Abstract—
The review considers complex, controversial, and individual effects of heparin and its derivatives on the bone and circulatory systems in dependence of the dose, the state of the cells and tissues of the recipient. General data on the anticoagulant activity of heparin and its derivatives are presented; special attention is paid to the effect of heparin on mesenchymal cells and tissues and its role in angiogenesis. We also discuss the ability of heparin to bind osteogenic and angiogenic biomolecules in the context of the development of systems for their delivery and sustained controlled release and propose a schematic representation of the positive and side effects of heparin as a delivery system for biomolecules in tissue engineering.
Similar content being viewed by others
INTROUCTION
Injuries of bones and surrounding soft tissues are inevitably accompanied by the dynamic activation of the coagulation and anticoagulation systems of the body. Damages of blood vessels result in hemorrhage leading to hematoma formation. The clotted blood fills the injured area and is thus associated with the bone marrow, endosteum, cortical bone, periosteum, and muscle tissue. A cellular and molecular environment, which plays an important role in the process of reparative bone tissue regeneration, is formed. In the focus of damage, immunocompetent cells, including macrophages, secrete inflammatory mediators such as TNF-α, IL-1, IL-6, IL-11, etc. These cytokines mediate the recruitment of mesenchymal stem cells and their differentiation into osteoblasts and chondroblasts [1, 2].
Activation of the hemostatic system in bone fractures and their surgical treatment can provoke hypercoagulation with the risk of systemic thrombosis and thromboembolism. Osteosynthesis, even with the use of biocompatible materials and implants, and, moreover, endoprosthetics of large joints, also increase the probability of thrombus formation due to numerous injuries of arterial and venous vessels, dissection of soft tissues, and the possible effect of the surface of medical devices on platelets and proteins of coagulation hemostasis. The balance between the risk of thromboembolism and bleeding is individual for each patient. As a rule aged patients with traumatological/orthopedic profile have chronic diseases (ischemic heart disease, angina pectoris, diabetes mellitus), vascular diseases (thrombophlebitis, deep vein thrombosis, pulmonary embolism). The presence of such complications requires thromboprophylaxis during treatment, at the preoperative and postoperative stages of management of this group of patients.
For the prevention of situations that threaten the patient’s life, the therapeutic interventions in the rehabilitation period (after operations with the use of bone-substituting materials) include direct anticoagulants, heparin derivatives, and various combinations of heparin itself.
1 ANTICOAGULANT ACTIVITY OF HEPARIN AND ITS DERIVATIVES
Unfractionated heparin (UFH) for therapeutic use in humans is usually prepared from extracts of intestinal mucosa or lung tissue of cattle and pigs; it consists of a heterogeneous mixture of glycosaminoglycans (GAGs) with slightly different structure and molecular mass [3, 4]. Therefore, regardless of the method of its production, UFH is capable of provoking hypersensitivity reactions accompanied by thrombocytopenia, vascular disorders, and an increase in serum alanine (ALT) and aspartate aminotransferase (AST) levels, which are observed after 4–8 days of therapy [3] (Fig. 1). Long-term administration of high doses of heparin increases a risk of systemic osteoporosis [5]. In this regard, UFH is fractionated with isolation of so-called low molecular weight heparins (LMWH) with a molecular mass of 1000–10 000 Da. These include the following well-known preparations: Fraxiparin (Aspen Notre Dame de Bondeville, France), Clexane (Sanofi Winthrop Industrie, France) and Fragmin (Vetter Pharma-Fertigung, Germany). One of the methods of purification is gel chromatography followed by precipitation in alcohol [4]. LMWH are less active than UFH in provoking the development of heparin-induced thrombocytopenia (HIT).
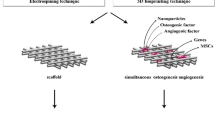
Positive and side effects of heparin as a delivery system for biomolecules in tissue engineering. AT-III—antithrombin III; BMP-2—bone morphogenetic protein 2; BMP-4—bone morphogenetic protein 4; BMP-6—bone morphogenetic protein 6; FGF—fibroblast growth factor; OPG, osteoprotegerin; TGF-β—transforming growth factor β-1; VEGF—vascular endothelial growth factor; MSCs are mesenchymal stem cells.
Heparin is not absorbed in the gastrointestinal tract and therefore it should be administered intravenously or subcutaneously; the parenterally administered heparin exhibits rapid but short-term anticoagulant activity and low bioavailability, especially at low doses [4]. UFH is an effective anticoagulant, and its effects begin to appear at low doses and with any route of administration (including oral, intravenous, intramuscular, subcutaneous, intrarectal, inhalation and in the form of ointments) [6]. It binds to all natural anticoagulants and, in particular, to antithrombin III, an inhibitor of activated blood coagulation factors (thrombin, IXa, Xa, XIa, XIIa). This is accompanied by suppression of activated blood coagulation factors II and X, decreased conversion of prothrombin to thrombin, inhibition of fibrin formation from fibrinogen, and a decrease of platelet aggregation. UFH is mainly used for the prevention and treatment of thromboembolic diseases, acute coronary syndrome, atrial fibrillation, and also as an anticoagulant in renal dialysis. Currently, it is also used for coronavirus infection COVID-19 [7]. UFH has a number of limitations and potential complications, including HIT, skin reactions, and long-term osteoporosis [8, 9]. LMWH were developed in the 1980s, and many of the disadvantages associated with unfractionated heparin were overcome by their use, including a lower risk of osteoporosis [9–11]. In addition, the use of LMWH reduces the risk of HIT, and monitoring of blood clotting parameters may be performed less frequently than in the case of therapy with UFH [9].
