Abstract
Coelastrella rubescens Kaufnerová & Eliás (Chlorophyceae) is a green, single-celled algae that lives in the terrestrial-air environment. Under stress conditions, its cells go into a state characterized by low photosynthetic activity and high content of reserve lipids and secondary carotenoids. For the first time, a comparative morphological, ultrastructural, and elemental analysis of vacuolar inclusions in the C. rubescens NAMSU R1 strain when cultivated on a mineral medium under conditions of low and high (causing stress) light intensity. Microalgae cells stained with the fluorescent dye DAPI showed signs of the presence of polyphosphates. Polarization microscopy in cells of C. rubescens has identified structures capable of refracting polarized light, which is typical of crystals. Cell analysis of C. rubescens with the transmission electron microscopy (TEM) method revealed the presence of various vacuoles with heterogeneous contents (autophagic bodies, crystalloids, and rounded globules of inhomogeneous electron density). With the exception of autophagic bodies noted in cells only in bright light, these inclusions were characteristic of microalgae cells, regardless of the intensity of illumination. The elemental composition of vacuolar inclusions was characterized by TEM in combination with energy-dispersive X-ray spectroscopy: the predominant content of nitrogen, phosphorus, or both elements simultaneously was established in them. The potential physiological role of C. rubescens vacuolar inclusions is discussed.
Similar content being viewed by others
INTRODUCTION
Unicellular green algae of the genus Coelastrella (Chlorophyceae, Sphaeropleales) are typical inhabitants of the terrestrial-air environment. They occur as a component of fouling of rocks, buildings, and tree bark and are widespread in temperate and polar latitudes and high mountain regions [1–5]. The ground-air environment is characterized by a combination of a number of environmental factors that are stressful for photosynthetic microorganisms, such as a high level of insolation, sharp fluctuations in humidity and temperature, and a lack of mineral nutrition [6]. Therefore, they have an extensive arsenal of adaptations to adverse conditions. Among them, one can single out the accumulation of light filter compounds that screen radiation in the visible and UV ranges (carotenoids, mycosporin-like amino acids, etc.) [7–10], DNA repair systems [7], enzymes for the elimination of reactive oxygen species [7], effective nonphotochemical quenching of excited states of chlorophyll [10, 11], and reversible reduction of the photosynthetic apparatus [11, 12].
Coelastrella rubescens is a typical member of the genus Coelastrella [1]. In a number of works, it is considered as a producer of astaxanthin [13, 14], a carotenoid of great practical importance. It is a component of various cosmetics, functional nutrition, and feed for fish and crustaceans in aquaculture. [15]. Astaxanthin accumulates in microalgae cells as a secondary carotenoid, i.e., structurally and functionally unrelated to the photosynthetic apparatus. For the NAMSU R1 strain studied in this work, the accumulation of a mixture of carotenoids, astaxanthin, and β-carotene was shown under stress [9]. This circumstance makes research of C. rubescens, in particular the NAMSU R1 strain, relevant.
A vacuole is a specific compartment of plant cells characterized by diversity both in terms of the composition of its components and the functions it performs. They can store biogenic elements and (or) deposit end products of metabolism. Vacuoles may contain inclusions of a different nature, such as oxalates, polyphosphates [16–19], and derivatives of purine nucleotides [17, 20, 21].
A special place in the functioning of the algae cell occupy vacuoles involved in the process of autophagy and selective isolation and degradation of damaged or abnormally folded molecules and organelles as well as components of the latter [11, 22]. Autophagy plays an important role in the acclimation of photoautotrophs to unfavorable conditions [11, 23, 24]. In C. rubescens cells, vacuoles containing chloroplast fragments and autophagosomes fusing with them have previously been described [9]. Moreover, inclusions of different structure and electron density, presumably different chemical nature, have been found in vacuoles of C. rubescens.
The purpose of the work is to study the localization, structure, and elemental composition of vacuolar inclusions in C. rubescens strain NAMSU R1 green microalgae.
