Article Text
Abstract
Objective Evidence indicates that multistrain probiotics benefit preterm infants more than single-strain (SS) probiotics. We assessed the effects of SS versus triple-strain (TS) probiotic supplementation (PS) in extremely preterm (EP) infants.
Design EP infants (gestational age (GA) <28 weeks) were randomly allocated to TS or SS probiotic, assuring blinding. Reference (REF) group was EP infants in the placebo arm of our previous probiotic trial. PS was commenced with feeds and continued until 37 weeks’ corrected GA. Primary outcome was time to full feed (TFF: 150 mL/kg/day). Secondary outcomes included short-chain fatty acids and faecal microbiota collected at T1 (first week) and T2 (after 3 weeks of PS) using 16S ribosomal RNA gene sequencing.
Results 173 EP (SS: 86, TS: 87) neonates with similar GA and birth weight (BW) were randomised. Median TFF was comparable (11 (IQR 8–16) vs 10 (IQR 8–16) days, p=0.92). Faecal propionate (SS, p<0.001, and TS, p=0.0009) and butyrate levels (TS, p=0.029) were significantly raised in T2 versus T1 samples. Secondary clinical outcomes were comparable. At T2, alpha diversity was comparable (p>0.05) between groups, whereas beta-diversity analysis revealed significant differences between PS and REF groups (both p=0.001). Actinobacteria were higher (both p<0.01), and Proteobacteria, Firmicutes and Bacteroidetes were lower in PS versus REF. Gammaproteobacteria, Clostridia and Negativicutes were lower in both PS versus REF.
Conclusion TFF in EP infants was similar between SS and TS probiotics. Both probiotics were effective in reducing dysbiosis (higher bifidobacteria and lower Gammaproteobacteria). Long-term significance of increased propionate and butyrate needs further studies.
Trial registration number ACTRN 12615000940572.
- probiotics
- intestinal microbiology
- neonatal gut
- infant/neonatal nutrition
Data availability statement
Data are available upon reasonable request. Our clinical trial was commenced in 2015 and completed in 2017.
This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated, and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/.
Statistics from Altmetric.com
Summary box
What is already known about this subject?
Evidence from systematic reviews suggests that multistrain probiotics may benefit enteral nutrition more than single-strain (SS) probiotics in preterm infants. There are limited data on the effect of multistrain probiotic on faecal short-chain fatty acids and microbiome, especially in extremely preterm (EP) infants.
What are the new findings?
This randomised trial compared the effect of SS versus triple-strain (TS) probiotic on the time to full feeds (TFF) in EP infants. TFF was comparable between the SS and TS probiotic groups. Both probiotics were effective in reducing dysbiosis (higher bifidobacteria and lower Gammaproteobacteria). Long-term significance of increased propionate and butyrate needs further evaluation.
How might it impact on clinical practice in the foreseeable future?
These results will help in designing future trials comparing SS versus multistrain probiotics in EP infants and assessing the long-term significance of increased propionate and butyrate in early life.
Introduction
Late-onset sepsis (LOS) and necrotising enterocolitis (NEC ≥stage II) contribute to significant mortality and morbidity, including long-term growth and neurodevelopment in preterm infants, especially those born before 28 weeks’ gestation.1–3 Recently, NEC and LOS have been shown to be preceded by gut dysbiosis.4–6 Preterm infants are at a high risk of gut dysbiosis due to gut immaturity further complicated by environmental exposures (eg, mode of delivery, chorioamnionitis and neonatal intensive care), feeding intolerance and antibiotic exposure.7 Probiotic supplementation (PS) has been proposed to reduce the risk of dysbiosis.5 8
Probiotics are live micro-organisms which, when administered in adequate amounts, confer a health benefit on the host.9 Systematic reviews of randomised controlled trials (RCTs) and non-RCTs have shown that probiotics significantly reduce the risk of all-cause mortality ((relative risk (RR) 0.76, 95% CI 0.65 to 0.89; 51 trials, n=10 170 infants; I²=0%; level of evidence (LoE): moderate),10 NEC (RR: 0.54, 95% /CI 0.45 to 0.65; 54 RCTs, n=10 604 infants; I2=17%; LoE: low),10 LOS (RR 0.89, 95% CI 0.82 to 0.97; 47 trials, n=9762 infants; I²=19%; LoE: moderate)10 and duration of hospitalisation while facilitating enteral nutrition in preterm infants.10–12 The mechanisms of benefits of probiotics include colonisation and normalisation of perturbed intestinal microbial communities, competitive exclusion of pathogens, bacteriocin production, increasing mucin production, modulating intestinal innate immunity and production of short-chain fatty acids (SCFAs), which strengthen the gut epithelial barrier and mediate anti-inflammatory, antimicrobial and immunomodulatory effects.13 14 Probiotics, particularly bifidobacteria, specifically use human milk oligosaccharides and facilitate establishment of a bifidobacteria dominant gut ecosystem while inhibiting pathogenic micro-organisms.15
