesis (symptom duration, weight change, electrolyte abnormalities) affecting TFEF.
How might it impact on clinical practice in the foreseeable future?
This study can be used to improve parental information. It also suggests that trials comparing different operation techniques or postoperative feeding regimens adjust their results for age at surgery and underlying diseases.
INTRODUCTION
Infantile hypertrophic pyloric stenosis (IHPS) is a relatively common disease with an incidence of 2–4 per 1000 live births in Western populations.1 Most studies regarding outcome after surgery for IHPS, in terms of time to full enteral feeding (TFEF) or length of hospital stay, focus on surgical method2 3 or method of postoperative feeding.4 5 Less is known whether patient characteristics, symptoms and signs of dehydration, or other variables in the preoperative phase can affect the outcome, and especially TFEF after pyloromyotomy. Therefore, the aim of this study was to examine which parameters could affect the TFEF in children operated on with pyloromyotomy for IHPS, with focus on patient’s characteristics, preoperative symptoms and signs of dehydration including laboratory values. Our hypothesis was that metabolic alkalosis and/or hypochloremia at admission, even if corrected before surgery, and symptom duration, increased the risk of longer duration to full enteral feeds. If influenceable parameters were proved to increase the risk of longer duration to full enteral feeds, a modified
preoperative treatment could possibly reduce the time to full feeds, and in the long run shorten length of postoperative hospital stay. Also, being able to better estimate time to full enteral feeds and duration of postoperative hospital stay is valuable when giving information to caregivers.
METHODS
Settings and children
All children were treated at a tertiary center for pediatric surgery with a catchment area of around 1.8 million inhabitants for specialized pediatric surgery in children up to 15 years. All patients with an International Classification of Diseases 10th Revision diagnosis code of Q.40.0 who underwent pyloromyotomy from 2001 to 2017 were eligible for inclusion. Inclusion criteria were diagnosis of isolated IHPS and isolated pyloromyotomy operation. Children with insufficient data for retrieving TFEF were excluded. Laparoscopic pyloromyotomy was not used at our department during the study period. The diagnosis of IHPS was based on clinical symptoms and ultrasound findings. The postoperative feeding was initiated by the operating surgeon and started 3–4 hours after surgery with either free feeding or according to a schedule. The specific feeding schedule started with 20% of the full amount (150 mL/kg), and increases with 20% for every meal; if significant vomiting occured, the same amount would be given again at the next meal.
Study design
This study is an institution-based retrospective study. Primary outcome was duration (hours) to full enteral feeding. This was defined by the time from operation to journal notes either stated ‘fully fed’ or describing correct amount of food intake without emesis. Independent variables were preoperative parameters such as demographical data, type of feeding (breastmilk/formula), symptoms and blood tests; surgical data such as method of operation; and postoperative parameters including method of feeding and complications. Prematurity was defined as a gestational age shorter than 37 weeks. Age at operation was corrected for prematurity. Small for gestational age was defined as a weight below the 10th percentile for the gestational age. Children with congenital heart disease (CHD) requiring surgery, and syndromes were included in the ‘severe underlying disease’ group. The cut-offs used for ultrasound were pyloric muscle thickness of 4 mm or more, and pyloric channel length of 16 mm or more.6 In infants small than 3 weeks the cut-off for thickness was 3.5 mm.7 Blood samples were taken from venous blood to perform analysis of sodium, potassium, chloride, base excess, pH, pCO2, pO2, hemoglobin, and liver function tests including alanine transaminase, aspartate transaminase, alkaline phosphatase, gamma-glutamyl transpeptidase, and bilirubin (total). Reference intervals for all analyses were age specific and followed international standards.8 The reference intervals for (total) bilirubin were: <2 days of age:<100 μmol/L; 2–6 days of age: <200 μmol/L; 7–20 days of age: <100 μmol/L; 21–29 days:<50 μmol/L; and >1 month of age: <22 μmol/L. Complications included were postoperative infection and reoperation. Length of postoperative hospital stay (hours) was counted from operation until discharge.
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics for Mac, V.24 (IBM). Continuous variables were presented as median or interquartile range (IQR) (no normal distribution) or mean±SD (normal distribution). Normal distribution was tested by evaluation of skewness and kurtosis. Dichotomous variables were presented as the absolute number and percentage of patients. The primary outcome of full enteral feeding was treated as a continuous parameter and different parameters were tested as independent variables, first in a univariate and then in a multivariate linear regression model. The results were presented as beta (B) with 95% confidence interval (CI). Reoperations and complications were also added to the regression analyses since they might have an impact on feeding.