MATERIALS AND METHODS
Strain and Cultivation Conditions
NAMSU R1 strain of green microalgae Coelastrella rubescens Kaufnerová & Eliás was isolated and described earlier [9]. The culture was grown in a periodic mode on the BG-11 medium [25] and under constant illumination of fluorescent lamps with cold white light 40 µmol photons/(m2s (hereinafter referred to as “low-light intensity”), temperature 24°C, and constant stirring at 85 rpm in a New Brunswick Innova 44 shaker-incubator (Eppendorf, Germany) for 15 days. During this period, samples were taken every 1–2 days to record the growth curve. The dry mass was measured gravimetrically according to [26]. Upon reaching the culture of the stationary phase of growth, the suspension was divided into two equal parts, one of which continued to be incubated under the same conditions without stirring. The other part of the suspension was incubated at high light intensity causing stress (150 µmol photons/(m2 s). The total incubation period was 36 days.
Light Microscopy
The presence of inclusions in cells was detected by optical microscopy. Staining of polyphosphates in cells was performed using a fluorescent dye solution. 4',6-diamidino-2-phenylindole (4',6-diamidino-2-phenylindole, DAPI) in dimethyl sulfoxide, concentration 1 mg/mL. We added 10 µL of DAPI stock solution to 90 µL of cell suspension and incubated it for 20 min in the dark. Polyphosphate fluorescence was visualized on a Leica DM2500 microscope. (Leica Microsystems, Germany) with a Leica DFC700T camera. Fluorescence was excited by radiation from an HXP 120 UV lamp. (Leica Microsystems, Germany) equipped with a filter D of the same manufacturer, in the range of 355–425 nm. Fluorescence emission was detected in the range of 455–700 nm. Visualization of crystalline inclusions in cells was carried out on a Leica DM2500 microscope with a Leica DFC495 camera in polarized light when the polarizer and analyzer are crossed at an angle 90°.
Transmission Electron Microscopy (TEM)
The cell ultrastructure and elemental composition of cell inclusions were studied by traditional transmission electron microscopy (TEM) and analytical TEM, respectively. Cell fixation and dehydration were performed according to the protocol described in Gorelova et al. [27]. Cells were first fixed in 2% (v/v) glutaraldehyde prepared in 0.1 mM sodium cacodylate buffer (pH 7.4) at room temperature for 30 min. Next, postfixation of the cells was carried out in a 1% (by mass) solution of osmium tetraoxide prepared in the same buffer for 4 h. The samples were embedded in the Araldite epoxy mixture (Sigma-Aldrich, United States). To study the ultrastructure of cells and the elemental composition of cell inclusions, ultrathin sections and semithin sections were prepared, respectively. Sections were prepared using a Leica EM UC7 ultratome (Leica Microsystems, Germany) and an Ultra 45° diamond knife (DiATOME, Switzerland). The sections were mounted on copper grids for electron microscopy with an ultrathin formvar substrate (Ted Pella, United States).
To study the ultrastructure by TEM, the sections were additionally contrasted with lead citrate solution [28]. The images were obtained using JEM-1011 and JEM-1400 electron microscopes (JEOL, Japan). The dimensions of cell structures were measured using TEM images obtained on ultrathin sections using the Fiji (ImageJ) v. 20200708-1553 (NIH, United States).
The elemental analysis of inclusions by analytical TEM was performed using energy dispersive X-ray spectroscopy (EDX), as described earlier [16], on a JEOL-2100 electron microscope (JEOL, Japan) equipped with a bright-field detector for operation in the scanning TEM (STEM) mode (JEOL, Japan) and X-Max X-ray detector (Oxford Instruments, United Kingdom). For each sample, spectra were obtained from dotted areas of at least ten cells. The spectra were processed using the INKA program (Oxford Instruments, United Kingdom) and presented in the range 0.1–4 keV.
RESULTS
Morphological Features of the Strain
At low light intensity, culture of microalgae C. rubescens NAMSU R1 is represented by solitary coccoid cells and autosporangia 10–15 µm in diameter, stained bright green (Figs. 1a, 2a). Under high-intensity light, the cells acquired an orange color, which indicates the accumulation of secondary carotenoids in the cells (Figs. 1c, 2c). Both under standard and stress conditions, autosporangia with autospores were observed (Fig. 1). On the 12th day of growth, the culture reached the stationary phase (Fig. 2).
Visualization of polyphosphate inclusions after DAPI staining in C. rubescens NAMSU R1 cells under illumination with (a, b) low- and (c, d) high-intensity light; (a, c) bright-field microscopy; (b, d) fluorescence microscopy. The glow of chlorophyll has a red tint, colored polyphosphates are yellow-green. Arrows indicate polyphosphate inclusions. The length of the scale bar corresponds to 2 µm.
Visualization of Cellular Inclusions using Light Microscopy
In the cells of the strain C. rubescens NAMSU R1 cultivated at both variants of the PAR flux intensity, various inclusions were detected using light microscopy (Fig. 1). In cells cultured in low-intensity light, we noted a few rounded structures with a diameter of 0.1–1.3 µm (Figs. 1a, 1b). Similar inclusions were observed in cells cultivated in high-intensity light (Figs. 1c, 1d). To determine the potential chemical nature of the inclusions, the cells were stained with DAPI, a dye that has an affinity for a number of cellular components, in particular, for polyphosphates. In both variants of the experiment in the cytoplasm of cells, fluorescence in the yellow-green region of the spectrum characteristic of polyphosphate granules was observed. The data obtained indicated the accumulation by C. rubescens cells of reserves of inorganic phosphorus in the form of polyphosphates in the light of both low and high intensity.
In addition, microalgae cells contained inclusions that did not exhibit fluorescence characteristic of polyphosphates after staining with DAPI (Fig. 1). The use of polarization microscopy made it possible to visualize these cell structures, which give a bright signal of polarized light (Fig. 3). By visual assessment, the occurrence and abundance of crystals in cells cultured under low intensity light was higher than in cells cultured under high-intensity light.
Visualization of crystal structures using polarizing microscopy in C. rubescens NAMSU R1 cells under illumination with (a, b) low- and (c, d) high-intensity light; (a, c) bright-field microscopy; (b, d) polarization microscopy. Crystals in the cells are visible as bluish-green inclusions. The length of the scale bar corresponds to 10 µm.
Ultrastructural Features of Vacuolar Inclusions
Cells of C. rubescens NAMSU R1 cultivated in low-intensity light had a relatively well-developed photosynthetic apparatus represented by a chloroplast (Fig. 4a) containing one (rarely two) pyrenoid and starch grains. The cells also contained one nucleus and vacuoles with different contents. In cells cultured in high-intensity light, the photosynthetic apparatus was less developed (Fig. 5a). A significant proportion of the area in the sections of such cells was occupied by lipid inclusions of low electron density (oleosomes).
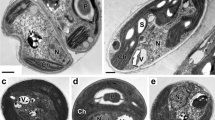
Variety of vacuolar inclusions in C. rubescens NAMSU R1 cells grown in low-intensity light: (a) general view of cells, (b–f) images of vacuoles with inclusions of different structures; (a, d–f) ultrathin sections; (b, c) semithin sections. V—vacuoles; Os—oleosomes; P—pyrenoid; Chl—chloroplast; R—nucleus. Scale bars: (e) 100 nm, (b, c, f) 200 nm, (a) 500 nm.
Variety of vacuolar inclusions in C. rubescens NAMSU R1 cells grown in high-intensity light: (a) general view of cells, (b–f) images of vacuoles with inclusions of different structures; (a, b, d–f) ultrathin sections; (c) semithin section. B—vacuoles; Os—oleosomes; Chl—chloroplast; N—nucleus. Scale bars: (d, f) 100 nm, (b, c, e) 200 nm, (a) 500 nm.
In cells and autospores in sporangia in the case of cultivation in the light of both low (Fig. 4) and high (Fig. 4) intensity, vacuoles with inclusions of various ultrastructure, packing, and electron density were revealed. In the first case, rounded globules 0.1–1.3 μm in size with a material of uniformly high electron density or similar globules with angular, less electron-dense lamellar structures embedded in them, morphologically similar to crystals, were noted (Fig. 4a). In some vacuoles, such small (Fig. 4b) or larger (Fig. 4c) inclusions dominated. Inclusions were also noted that combined, in different proportions, parts of lamellar structures and ordered regions with alternating electron-dense and electron-transparent bands of equal width 4.1 ± 0.2 nm as well as randomly distributed granules (Figs. 4d, 4e). We also encountered vacuoles with electron-dense granules of different sizes, where there were no crystal-like structures (Fig. 4f).