Evidence suggests that a mixture of probiotic strains may confer more benefits compared with single-strain (SS) probiotics.16–18 Multistrain probiotics containing bifidobacteria have been shown to be effective in preventing NEC, LOS and other morbidities in preterm infants.19 Ishizeki et al have reported that in preterm very low birthweight (VLBW) infants, supplementation with a mixture of three strains (5×108 colony-forming unit (CFU) of each strain, three strains/intervention group) for 6 weeks significantly increased and prolonged detection rates and colony counts of faecal bifidobacteria compared with the SS Bifidobacterium breve M-16V group.20
Based on the evidence in totality and the results of our clinical trial of SS (B. breve M-16V) supplementation, we have been providing routine probiotic prophylaxis using this strain for all preterm infants born <34 weeks’ gestation since 2011.21 22 Considering that multistrain probiotics may be better than SS probiotics, we decided to study this issue in our population of preterm infants. Probiotics are known to improve gut motility and feeding intolerance in preterm infants.23 24 Rapid attainment of full feeds is associated with shorter hospital stay, improved postnatal growth and potentially improved long-term neurodevelopmental outcomes.16 18 25 26 Given these data, we focused on the time to full feeds (TFFs) in our study comparing SS with multistrain bifidobacteria probiotic. We also aimed to assess gut microbiota and faecal SCFA as potential pathways of benefits of probiotics.
Methods
Hypothesis and aim
Our primary aim was to assess the TFF in extremely preterm (EP, gestation <28 weeks) infants supplemented with either an SS or triple-strain (TS) probiotic. Secondary aims included faecal SCFA and microbiota in the SS and TS probiotic groups. We hypothesised that (1) compared with SS, the TS probiotic will reduce TFF by improving gut motility; (2) infants supplemented with SS or TS probiotic will significantly reduce dysbiosis compared with EP infants who received placebo in our previous probiotic trial serving as the reference (REF) group (online supplemental appendix 1).22
Supplemental material
Participant recruitment
Design and setting: A double-blind RCT in EP infants in our tertiary neonatal intensive care unit.
Eligibility criteria: (1) gestation of <28 weeks, (2) readiness to commence on feeds/on feeds for <12 hours and (3) informed parental consent.
Exclusion criteria: (1) congenital malformations, (2) chromosomal aberrations, (3) not being ready for feeds/on feeds for ≥12 hours.
SS probiotic group: B. breve M-16V (3×109 CFU/day).
TS probiotic group: mixture of B. breve M-16V, B. longum subsp. infantis M-63 and B. longum subsp. longum BB536 (3×109 CFU/day).
REF (ie, no probiotic) group: EP infants from the placebo arm of a previous RCT were used as a REF group only for the microbiome analysis in this study.22
Valid comparison: The comparison of SS versus TS probiotic was robust as (1) the SS (B. breve M-16V) was one of the components of the TS product, and (2) the total probiotic dose (3×109 CFU/day) was identical in both groups.
Rationale for selecting the three Bifidobacterium strain products: This was based on clinical and preclinical studies reporting benefits of multistrain probiotic, particularly of Bifidobacterium species in preterm infants.18 19 27 28
Sample size: The mean (±SD) TFF in EP infants was 24 (±14.8) days in our unit.22 Sample size of 75 neonates per group was estimated to achieve 80% power (alpha 0.05) to detect a 30% reduction (clinically significant) in TFF in the SS versus TS probiotic group. To allow for attrition (15%), the sample size was increased to 172.
Primary outcome: TFF measured as the time to reach 150 mL/kg/day feeds from the time feeding was commenced.
Secondary outcomes:
Clinical: NEC ≥stage II, all-cause mortality, duration of parenteral nutrition (PN), length of hospital stay, LOS, intestinal transit time (ITT) using carmine red dye29 and growth at discharge.
Laboratory based: (1) faecal SCFA levels assessed by modified gas chromatography–mass spectrometry30; (2) faecal microbiota assessed using 16S ribosomal RNA gene sequencing. A subset of samples was assessed using next-generation sequencing.