Heparin composed of repeating disaccharide units (1 → 4) of α-D-glucosamine and α-iduric (less often α-D-glucuronic) acid, belongs to the GAG family. It has been found in the intercellular substance, tissues of the lungs, liver, heart and is usually stored in the secretory granules of mast cells; it is released into the vascular network at the sites of tissue damage, which indicates heparin participation in the protection against penetration of bacteria and foreign elements [12]. In this regard, heparin is able to modulate the state of cells and, thus, when administered for a long time after implantation and endoprosthetics, affect the osseointegration of medical devices.
2 THE EFFECT OF HEPARIN ON MESENCHEMIC CELLS AND TISSUES
Numerous biochemical, cellular biological, and genetic studies have shown that GAGs play a crucial role in the regulation of most signaling pathways associated with growth factors, including the transforming growth factor-β (TGF-β) family and fibroblast growth factors (FGFs) [13].
Long-term studies of the effect of heparin on mesenchymal cells performed using animal models and in in vitro studies, gave controversial results. However, it was found that the effects of heparin on healthy proliferating cells and tissues depended on its concentration. For example, heparin has a stimulating effect on cell proliferation at low and very low concentrations, while increased concentrations inhibit cell proliferation [14] (Fig. 1). On the contrary, Khan et al., demonstrated that tumor cells responded to heparin by increased proliferation and attenuated differentiation mediated via BMP-4 signaling pathways [15] (Fig. 1).
Binding of heparin to bone morphogenetic proteins (BMPs) resulted in a limitation of the osteogenic activity of BMP-6 in a 72-h culture of myoblasts, recorded by a decrease in the expression of alkaline phosphatase and osteocalcin [16]. In a test for ectopic bone and cartilage formation in osteoporotic mice, heparin also significantly inhibited regenerative activity of BMP-6. This suggests that interaction with BMP-6 is one of the mechanisms of heparin-associated osteoporosis [16] (Fig. 1).
On the other hand, in vitro heparin stimulated osteogenic differentiation of human dental pulp cells; this effect appeared as early as 3 days of cultivation and was characterized by an increase in alkaline phosphatase activity, expression of bone morphogenetic protein (BMP-2) and osteocalcin, and formation of mineralization nodules in the extracellular matrix (ECM) [17] ( Fig. 1).
Modification with heparin of porous composite scaffolds of type I collagen and hydroxyapatite (HAP) showed a dose dependence but not on the mode dependence of inclusion in the scaffold composition. In a low concentration (30 mg/g collagen) heparin stimulated proliferation, while in a high concentration (150 mg/g collagen) it stimulated osteogenic differentiation of human mesenchymal stem cells (MSC) [18].
Proliferation of human bone marrow MSCs obtained from different donors demonstrated individual sensitivity to low doses of heparin (less than 200 ng/mL) and its pleutropic effect on signaling pathways of cell growth and differentiation (including the superfamilies of transforming growth factor-beta (TGF-β) and BMPs, Fibroblast Growth Factors (FGFs), Wnts). High doses of heparin (100 µg/mL or more) had a clear inhibitory effect on cell growth [5].
Clinical studies of the effect of heparin on human bone tissue, conducted mainly in pregnant women gave controversial results. On the one hand, long-term use of LMWH during pregnancy for 3 months or more led to bone mass loss and fractures [19]. Other authors reported that the absolute risk of fractures in this population of patients was rather small (1–2%) [20]. The decrease in mean bone mineral density by 2–4% caused by prophylactic doses of LMWH or UFH was comparable to the bone mass loss during physiological pregnancy [21].
These studies confirm the possible role of long-term therapy with UFH in changes in bone metabolism, as well as a decreased risk of fractures in patients after UFH replacement for LMWH. However, decrease in bone mineral density by 2–4% or an increase in the fracture risk of up to 2% has clinical implications for other populations, including cancer patients or the elderly, who may require a long-term course of LMWH therapy and also for individuals with a higher baseline fracture risk due to for aging or concomitant somatic diseases [22–24].
There are a few studies performed in non-pregnant adult populations, which gave controversial results. Gajic-Veljanoski et al. reported that long-term therapy with LMWH in patients with venous thromboembolism associated with oncology and other diseases reduced the mean bone mineral density to 4.8% after 3–24 months in 2 of 5 prospective observational studies [25]. The meta-analysis did not reveal an increase in the risk of fractures in the LMWH group compared with the control group, where patients took mainly UFH or vitamin K antagonists (VKA). LMWH therapy for 3–6 months may not increase the risk of fractures, but long-term exposure up to 24 months can negatively affect bone mineral density [26]. Important considerations are the need for long-term LMWH therapy to obtain sufficient amounts of calcium and vitamin D to minimize bone loss and monitoring bone mineral density in those patients who are at increased risk of bone loss or fractures [25].
However, using histomorphometric analysis Muir et al. demonstrated that UFH and LMWH caused a dose-dependent decrease in the volume of cancellous bone; the effects of UFH were more potent than those of LMWH. