Abstract
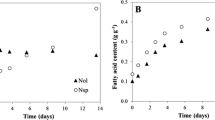
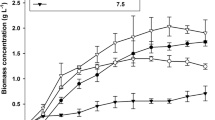
We examined responses of batch cultures of the marine microalga Nannochloropsis sp. to combined alterations in salinity (13, 27, and 40 g/l NaCl) and light intensity (170 and 700 μmol photons/m2·s). Major growth parameters and lipid productivity (based on total fatty acid determination) were determined in nitrogen-replete and nitrogen-depleted cultures of an initial biomass of 0.8 and 1.4 g/l, respectively. On the nitrogen-replete medium, increases in light intensity and salinity increased the cellular content of dry weight and lipids due to enhanced formation of triacylglycerols (TAG). Maximum average productivity of ca. 410 mg TFA/l/d were obtained at 700 μmol photons/m2·s and 40 g/l NaCl within 7 days. Under stressful conditions, content of the major LC-PUFA, eicosapentaenoic acid (EPA), was significantly reduced while TAG reached 25% of biomass. In contrast, lower salinity tended to improve major growth parameters, consistent with less variation in EPA contents. Combined higher salinity and light intensity was detrimental to lipid productivity under nitrogen starvation; biomass TFA content, and lipid productivity amounted for only 33% of DW and ca. 200 mg TFA/l/day, respectively. The highest biomass TFA content (ca. 47% DW) and average lipid productivity of ca. 360 mg TFA/l/day were achieved at 13 g/l NaCl and 700 μmol photons/m2·s. Our data further support selecting Nannochloropsis as promising microalgae for biodiesel production. Moreover, appropriate cultivation regimes may render Nannochloropsis microalgae to produce simultaneously major valuable components, EPA, and TAG, while sustaining relatively high biomass growth rates.




Similar content being viewed by others
References
Bell JG, Sargent JR (2003) Arachidonic acid in aquaculture feeds: current status and future opportunities. Aquaculture 218:491–499
Boussiba S, Vonshak A, Cohen Z, Avissar Y, Richmond A (1987) Lipid and biomass production by the halotolerant microalga Nannochloropsis salina. Biomass 12:37–47
Chini Zittelli G, Lavista F, Batianini A, Rodolfi L, Vincenzini M, Tredici MR (1999) Production of eicosapentaenoic acid (EPA) by Nannochloropsis sp. cultures in outdoor tubular photobioreactors. J Biotechnol 70:299–312
Chiu SY, Kao CY, Tsai MT, Ong SC, Chen CH, Lin CS (2009) Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour Technol 100:833–838
Cohen Z, Khozin-Goldberg I (2005) Searching for PUFA-rich microalgae. In: Cohen Z, Ratledge C (eds) Single cell oils. American Oil Chem Society, Champaign, pp 53–72
Fietz S, Bleiß W, Hepperle D, Koppitz H, Krienitz L, Nicklisch A (2005) First record of Nannochloropsis limnetica (Eustigmatophyceae) in the autotrophic picoplankton from Lake Baikal. J Phycol 41:780–790
Fisher T, Berner T, Iluz D, Dubinsky Z (1998) The kinetics of the photoacclimation response of Nannochloropsis sp. (Eustigmatophyceae): a study of changes in ultrastructure and PSU density. J Phycol 34:818–824
Guillard R, Ryther JH (1962) Studies of marine planktonic diatoms. I. Cyclotella nana Husted and Detonula confervacea (Cleve) Gran (“F” medium). Can J Microbiol 8:229–239
Guschina IA, Harwood JL (2006) Lipids and lipid metabolism in eukaryotic algae. Prog Lipid Res 45:160–186
Hibberd D (1980) Eustigmatophytes. In: Cox E (ed) Phytoflagellates: developments in marine biology. Elsevier, New York, pp 319–334
Hodgson P, Henderson R, Sargent J, Leftley J (1991) Patterns of variation in the lipid class and fatty acid composition of Nannochloropsis oculata (Eustigmatophyceae) during batch culture. J Appl Phycol 3:169–181
Hu H, Gao K (2006) Response of growth and fatty acid compositions of Nannochloropsis sp. to environmental factors under elevated CO2 concentration. Biotechnol Lett 28:987–992
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Khozin-Goldberg I, Bigogno C, Shrestha P, Cohen Z (2002) Nitrogen starvation induces the accumulation of arachidonic acid in the freshwater green alga Parietochloris incisa (Trebouxiophyceae). J Phycol 38:991–994
Krienitz L, Wirth M (2006) The high content of polyunsaturated fatty acids in Nannochloropsis limnetica (Eustigmatophyceae) and its implication for food web interactions, freshwater aquaculture and biotechnology. Limnologica 36:204–210
Li Y, Han D, Sommerfeld M, Hu Q (2011) Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresour Technol 102:123–129
Radakovits R, Jinkerson RE, Darzins A, Posewitz MC (2010) Biofuels from eukaryotic microalgae. Eukaryot Cell. doi:https://doi.org/10.1128/EC.00364-09
Renaud S, Parry D, Thinh L, Kuo C, Padovan A, Sammy N (1991) Effect of light intensity on the proximate biochemical and fatty acid composition of Isochrysis sp., and Nannochloropsis oculata for use in tropical aquaculture. J Appl Phycol 3:43–53