Research of C. rubescens NAMSU R1 cells cultured in high light (Fig. 4a) showed the presence of vacuoles with inclusions similar to those found in cells cultured in low light. In particular, vacuoles were noted that contained lamellar crystal-like structures (Figs. 5a, 5b, 5c), vacuoles, in which, in addition to such inclusions, there were formations characterized by alternating bands of low and high electron density (Figs. 5d, 5e) and, in some cases, there were also small globules (from 15 to 50 nm) with an increased electron density (Figs. 5e, 5f). A distinctive feature of the heterogeneous content of vacuoles in cells grown in high-intensity light was the detection of autophagic bodies (Figs. 5a, 5b, 5d).
Analysis of the Elemental Composition of the Detected Vacuolar Inclusions
Research of C. rubescens NAMSU R1 cells cultivated in light of different intensity revealed the presence of vacuolar inclusions of different types. Figure 6 shows representative spectra for vacuolar structures characterized by the content of predominantly phosphorus (P) (the characteristic X-ray energy is 2.013 keV) (Fig. 6a), nitrogen (N) (0.392 keV) (Fig. 6b), and the joint presence of these chemical elements (Fig. 6c). The spectra of phosphorus-containing inclusions also included peaks of magnesium (Mg) (1.253 keV) and calcium (Ca) (main signal at 3.690 keV) (Fig. 6a), while only the Ca peak was present in the spectra of mixed-type inclusions (Fig. 6c). In the spectra of both phosphorus-containing and mixed-type inclusions, the P peak partially overlapped with the osmium (Os) peak. Osmium tetroxide was used to fix the samples (see Materials and Methods). Spectra with a predominant content of P (Fig. 6a) corresponded to inclusions of high electron density, namely, large vacuolar globules in cells exposed to low-intensity light (Fig. 4a), smaller globules in mixed inclusions (Figs. 4f, 5f), and areas of inclusions with alternating bands of high and low electron density (Figs. 4d, 4e; 5e, 5f) in the light of both illumination levels. The spectra corresponding to a high N content (Fig. 6b) were characteristic of inclusions of a crystalline type noted for both cultivation conditions (Figs. 4a–4e, 5). In this case, the point spectra of X-rays containing peaks N and P (Fig. 6c) were obtained from cellular inclusions of a mixed type in the region of boundaries of more electron-dense P-containing structures and less electron-dense (in the case of ultrathin sections) N-containing crystals (Figs. 4b, 4d, 4e, 5b, 5e, 5f).
DISCUSSION
In general, features of cell morphology and ultrastructure of C. rubescens NAMSU R1 under the described cultivation conditions were similar to those for representatives of the genus Coelastrella [1, 3, 9, 13, 14]. In the case of culturing in low-intensity light, the cells exhibited features characteristic of a culture with an active metabolism: a developed photosynthetic apparatus and the presence of a large number of dividing cells. Previously, it was shown that increasing the light intensity to 150 µmol photons/(m2 s) (denoted in this work as high-intensity light) led to a decrease in the maximum photochemical efficiency FSII, the reduction of the photosynthetic apparatus, and the appearance of details of secondary carotenoids in the absorption spectra [9]. This is a typical response of terrestrial air microalgae to stress [6, 29]. An indirect indication that the C. rubescens NAMSU R1 cells cultivated in high-intensity light were under stress is that they had a characteristic orange color, indicating the accumulation of secondary carotenoids.
Regardless of light intensity, culture research of C. rubescens NAMSU R1 using a combination of microscopy methods revealed the presence of vacuolar inclusions of different structure and chemical composition. In particular, vacuolar inclusions were noted, which accumulated mainly one of the elements, P or N, and inclusions enriched both of these elements.
According to the results of standard and analytical TEM, no large inclusions containing predominantly P were noted. In this case, the point spectra in the region of electron-dense regions of heterogeneous inclusions had a form typical of phosphorus-enriched structures identified as polyphosphates [16]. Vacuolar structures, represented by alternating bands with high and low electron density and characterized by a P peak in the EDRS spectra, were apparently organized according to the previously described type of “multicore cable” proposed for polyphosphate inclusions [16, 17]. The accumulation of the P reserve in this form is known for many microalgae [16, 17, 30, 31]. The formation of polyphosphates can occur under different conditions of the availability of this element in the medium, both in its deficiency and in its excess (as a result of excessive absorption of P) [30–33].