Safety: (1) sepsis due to administered bifidobacteria; (2) abdominal distension, diarrhoea and vomiting leading to cessation of PS. An independent data safety committee monitored all outcomes from enrolment until death or corrected gestational age (CGA) of 37 weeks.
Preplanned subgroup: infants small for gestational age (SGA: birth weight (BW) <10th centile for gestational age (GA)) due to intrauterine growth restriction, considering they are at high risk of mortality and morbidities (eg, NEC, LOS and feed intolerance).31 32
Randomisation, allocation concealment and blinding
Group allocation was based on computer-generated randomisation sequence in random block sizes of 2 and 4. Opaque, sealed and coded envelopes were used for randomisation. Allocation concealment was optimised by prescribing allocation only after obtaining informed parental consent and recording basic neonatal data. The clinical trial pharmacist supplied the randomisation sequence and the sachets (identical design, weight, smell and taste) containing either the SS (B. breve M-16V, 6×109 /g sachet) or the TS (B. breve M-16V, B. longum subsp. infantis M-63 and B. longum subsp. longum BB536; 2×109 of each strain/g sachet) probiotic manufactured by Morinaga Milk Industry Co., Japan, to the nursing staff. This assured masking of all investigators, including outcome assessors, nursing staff and parents.
Probiotic protocol
When ready for feeds, enrolled infants were supplemented with freshly reconstituted contents of the allocated sachets every day and continued until CGA of 37 weeks. The dry lyophilised powder in the sachets was reconstituted using mum’s own milk (first choice) or sterile water for injection. During reconstitution, care was taken to reduce the risk of cross-contamination by adhering to strict hand hygiene, separate preparation of individual doses and avoiding contact with indwelling central lines, tubes and catheters. The single dose (1.5×109 CFU/day as 1 mL of the reconstituted solution) was given via the feeding tube until reaching feeds of 50 mL/kg/day.21 22 It was increased thereafter to 3×109 CFU/day (1 mL reconstituted solution two times per day) once feeds exceeded 50 mL/kg/day. Considering the risk of probiotic sepsis, supplementation was discontinued when feeds were stopped for suspected or proven sepsis and NEC.
Data handling, storage and confidentiality
The National Health and Medical Research Council (NHMRC) Australian guidelines were followed for confidentiality and data storage.33
Reporting
The revised Consolidated Standards of Reporting Trials (CONSORT) guidelines34 were used for reporting the results as highlighted on the EQUATOR network (https://www.equator-network.org/reporting-guidelines/, accessed October 2021).
Faecal sample collection, DNA extraction, SCFA assessment and microbiota analysis details are included in online supplemental appendix 1.
Approach to statistical analysis of clinical, SCFA and microbiome data are included in online supplemental appendix 2.
Results
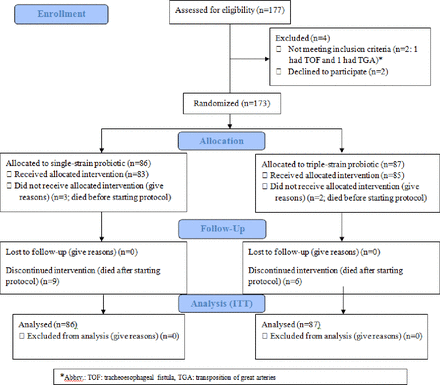
A total of 173 neonates were randomised (SS: 87, TS: 86) between September 2015 and May 2017. Figure 1 outlines the CONSORT flow diagram. Maternal and neonatal demographic characteristics were comparable between the two probiotic groups (table 1).
Consolidated Standards of Reporting Trials flow diagram showing details of the study recruitment. TGA, Transposition of Great Arteries; TOF, Tracheo-oesophageal fistula; ITT: Intention To Treat.
Baseline characteristics of probiotic supplemented (SS and TS) and REF groups
Primary outcome (TFF: 150 mL/kg/day)
The median TFF was comparable between the SS and TS groups (11 (IQR 8–16) vs 10 (IQR 8–16) days; HR 1.02, 95% CI 0.74 to 1.40; p=0.920).