Collinearity between the continuous parameters was evaluated. If moderate to strong correlation existed, the final regression model would be tested with and without the different variable(s). Further, we checked for heteroscedasticity by plotting the residuals of the final regression model against patients’ age. A p value <0.05 was considered to be statistically significant.
RESULTS
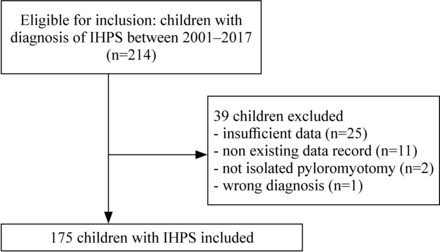
From 2001 to 2017, a total of 214 children were diagnosed and operated on for IHPS. A total of 39 (18%) patients were excluded; 25 for missing data, 11 for non-existing data record, 2 for non-isolated pyloromyotomy, and 1 for wrong diagnosis; leaving a total of 175 children included in the study (figure 1).
Flow chart of inclusion and exclusion of children operated for infantile hypertrophic pyloric stenosis (IHPS) 2001–2017.
The included cohort consisted of 148 (85%) boys, with a mean corrected age of 37 days, a mean weight of 3925 g, 24 (14%) of which were premature, and 12 (7%) of which had a severe underlying disease (online supplementary table 1). Median duration of symptoms was 4 days (IQR: 7); 128 patients (74%) experienced weight loss; and increased bilirubin was seen in 25 patients (14%) (table 1).
Supplemental material
Demographics, preoperative data and surgical method in 175 children with infantile hypertrophic pyloric stenosis
Mean TFEF for the whole cohort was 47±35 hours and mean length of postoperative hospital stay was 66±44 hours. The majority of children (88%) were fed according to a feeding schedule. No child required intravenous nutrition. There were seven children(4%) presented with postoperative complications, of which four required reoperation (table 2).
Postoperative data and outcome in 175 children operated for infantile hypertrophic pyloric stenosis
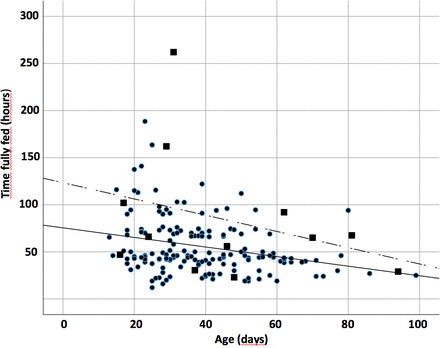
In the univariate analysis, age, weight, severe underlying disease increased bilirubin level, and duration of symptoms were found to affect TFEF (table 3). In the multivariate analysis, only age (B: −0.62 (95% CI −1.05 to −0.19), p=0.005) (figure 2) and severe underlying disease (B: 26.5 (95% CI 3.3 to 49.7), p=0.026) remained as variables significantly affecting TFEF (table 4). Hence, for every day of age, the time to fully fed decreased with 0.6 hour, and the presence of an underlying disease increased the time to fully fed with over 1 day. TFEF did not seem to be affected by prematurity, preoperative weight loss, symptom duration, preoperative acid/base balance or electrolyte values, surgical method, or method of postoperative feeding (tables 3 and 4).
Univariate regression of demographics, preoperative and postoperative data and the effect on time in hours to full enteral feeding
Scatterplot over the correlation between age in days and hours to fully fed in 175 children operated for infantile hypertrophic pyloric stenosis. ■Patients with underlying disease; — denotes age; --- denotes underlying diseases.
Multivariate linear regression of variables predicting longer time in hours to full enteral feeding in 175 children operated for infantile hypertrophic pyloric stenosis
The correlation between the different continuous parameters was overall weak (Spearman’s correlation coefficient (rs) between −0.1 and 0.1), except the correlation between age and weight(rs: 0.41). The final regression model was tested with and without the weight included and there was little difference in the beta for age (−0.62, 95% CI −1.05 to −0.19 and −0.61, 95% CI −0.98 to −0.25, respectively). When checking for heteroscedasticity, the residuals of the final regression model seemed to have constant variance (data not shown).
DISCUSSION
In children who underwent pyloromyotomy for IHPS, a higher age decreased TFEF, while presence of underlying disease was associated with increased duration.