In cells incubated in low and high light intensity, vacuolar inclusions were noted containing predominantly N and having a crystalline structure (Fig. 2). It is known that the reserve of this biogenic element in microalgae cells can be represented by various derivatives of purine bases, such as microcrystalline inclusions of uric acid [34] or guanine [20, 21]. In recent years, interest has been growing in the deposition of N in the cells of eukaryotic microalgae in the form of crystals of purine bases. Moreover, this form of N storage is found in different taxonomic groups of microalgae [20, 21]. In this work, crystals with a high content of N in the composition of vacuolar inclusions were first discovered in Coelastrella. Among the representatives of the Scenedesmaceae family, to which this genus belongs, the formation of N-containing crystals in the cells of microalgae of the genera Desmodesmus and Tetradesmus was observed [16, 18–21]. At the same time, it was shown that the formation of these vacuolar inclusions occurs under conditions of sufficient or excess N content in the medium for subsequent use under conditions of a deficiency of this element [16, 19, 21]. In this work, the formation of N-containing crystals was noted under conditions of the late stationary phase of C. rubescens culture growth at different light intensities. Since an increase in biomass by less than two times was recorded during the cultivation period, which preceded the transfer of cell biomass under conditions of different levels of illumination, it can be assumed that there is an excess content of nutrients in the medium due to the low cell density. Excessive absorption of nitrates and phosphates from the environment could be toxic to cells without converting them into poorly soluble compounds deposited in their isolating compartment (vacuoles). In addition, it is assumed that guanine crystals have a wider range of functions than N depot, in particular, modulation of the intensity and spectral composition of light available for photosynthesis [21].
Among the representatives of the genus Coelastrella, carotenogenic microalgae [3, 13, 14] capable of accumulating high amounts of secondary carotenoids are known [15]. The presence of rounded electron-dense inclusions in cell vacuoles was shown previously for another carotenogenic algae, Haematococcus lacustris (Gir.-Chantr.) Rostaf. (Volvocales, Chlorophyceae) [35]. The authors of the cited publication suggested that that they corresponded to inclusions of astaxanthin (English astaxanthin granules) and were involved in the metabolism of secondary carotenoids. Inclusions similar in localization, structure, and electron density were also found in C. rubescens in the present work. However, based on the TEM-EDRS analysis of the elemental composition, it can be concluded that they are polyphosphates rather than carotenoid inclusions. Thus, we can conclude that it is necessary to use analytical TEM to characterize vacuolar inclusions and clarify the existing ideas about the nature of certain types of intracellular inclusions in carotenogenic microalgae.
This work characterizes the vacuoles that form in the C. rubescens NAMSU R1 cells cultured under normal- and high-light stress conditions. The polyfunctionality of these organelles is shown in connection with the accumulation in them of inclusions differing in structure, functions, and elemental composition. Probably, the vacuoles in this species of microalgae are involved in acclimation to unfavorable environmental conditions, i.e., play the role of “adaptive organelles.” Such organelles provide intracellular homeostasis due to the formation and consumption of intracellular reserves of biogenic elements and also implement emergency rearrangements in the cell according to the mechanism of stress-induced autophagy.
Change history
21 September 2022
An Erratum to this paper has been published: https://doi.org/10.1134/S1021443722330020
REFERENCES
Kaufnerová, V. and Eliáš, M., The demise of the genus Scotiellopsis Vinatzer (Chlorophyta), Nova Hedwigia, 2013, vol. 97, p. 415. https://doi.org/10.1127/0029-5035/2013/0116
Skrebovs’ka, S.V. and Kostikov, I.Yu., Scotielleopsis levicostata (Chlorophyta) in the Scenedesmaceae system, Chornomorsk. Bot. Zh., 2012, vol. 8, p. 401.