Secondary outcomes
Clinical
There were no significant differences between the SS and TS groups in all-cause mortality (12/86 (14%) vs 8/87 (9.2%), p=0.328); NEC ≥stage II (3/86 (3.5%) vs 3/87 (3.4%), p=1.000); time until NEC or death (HR 0.59, 95% CI 0.24 to 1.46; p=0.253); median duration of PN (10 days in both groups, p=0.265); hospital stay (114 vs 116 days, p=0.750); suspected LOS (44/85 (51.8%) vs 41/86 (47.7%); p=0.593); and blood culture positive LOS (21/85 (24.7%) vs 15/86 (17.4%), p=0.244). Median ITT was comparable (17 hours vs 18 hours, p=0.826). Two infants in the SS group needed surgery for NEC compared with none in the TS group.
Of the 20 deaths in the enrolled infants, five occurred before commencing trial supplementation (SS: 3, TS: 2). Median age at death was similar between groups (10 (IQR 6–24) vs 10 (IQR 4–20) days, p=0.740).
There were no significant differences between the SS and TS groups for other neonatal outcomes such as anthropometry at discharge or incidence of postnatal growth restriction (table 2). Results did not change significantly when analyses were adjusted for multiple births. A ‘per protocol’ analysis including only infants who had at least one treatment dose showed no change in results.
Primary and secondary outcomes
Safety
There were no cases of probiotic sepsis or related adverse effects during the trial period.
Laboratory based
Faecal SCFA
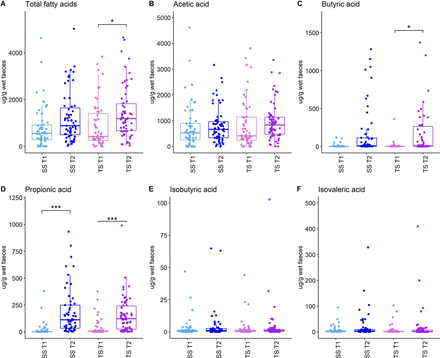
Total fatty acid levels were comparable between the groups at both time points (T1, p=0.92, and T2, p=0.151). Levels were higher in the SS (T2: median 885 (IQR 501–1643) vs T1: median 557 (IQR 290–919) µg/g of wet faeces, p=0.059) and the TS (T2: median 1189 (IQR 674–1825) vs T1: median 422 (IQR 245–1291) µg/g of wet faeces, p=0.014) groups, reaching significance only in the TS group (p=0.014) but not in the SS group (p=0.058). Propionic acid levels were significantly higher at T2 compared with T1 in both SS (T2: median 112 (IQR 34–249) vs T1: median 2 (IQR 1–2), p<0.001) and TS (T2: median 123 (IQR 26–241) vs T1: median 2 (IQR 1–12), p<0.001) groups, whereas butyric acid levels were significantly higher at T2 compared with T1 only in the TS group (T2: median 8 (IQR 2–263) vs T1: median 1 (IQR 1–3), p=0.029) but not in the SS group (T2: median 8 (IQR 3–111) vs T1: median 2 (IQR 1–3), p=0.188) (figure 2A–F).
Combined SCFA (butyric acid, propionic acid and acetic acid) and BCFA (isobutyric and isovaleric acid) for all groups. SCFA and BCFA levels. SCFA (acetic acid (B), butyric acid (C) and propionic acid (D)) and BCFA (isobutyric (E) and isovaleric acid (F)) levels in SimPro groups (SS and TS). Box shows IQR; the line, median and the dots represent an individual sample. Differences between groups and time were calculated using linear mixed effects test with Tukey correction to adjust for multiple testing. Significant differences are indicated by *p<0.05, ***p<0.001. BCFA, branched-chain fatty acid; SCFA, short-chain fatty acid; SS, single strain; TS, triple strain.
Subgroup analysis
Clinical outcomes were comparable in the subgroup of 12 SGA infants (six per group) (online supplemental table 1). Highly variable SCFA levels and small numbers in each group made it difficult to reach any conclusion. Linear mixed effects model test showed non-significance for all cross-sectional and longitudinal analyses.
Supplemental material
Faecal microbiome analysis
Prior to using EP infants in the placebo arm from our previous clinical trial35 as the REF group, diversity analyses were conducted to ensure that faecal community structures of infants at T1 from both studies were similar (online supplemental figures 1 and 2). Alpha-diversity measures: Simpson (REF vs SS, p=0.84; REF vs TS, p=0.84), Shannon (REF vs SS, p=0.74; REF vs TS, p=0.63), ACE (REF vs SS, p=0.27; REF vs TS, p=0.29) and Chao (REF vs SS, p=0.20; REF vs TS, p=0.20) showed no differences (online supplemental figure 1A–D). Beta-diversity measures: weighted Unifrac (REF vs SS, p=0.114; REF vs TS, p=0.429) and Bray Curtis (REF vs SS, p=0.204; REF vs TS, p=0.325) analyses demonstrated that infants from both studies had similar community structures at T1 (online supplemental figure 2A,B).