Renaud SM, Parry DL (1994) Microalgae for use in tropical aquaculture II: Effect of salinity on growth, gross chemical composition and fatty acid composition of three species of marine microalgae. J Appl Phycol 6:347–356
Richmond A, Cheng-Wu Z, Zarmi Y (2003) Efficient use of strong light for high photosynthetic productivity: interrelationships between the optical path, the optimal population density and cell-growth inhibition. Biomol Eng 20:229–236
Rodolfi L, Chini Zittelli G, Barsanti L, Rosati G, Tredici MR (2003) Growth medium recycling in Nannochloropsis sp. mass cultivation. Biomol Eng 20:243–248
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Roessler PG (1990) Environmental control of glycerolipid metabolismin microalgae: commercial implications and future research directions. J Phycol 26:393–399
Roncarati A, Meluzzi A, Acciarri S, Tallarico N, Meloti P (2004) Fatty acid composition of different microalgae strains (Nannochloropsis sp., Nannochloropsis oculata (Droop) Hibberd, Nannochloris atomus Butcher and Isochrysis sp.) according to the culture phase and the carbon dioxide concentration. J World Aquac Soc 35:401–411
Shifrin NS, Chisholm SW (1981) Phytoplankton lipids: inter-specific differences and effects of nitrate, silicate and light-dark cycles. J Phycol 17:374–384
Solovchenko A, Khozin-Goldberg I, Cohen Z, Merzlyak M (2009) Carotenoid-to- chlorophyll ratio as a proxy for assay of total fatty acids and arachidonic acid content in the green microalga Parietochloris incisa. J Appl Phycol 21:361–366
Solovchenko A, Khozin-Goldberg I, Didi-Cohen S, Cohen Z, Merzlyak M (2008) Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. J Appl Phycol 20:245–251
Solovchenko A, Khozin-Goldberg I, Recht L, Boussiba S (2010) Stress-induced changes in optical properties, pigment and fatty acid content of Nannochloropsis sp.: implications for non-destructive assay of total fatty acids. Mar Biotech. doi:https://doi.org/10.1007/s10126-010-9323-x
Stephenson AL, Dennis JS, Howe CJ, Scott SA, Smith AG (2010) Influence of nitrogen-limitation regime on the production by Chlorella vulgaris of lipids for biodiesel feedstocks. Biofuels 1:47–58
Suen Y, Hubbard JS, Holzer G, Tornabene TG (1987) Total lipid production of the green alga Nannochloropsis sp. QII under different nitrogen regimes. J Phycol 23:289–296
Sukenik A (1999) Production of EPA by Nannochloropsis. In: Cohen Z (ed) Chemicals from microalgae. Taylor and Francis, London, pp 41–56
Sukenik A, Beardall J, Kromkamp JC, Kopeck J, Masojídek J, van Bergeijk S, Gabai S, Shaham E, Yamshon A (2009) Photosynthetic performance of outdoor Nannochloropsis mass cultures under a wide range of environmental conditions. Aquat Microb Ecol 56:297–308
Sukenik A, Carmeli Y (1990) Lipid synthesis and fatty acid composition in Nannochloropsis sp. (Eustigmatophyceae) grown in a light-dark cycle. J Phycol 26:463–469
Sukenik A, Carmeli Y, Berner T (1989) Regulation of fatty acid composition by irradiance level in the eustigmatophyte Nannochloropsis sp. J Phycol 25:686–692
Sukenik A, Yamaguchi Y, Livne A (1993a) Alterations in lipid molecular species of the marine eustigmatophyte Nannochloropsis sp. J Phycol 29:620–626
Sukenik A, Zamora O, Carmeli Y (1993b) Biochemical quality of marine unicellular algae with emphasis on lipid composition. II. Nannochloropsis sp. Aquaculture 117:313–326
Thompson GA (1996) Lipids and membrane function in green algae. Biochim Biophys Acta 1302:17–45
Tredici M (2010) Photobiology of microalgae mass cultures: understanding the tools for the next green revolution. Biofuels 1:143–162
Vieler A, Wilhelm C, Goss R, Süß R, Schiller J (2007) The lipid composition of the unicellular green alga Chlamydomonas reinhardtii and the diatom Cyclotella meneghiniana investigated by MALDI-TOF MS and TLC. Chem Phys Lipids 150:143–155
Zhekisheva M, Boussiba S, Khozin-Goldberg I, Zarka A, Cohen Z (2002) Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters. J Phycol 38:325–331
Zou N, Zhang C, Cohen Z, Richmond A (2000) Production of cell mass and eicosapentaenoic acid (EPA) in ultrahigh cell density cultures of Nannochloropsis sp. (Eustigmatophyceae). Eur J Phycol 35:127–133
Acknowledgements
We would like to thank two anonymous reviewers for their critical and helpful evaluation of the manuscript and Ms. S. Didi-Cohen for her dedicated technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pal, D., Khozin-Goldberg, I., Cohen, Z. et al. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp.. Appl Microbiol Biotechnol 90, 1429–1441 (2011). https://doi.org/10.1007/s00253-011-3170-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3170-1