Chekanov, K., Fedorenko, T., Kublanovskaya, A., Litvinov, D., and Lobakova, E., Diversity of carotenogenic microalgae in the White Sea polar region, FEMS Microbiol. Ecol., 2020, vol. 96, p. 183. https://doi.org/10.1093/femsec/fiz183/5632105
Goecke, F., Noda, J., Paliocha, M., and Gislerød, H.R., Revision of Coelastrella (Scenedesmaceae, Chlorophyta) and first register of this green coccoid microalga for continental Norway, World J. Microbiol. Biotechnol., 2020, vol. 36, p. 1. https://doi.org/10.1007/s11274-020-02897-0
Stewart, A., Rioux, D., Boyer, F., Gielly, L., Pompanon, F., Saillard, A., Thuiller, W., Valay, J., Maréchal, E., and Coissac, E., Altitudinal zonation of green algae biodiversity in the French Alps, Front. Recent Dev. Plant Sci., 2021, vol. 12, p. 1066. https://doi.org/10.3389/fpls.2021.679428
Holzinger, A. and Karsten, U., Desiccation stress and tolerance in green algae: consequences for ultrastructure, physiological and molecular mechanisms, Front. Plant Sci., 2013, vol. 4, p. 327. https://doi.org/10.3389/fpls.2013.00327
Ugya, A., Imam, T., Li, A., Ma, J., and Hua, X., Antioxidant response mechanism of freshwater microalgae species to reactive oxygen species production: a mini review, Chem. Ecol., 2020, vol. 36, p. 174. https://doi.org/10.1080/02757540.2019.1688308
Rastogi, R.P., Sonani, R.R., Incharoensakdi, A., and Madamwar, D., Sun-screening biomolecules in microalgae: role in UV-photoprotection, in UV-B Radiation: From Environmental Stressor to Regulator of Plant Growth, Singh, V.P., Singh, S., Prasad, S.M., and Parihar, P., Eds., Chichester: Wiley, 2017, ch. 11, p. 197. https://doi.org/10.1002/9781119143611.ch11
Zaytseva, A., Chekanov, K., Zaytsev, P., Bakhareva, D., Gorelova, O., Kochkin, D., and Lobakova, E., Sunscreen effect exerted by secondary carotenoids and mycosporine-like amino acids in the aeroterrestrial chlorophyte Coelastrella rubescens under high light and UV-A irradiation, Plants, 2021, vol. 10, p. 2601. https://doi.org/10.3390/plants10122601
Chekanov, K., Schastnaya, E., Neverov, K., Leu, S., Boussiba, S., Zarka, A., and Solovchenko, A., Non-photochemical quenching in the cells of the carotenogenic chlorophyte Haematococcus lacustris under favorable conditions and under stress, Biochim. Biophys. Act-a, Gen. Subj., 2019, vol. 1863, p. 1429. https://doi.org/10.1016/j.bbagen.2019.05.002
Solovchenko, A., Baulina, O., Ptushenko, O., and Gorelova, O., Ultrastructural patterns of photoacclimation and photodamage to photosynthetic algae cell under environmental stress, Physiol. Plant., 2019, vol. 166, p. 251. https://doi.org/10.1111/ppl.12912
Chekanov, K., Vasilieva, S., Solovchenko, A., and Lobakova, E., Reduction of photosynthetic apparatus plays a key role in survival of the microalga Haematococcus pluvialis (Chlorophyceae) at freezing temperatures, Photosynthetica, 2018, vol. 56, p. 1268. https://doi.org/10.1007/s11099-018-0841-5
Minyuk, G., Chelebieva, E., Chubchikova, I., Dantsyuk, N., Drobetskaya, I., Sakhon, E., Chivkunova, O., Chekanov, K., Lobakova, E., Sidorov, R., and Solovchenko, A., pH and CO2 effects on Coelastrella (Scotiellopsis) rubescens growth and metabolism, Russ. J. Plant Physiol., 2016, vol. 63, p. 566. https://doi.org/10.1134/S1021443716040105
Minyuk, G., Chelebieva, E., Chubchikova, I., Dantsyuk, N., Drobetskaya, I., Sakhon, E., Chekanov, K., and Solovchenko, A., Stress-induced secondary carotenogenesis in Coelastrella rubescens (Scenedesmaceae, Chlorophyta), a producer of value-added keto-carotenoids, Algae, 2017, vol. 32, p. 245. https://doi.org/10.4490/algae.2017.32.8.6
Liu, C., Hu, B., Cheng, Y., Guo, Y., Yao, W., and Qian, H., Carotenoids from fungi and microalgae: A review on their recent production, extraction, and developments, Bioresour. Technol., 2021, vol. 337, art. ID 125398. https://doi.org/10.1016/j.biortech.2021.125398
Shebanova, A., Ismagulova, T., Solovchenko, A., Baulina, O., Lobakova, E., Ivanova, A., Moiseenko, A., Shaitan, K., Polshakov, V., Nedbal, L., and Gorelova, O., Versatility of the green microalga cell vacuole function as revealed by analytical transmission electron microscopy, Protoplasma, 2017, vol. 254, p. 1323. https://doi.org/10.1007/s00709-016-1024-5
Pugacheva, T.T., Monitoring the formation, subcellular distribution, and consumption of phosphorus and nitrogen reserves in phototrophic microorganisms by analytical transmission electron microscopy, Cand. Sci. (Biol.) Dissertation, Moscow: Moscow State Univ., 2019.