Richness and diversity
Alpha-diversity analysis showed all groups (REF, SS and TS: SiMPro) had significantly increased bacterial richness at T2 versus T1 (all p<0.001, online supplemental figure 3A,B). However, increased bacterial evenness was observed in infants only in the SS group (p<0.001; online supplemental figure 3C,D). At T2, bacterial richness indices were comparable between all groups (ACE (REF vs SS, p=0.38; REF vs TS, p=0.93; SS vs TS; p=0.38) and Chao1 (REF vs SS, p=0.54; REF vs TS, p=0.58; SS vs TS, p=0.58); figure 3A–D). Alpha-diversity measures showed no difference in within-group variability between REF and SiMPro groups (Shannon (REF vs SS, p=0.79; REF vs TS, p=0.17) and Simpson (REF vs SS, p=0.70; REF vs TS, p=0.16); figure 3C,D). However, TS demonstrated reduced within-group variability compared with SS (Shannon, p=0.02; Simpson, p=0.005; figure 3C,D).
(A–I) Alpha and beta diversity at T2. Box plots of bacterial richness measures; Ace (A) and Chao1 (B) and alpha evenness measures; Shannon (C) and Simpson index (D). Box shows IQR; the line, median and the error bars; the range and the dots are outliers. Differences between groups were calculated using Wilcoxon rank-sum test with Benjamini-Hochberg correction to adjust for multiple testing. Significant differences are indicated by *p<0.05, ** <0.01. (E) Principal coordinate analysis based on weighted Unifrac distances. Each sample is depicted as a dot. (F) Permutational Analysis of Variance (PERMANOVA) was used to identify if there were differences in the community structures by group and time followed by pairwise Adonis test for comparisons between the groups. REF, reference; SS, single strain; TS, triple strain.
Beta-diversity analysis showed significant difference in bacterial community structure of all groups at T2 versus T1 (all p<0.05, online supplemental figure 3E–I). At T2, community structures were significantly different in REF versus both SiMPro groups (SS vs REF: R2=0.135, p=0.001; TS vs REF: R2=0.194, p=0.001; figure 3E) but comparable between SiMPro groups (p=0.149, figure 3E). Community structure in groups was not affected by factors such as ethnicity, gender, mode of delivery and GA but was influenced by duration of antibiotic exposure (PERMANOVA: ethnicity, p=0.93; gender, p=0.50; delivery, p=0.677; GA, p=0.109; duration of antibiotic, p=0.001; online supplemental figure 4).
Relative abundance (RA) of bacterial taxa
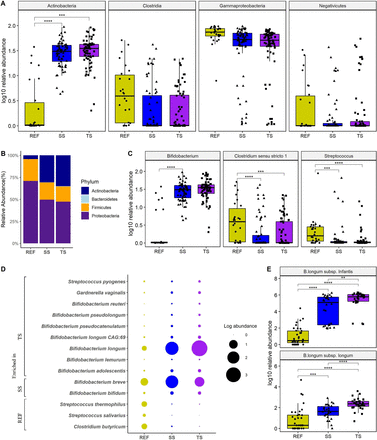
Actinobacteria, Proteobacteria, Bacteroides and Firmicutes were the most prevalent phyla in the faecal samples (figure 4B). Analysis of Composition of Microbiomes with bias correction (ANCOM) analyses revealed Actinobacteria to be significantly enriched in the SiMPro groups compared with the REF group at T2 (online supplemental table 2). At T2, SiMPro groups had increased RA of Actinobacteria compared with the REF group (both p<0.001, online supplemental table 2). Although not significant, SiMPro groups also exhibited decreased RA of Proteobacteria (SS (median 51.7, IQR 38.3–69.8) and TS (median 50.2, IQR 37.2–66.6) compared with the REF group (median 73.2, IQR 60.8–86.5). RA of Firmicutes (SS (median 11.4, IQR 3.9–27.9) and TS (median 10.4, IQR 6.5–21.9) was also reduced in the SiMPro group versus the REF group (median 20.3, IQR 12.4–33.6). At class level, ANCOM analyses revealed Actinobacteria to be significantly different between the groups (online supplemental table 3). At T2, ANCOM analysis showed both SiMPro groups to have significantly increased RA of Actinobacteria (both p<0.0001, figure 4A). Although not significant, Clostridia levels were reduced in both SS (median 0.01, IQR 0–3.04) and TS (median 0, IQR 0–0.01) compared with REF (median 2.95, IQR 0.14–9.41). In addition, Gammaproteobacteria levels were reduced in SS (median 51.7, IQR 38.3–69.8) and TS (median 50.2, IQR 37.7–66.6) compared with REF (median 73.1, IQR 60.8–86.5) (online