There is a significant value in evaluating preoperative factors as predictors for worse postoperative outcome. Such evaluations improve preoperative and postoperative information to caregivers; they also provide a way to improve preoperative and postoperative care. Further, being able to stratify patients in different risk groups would be of great importance in future studies evaluating different surgical methods or different postoperative feeding regimens, as described earlier.4 We evaluated several parameters of patient characteristics, preoperative symptoms and signs of dehydration, including laboratory values, feeding method and operation method, but only two of these parameters appeared to affect TFEF. Our hypothesis that metabolic alkalosis and/or hypochloremia at admission, and symptom duration, would increase the duration of reaching goal feeds was not supported. In addition, neither prematurity, preoperative weight loss, other preoperative acid/base balance or electrolyte values, surgical method, nor method of postoperative feeding had an effect on TFEF. The finding of higher age decreasing and severe underlying disease increasing TFEF is not unexpected. Unfortunately, these parameters also cannot be influenced and we therefore cannot modify our preoperative or postoperative management of children with IHPS based on this study. The strength of this study is that all children were treated at the same center and that the management of children with IHPS, besides operation technique, did not change during the study period. Another strength is the full evaluation of several parameters and a multivariate analysis which in comparison with the literature is sparsely conducted before.
A few studies before the present one have tried to evaluate how different preoperative parameters affect postoperative outcome, either defined as emesis, time to goal intake, or length of postoperative hospital stay. A prospective study found that the degree of hypokalemic, hypochloremic, and metabolic alkalosis correlated with the number of postoperative emesis episodes and TFEF.9 The authors also concluded that the duration of dehydration and failure to thrive was correlated with poor outcome since there were inverse correlations between the number of episodes of postoperative emesis and time to goal intakes with weight at admission. Results from a randomized trial found that low chloride levels increased TFEF.10 Our results do not support either of these results despite roughly the same number of included patients; hence the present study was in that sense not underpowered. The parameters found to cause longer time to full goals in previous studies9 10 were mainly laboratory values that should be less affected by our retrospective design. On the other hand, the primary outcome of TFEF was in the present study extracted from charts and could of course be subject to the disadvantages of a retrospective design. Other studies have found a correlation of postoperative emesis or longer hospital stay (not specifically TFEF) with weight11 and age at surgery,12 13 symptom duration14 15 and pyloric thickness on ultrasound.12
None of these results were supported by our study. However, none of these retrospective studies included a multivariate analysis and the results could therefore be questioned.
A severe underlying disease, defined in our study as CHD requiring surgery, or a syndrome, was also associated with increased risk of longer TFEF. A study of IHPS comparing postoperative outcomes between patients with and without CHD showed that a comorbidity lengthened hospital stay.16 In isolated cases of IHPS, time until full feed often equals length of hospital stay, but it seems harder to distinguish the two when a comorbidity is the evaluated factor. We believe that this is due to other treatment requirements which may prolong or hinder the advancement of reaching goal feeds. Children with CHD may also need extra calories.
All children did not have the same postoperative feeding regimen which was known to affect times to full enteral feeds.4 17 18 Although this was adjusted in the multivariate analysis, it remained a true limitation since this might be slow down feeding. With the present feeding protocol, no infant could reach full feeds in less than 5 feeds—that is, 15 hours. Further, the retrospective study design which may have affected the precision of which time until full enteral feed could be retrieved. A prospective study would of course perform better regarding exact hours. A third weakness is the sample size of study population which compared with other studies is relatively small despite a long study period.
In conclusion, TFEF decreased with higher age and increased in children with a severe underlying disease. No evidence was found for parameters reflecting preoperative emesis (symptom duration, weight change, electrolyte abnormalities) affecting TFEF. Parental information may be improved by increased knowledge about what affect, and what does not affect, time to full feeds and length of postoperative hospital stay. We suggest that future research examining preoperative, perioperative, and postoperative parameters, or trials comparing different operation techniques or postoperative feeding regimens, when adjusted for corrected age at surgery and presence of underlying diseases.
Acknowledgments
The authors thank Anna Åkesson (statistician, Clinical Trial Unit of Forum South, Lund, Sweden) for help with the statistical analyses.
References
Footnotes
Contributors DE collected the data, drafted the initial manuscript and helped revise the final manuscript. MS conceptualized the design, gathered the cohort, performed the statistical analyses and revised the manuscript. Both authors approved the final manuscript.
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests None declared.
Patient consent for publication Not required.
Ethical approval This study has been approved by the Regional Ethical Review Board (DNR number 2010/49) and by the regional hospital ethics committee and was done in accordance with the principles outlined in the Declaration of Helsinki.
Provenance and peer review Not commissioned; externally peer reviewed.
Data availability statement Data are available upon reasonable request. Data are available upon request to the author (martin.salo@med.lu.se). The data are in form of deidentified participant data.