Ismagulova, T., Chekanov, K., Gorelova, O., Baulina, O., Semenova, L., Selyakh, I., Chivkunova, O., Lobakova, E., Karpova, O., and Solovchenko, A., A new subarctic strain of Tetradesmus obliquus, Part I: Identification and fatty acid profiling, J. Appl. Phycol., 2018, vol. 30, p. 2737. https://doi.org/10.1007/s10811-017-1313-1
Ismagulova, T., Shebanova, A., Gorelova, O., Baulina, O., and Solovchenko, A., A new simple method for quantification and locating P and N reserves in microalgal cells based on energy-filtered transmission electron microscopy (EFTEM) elemental maps, PLoS One, 2018, vol. 13, p. E0208830. https://doi.org/10.1371/journal.pone.0208830
Moudříková, Š., Mojzeš, P., Zachleder, V., Pfaff, C., Behrendt, D., and Nedbal, L., Raman and fluorescence microscopy sensing energy-transducing and energy-storing structures in microalgae, Algal Res., 2016, vol. 16, p. 224. https://doi.org/10.1016/j.algal.2016.03.016
Mojzeš, P., Gao, L., Ismagulova, T., Pilátová, J., Moudříková, Š., Gorelova, O., Solovchenko, A., Nedbal, L., and Salih, A., Guanine, a high-capacity and rapid-turnover nitrogen reserve in microalgal cells, Proc. Natl. Acad. Sci. U.S.A., 2020, vol. 117, p. 32722. https://doi.org/10.1073/pnas.2005460117
Zhang, Z., Sun, D., Cheng, K.W., and Chen, F., Inhibition of autophagy modulates astaxanthin and total fatty acid biosynthesis in Chlorella zofingiensis under nitrogen starvation, Bioresour. Technol., 2018, vol. 247, p. 610. https://doi.org/10.1016/j.biortech.2017.09.133
Wang, X., Song, Y., Liu, B., Hang, W., Li, R., Cui, H., Li, R., and Jia, X., Enhancement of astaxanthin biosynthesis in Haematococcus pluvialis via inhibition of autophagy by 3-methyladenine under high light, Algal Res., 2020, vol. 50, p. 101991. https://doi.org/10.1016/j.algal.2020.101991
Gorelova, O., Baulina, O., Ismagulova, T., Kokabi, K., Lobakova, E., Selyakh, I., Semenova, L., Chivkunova, O., Karpova, O., Scherbakov, P., Khozin-Goldberg, I., and Solovchenko, A., Stress-induced changes in the ultrastructure of the photosynthetic apparatus of green microalgae, Protoplasma, 2019, vol. 256, p. 261. https://doi.org/10.1007/s00709-018-1294-1
Stanier, R., Kunisawa, R., Mandel, M., and Cohen-Bazire, G., Purification and properties of unicellular blue-green algae (order Chroococcales), Bacteriol. Rev., 1971, vol. 35, p. 171. https://doi.org/10.1128/br.35.2.171-205.1971
Pal, D., Khozin-Goldberg, I., Cohen, Z., and Boussiba, S., The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp., Appl. Microbiol. Biotechnol., 2011, vol. 90, p. 1429. https://doi.org/10.1007/s00253-011-3170-1
Gorelova, O., Baulina, O., Solovchenko, A., Selyakh, I., Chivkunova, O., Semenova, L., Scherbakov, P., Burakova, O., and Lobakova, E., Coordinated rearrangements of assimilatory and storage cell compartments in a nitrogen-starving symbiotic chlorophyte cultivated under high light, Arch. Microbiol., 2015, vol. 197, p. 181. https://doi.org/10.1007/s00203-014-1036-5
Reynolds, E., The use of lead citrate at high pH as an electron-opaque stain in electron microscopy, J. Cell Biol., 1963, vol. 17, p. 208. https://doi.org/10.1083/jcb.17.1.208
Solovchenko, A. and Neverov, K., Carotenogenic response in photosynthetic organisms: a colorful story, Photosynth. Res., 2018, vol. 133, p. 31. https://doi.org/10.1007/s11120-017-0358-y