supplemental table 3). Potentially pathogenic families of Clostridiaceae and Streptococcaceae were significantly lower in both SiMPro groups and REF group at T2 (all p<0.05, online supplemental table 4), while Bifidobacteriaceae (both p=0.00062) was increased (online supplemental table 4). At the genus level, the SiMPro groups had significantly increased RA of Bifidobacterium (all p<0.0001, figure 4C) and decreased RA of Streptococcus (both p<0 .0001, figure 4C) and Clostridium sensu stricto 1 (both p<0 .0001, figure 4C) compared with the REF group (online supplemental table 5). At the species level (using metagenomics data), 28 species were found to be differentially distributed; most of them were of Bifidobacterium species (online supplemental table 6). Of these, 14 species had a mean RA of >0.01% (figure 4D). The REF group was enriched in Clostridium butyricum, Streptococcus salivarius and S. thermophilus (all p<0.01) compared with the SiMPro groups. At this level, differences were found in the SiMPro groups. At T2, the SS group had significantly higher B. breve (p<0.01) and B. bifidum (p<0.05) compared with the TS group, whereas the TS group had significantly increased B. longum (p=0.005), B. longum CAG:69 (p=0.012), B. reuteri (p=0.024), B. pseudocatenulatum CAG:263 (p=0.043), B. pseudocatenulatum (p=0.047), S. pyogenes (p=0.005) and Gardnerella vaginalis (p=0.048) compared with SS group (figure 4D). Subspecies analysis revealed that the SiMPro groups had significantly increased B. longum subsp. infantis and B. longum subsp. longum (all p<0.0001) versus the REF group. However, the TS group had significantly increased B. longum subsp. infantis (p=0.0024) and B. longum subsp. longum (p=0.0001) compared with the SS group (figure 4E).
(A–E) Infant gut microbiota by taxa at T2. Box plots showing RA of class (A), genus (C) and subspecies (E). Box shows IQR; the line, median and the dots represent an individual sample. (B) Shows phylum level composition. (D) Bubble plot of significantly different Species with RA >0.01%. Differences between groups were calculated using Wilcoxon rank-sum test with Benjamini-Hochberg correction to adjust for multiple testing. Significant differences are indicated by **p<0.01, ***p<0.001, ****p<0.0001. RA, relative abundance; REF, reference; SS, single-strain; TS, triple-strain.
Discussion
The results of our double-blind RCT conducted exclusively in EP infants showed that TFF was comparable in SS and TS bifidobacteria-supplemented groups. Furthermore, there were no significant differences in NEC ≥stage II, LOS, all-cause mortality, duration of hospitalisation and ITT. Both groups showed comparable SCFA levels. At T2, propionic acid levels were significantly higher in both SiMPro groups, whereas butyric acid levels were significantly higher only in the TS group. When compared with the REF group (placebo arm of the PANTS trial), both SiMPro groups showed significantly higher bifidobacteria and lower Gammaproteobacteria. Microbial profiles were different at species levels between the SS and TS groups. SiMPro supplements were well tolerated without any adverse effects, including sepsis, due to the administered probiotic strains.
Our study showed no significant difference in the median (IQR) TFF between the infants in the SS and TS groups. It is important to note that sample size for this study was based on the mean (±SD) TFF (24±14.8 days) from our previous placebo controlled RCT assessing product quality and effect of B. breve M-16V supplementation on faecal bifidobacteria counts before introducing routine PS from June 2012.22 Updated data showed significant reduction in mean (±SD) TFF (12±6.5 days) following routine probiotic supplementation (RPS) with B. breve 3 M-16V in infants of <29 weeks’ gestation.21 Using the new estimate of TFF, the SiMPro trial has 95% power to detect a 30% reduction in TFF as desired originally during planning.
It is possible that the median duration of 10–11 days represents the shortest possible TFF considering the strategies for optimising enteral nutrition of EP infants in our unit.35–37 However, it is equally possible that TS probiotic was not superior in reducing TFF compared with SS, or its effect size was smaller than expected. It is important to note that other investigators have reported TFF of 11±3.6,38 12 (9–16)39 and 14 (10–22)40 days in very preterm infants. Boscarino et al concluded that high-energy intake administered through the enteral route was positively correlated to cerebral growth, whereas energy intake via the parenteral route resulted in poorer cerebral growth.41
Gómez-Rodriguez et al conducted an RCT assessing the effect of SS versus multistrain probiotic in 90 very preterm infants.42 Median TFF was 18 (0–56) days vs 15 (0–39) days for the SS group versus multistrain group, respectively. NEC incidence and faecal sIgA levels were comparable between groups.42 Compared with their study,42 exposure to antenatal steroids was higher, and incidence of caesarean delivery, median gestation and BW of study participants and median duration of antibiotics for LOS was lower in our trial. Additionally, median TFF was significantly shorter (15–18 days vs 10–11 days); postnatal age at starting probiotics was earlier (5 days vs 3 days); and exclusive human milk feeding was higher. Priyadarshi et al reported no difference in NEC, TFF and LOS in their retrospective observational study comparing 180 very preterm (gestation <32 weeks) infants (two-strain probiotic group: B. bifidum+Lactobacillus acidophilus, 1×109 CFU each) vs 196 very preterm infants (SS: B. breve M-16V, 2.5×109 CFU/day). TFF was 17.4±11 vs 15±9 days for the two-strain and SS groups, respectively.43
A Cochrane review in 2014 had reported that probiotics reduce the risk of NEC in preterm infants.12 The updated (year 2020) Cochrane review (56 RCTs, n=10 812) also found that probiotics reduce the risk of NEC in very preterm (VP) VLBW infants (evidence grade: ‘low certainty’; RR: 0.54, 95% CI 0.45 to 0.65). Trials using multistrain probiotics showed larger effect size for NEC.10 Evidence for LOS and mortality was graded as ‘moderate certainty’.10 Furthermore, in their systematic review and network meta-analysis, Morgan et al reported moderate to high evidence for superiority of combinations of probiotics containing one or more Lactobacillus or Bifidobacterium compared with other multistrain probiotics.18 It is important to note that probiotic effects are strain-specific, and different strains in a mixture can have synergistic, compatible or antagonistic effects.12 27 44 The reported improved efficacy of multistrain probiotics could simply be due to the higher cumulative dose. Hence, ideal comparison involves identical total dose in SS versus multistrain arms of the trial where SS is also a component of the multistrain probiotic. Our trial involves such a comparison.
Comparing our results with previous studies of SCFA in preterm infants exposed to probiotics is important.45 46 Wang et al randomised 66 preterm infants (extremely low birth weight (ELBW), VLBW, low birth weight: 22 per group) to receive probiotic (B. breve M-16V, 1.6×108 CFU two times per day) or no probiotic (control).45 Compared with birth, faecal acetate and total SCFA were significantly higher at 2 and 4 weeks, but butyric acid levels were significantly lower.45 Considering the differences in eligibility criteria (ELBW vs EP), method of SCFA assay and units of measurement, comparing results of the 22 ELBW infants in Wang et al with 86 infants (SS group) in our study is difficult. Infants in our study received exclusive human milk diet and higher probiotic dose for longer duration.
Long-term follow-up of our cohort is important, considering the clinical significance of increased propionate and butyrate levels (reduced allergy, asthma, obesity, metabolic syndrome and improved neurodevelopment).47–49 SCFAs benefit through their influence on Treg biology, epithelial integrity, gut homeostasis, dendritic cell biology, gene transcription and IgA antibody responses.13 14 Gut microbiota may regulate neurodevelopment and neurobehaviour by various mechanisms including SCFA modulation through the gut–brain axis.49
To our knowledge, there are no previous RCTs reporting on faecal microbiota of SS versus multistrain probiotics in preterm infants. Previous RCTs of SS40 50 51 or multistrain probiotic39 versus placebo have reported variable effects on gut colonisation. Previous studies have reported increased Proteobacteria, Firmicutes and coagulase-negative staphylococci (CONS) in infants with NEC.4 Both SiMPro groups had decreased Gammaproteobacteria and Clostridia and reduced CONS, especially in the TS group versus REF group.