Kokabi, K., Gorelova, O., Ismagulova, T., Itkin, M., Malitsky, S., Boussiba, S., Solovchenko, A., and Khozin-Goldberg, I., Metabolomic foundation for differential responses of lipid metabolism to nitrogen and phosphorus deprivation in an arachidonic acid-producing green microalga, Plant Sci., 2019, vol. 283, p. 95. https://doi.org/10.1016/j.plantsci.2019.02.008
Kokabi, K., Gorelova, O., Zorin, B., Didi-Cohen, S., Itkin, M., Malitsky, S., Solovchenko, A., Boussiba, S., and Khozin-Goldberg, I., Lipidome remodeling and autophagic response in the arachidonic-acid-rich microalga Lobosphaera incisa under nitrogen and phosphorous deprivation, Front. Plant Sci., 2020, vol. 11. https://doi.org/10.3389/fpls.2020.614846
Powell, N., Shilton, A., Chisti, Y., and Pratt, S., Towards a luxury uptake process via microalgae-defining the polyphosphate dynamics, Water Res., 2009, vol. 43, p. 4207. https://doi.org/10.1016/j.watres.2009.06.011
Solovchenko, A., Khozin-Goldberg, I., Selyakh, I., Semenova, L., Ismagulova, T., Lukyanov, A., Mamedov, I., Vinogradova, E., Karpova, O., Konyukhov, I., Vasilieva, S., Mojzeš, P., Dijkema, C., Vecherskaya, M., Zvyagin, I., et al., Phosphorus starvation and luxury uptake in green microalgae revisited, Algal Res., 2019, vol. 43, p. 101651. https://doi.org/10.1016/j.algal.2019.101651
Clode, P.L., Saunders, M., Maker, G., Ludwig, M., and Atkins, C.A., Uric acid deposits in symbiotic marine algae, Plant Cell Environ., 2009, vol. 32, p. 170. https://doi.org/10.1111/j.1365-3040.2008.01909.x
He, B., Hou, L., Zhang, F., Cong, X., Wang, Z., Guo, Y., Shi, J., Jiang, M., Zhang, X., and Zang, X., Ultrastructural changes of Haematococcus pluvialis (Chlorophyta) in process of astaxanthin accumulation and cell damage under condition of high light with acetate, Algae, 2020, vol. 35, p. 253. https://doi.org/10.4490/algae.2020.35.5.22
ACKNOWLEDGMENTS
We are grateful to the staff of the Faculty of Biology of Moscow State University: Researcher, Ph.D. D.A. Chudaev for help in conducting polarizing microscopy; Professor, d.b.s. A.E. Solovchenko; and leading researcher, Ph.D. K.A. Chekanov for a critical discussion of the results obtained and valuable comments. Electron microscopic studies were carried out using the equipment of the Moscow State University Shared Use Center.
Funding
Work on the cultivation of microalgae cultures and their physiological and biochemical characteristics was carried out with the financial support of the Megagrant of the Government of the Russian Federation (Agreement no. 075-15-2019-1882). Work on the cell research using scanning transmission electron microscopy and energy-dispersive X-ray spectroscopy was supported by a grant from the president of the Russian Federation (no. MK-1952.2021.1.4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare they have no conflicts of interest. This article does not contain any research involving humans and animals as research objects.
Additional information
Abbreviations: TEM—transmission electron microscopy; STEM—scanning transmission electron microscopy; EDRS—energy dispersive X-ray spectroscopy; DAPI—4',6-diamidino-2-phenylindole.
The original online version of this article was revised: Due to a retrospective Open Access order.
Rights and permissions
Open Access. This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zaitseva, A.A., Zaitsev, P.A., Gorelova, O.A. et al. Characteristics of Vacuolar Inclusions in Coelastrella rubescens Namsu R1 Green Microalgae Cells in Low- and High-Intensity Light. Russ J Plant Physiol 69, 78 (2022). https://doi.org/10.1134/S1021443722040227
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1134/S1021443722040227