To our knowledge, SiMPro is the first RCT with robust design for comparing SS and multistrain probiotics with adequate power (95%) for a clinically important primary outcome (TFF) in EP infants.41 Our comprehensive microbiome analysis was based on 16SrRNA gene and metagenomic sequencing. Our trial was not powered for NEC as primary outcome considering its low incidence in our unit.21 22 SiMPro groups had significantly increased levels of Bifidobacterium at T2 versus T1 compared with REF clearly demonstrating effect of PS on infant gut microbiome. The absence of a placebo arm in our RCT due to ethical difficulties was another limitation. Although we used the placebo arm of the PANTS study as our REF group, we cannot discount the possibility of batch effect as a confounder in our study. Furthermore, this also resulted in being unable to provide REF SCFA levels in EP infants not receiving probiotic. Previous studies are not helpful in this context due to methodological differences.45 46
In conclusion, TFF and other clinical outcomes in EP infants were similar between SS and TS strain probiotics. The long-term significance of raised propionate and butyrate needs to be studied.
Data availability statement
Data are available upon reasonable request. Our clinical trial was commenced in 2015 and completed in 2017.
Ethics statements
Patient consent for publication
Ethics approval
This study involves human participants and was approved by Women and Newborn Health Ethics Committee. Participants gave informed consent to participate in the study before taking part.
Acknowledgments
We sincerely thank the following: Professor Fumiaki Abe and Noriyuki Iwabuchi of Morinaga Milk Industry, Japan, for providing trial supplements free of cost; Annie Chang, Melanie McDougall and Chooi Yen Kok: nursing research assistants, data collection, recruitment, sample collection and preparation for storage; Nabeelah Mukadam and Michael Petrovski, KEMH pharmacy department; Dr J Tan, consultant neonatologist at Princess Margaret and Perth Children’s Hospital for his role on the data monitoring committee for the study duration; nursing staff at King Edward Memorial and Princess Margaret Hospital for collecting stool samples and administering the trial supplements to study infants; parents for providing informed consent for participation of their infants in the randomised trial; Mr Rikky Purbojati, Nanyang Technological University, Singapore, and Dr Daniela Moses, Deputy Research Director, Meta-‘omics and Microbiomes, Nanyang Technological University, Singapore: assistance with bioinformatics analysis and interpretation. Dr J Tan and Dr R Jois, Department of Neonatology, Joondalup Health Campus (JHC), Perth, Western Australia: assistance with trial protocol continuation at JHC in trial participants who were transferred before 37 weeks completed GA and for provision of relevant data at discharge. Dr M Deshmukh, Dr. J Du-Plessis and Dr S Mehta, Department of Neonatology, Fiona Stanley Hospital (FSH), Perth, Western Australia: assistance with trial protocol continuation at FSH in trial participants who were transferred before 37 weeks completed GA and for provision of relevant data at discharge.
References
Supplementary materials
Supplementary Data
This web only file has been produced by the BMJ Publishing Group from an electronic file supplied by the author(s) and has not been edited for content.
Footnotes
Twitter @GayatriJape
Contributors GJ contributed to data acquisition, infant recruitment, ethics, governance and TGA application, funding application, setting up the clinical trial including liaising with pharmacy, ordering trial equipment for storage of faecal samples, supervision of project running, data interpretation, writing first and final draft of manuscript and revision of manuscript for critically important intellectual content. ME contributed to analysis and interpretation of data including bioinformatics details, critical revision of the manuscript for important intellectual content. SP and KS contributed to conception and design, data interpretation, revision of manuscript for critical important intellectual content, supervision of project running and manuscript writing. SP was also the guarantor for the work conducted in this study. EN and DD contributed to statistical input into design, analysis and interpretation of clinical and short-chain fatty acid (SCFA) data and revision of the manuscript for critical important intellectual content. AK contributed to conception and design, and revision of the manuscript for critical important intellectual content, assuring independent safety check of trial probiotic products. SR assisted with funding application and revision of the manuscript for critically important intellectual content. LChe contributed to sample analysis for SCFA, SCFA data analysis and interpretation and revision of manuscript for critically important intellectual content. LCha and SS contributed to sample analysis for metagenomic sequencing and data interpretation; CK contributed as research assistant and for data collection, data cleaning, participant recruitment, sample collection and preparation for storage. PC contributed to critical revision of the manuscript for important intellectual content. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work.
Funding Telethon-WIRF Channel-Seven Trust Grant and Princess Margaret Hospital Foundation (PMHF) Translational Grant.
Disclaimer The funding organisations played no role in the design and conduct of the study. The manufacturer Morinaga Milk Industry Co, Japan, was not the sponsor but only supplied the probiotic products free of cost for the trial and was not involved in the design, conduct, analysis and reporting of the trial.
Competing interests None declared.
Provenance and peer review Not commissioned; externally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.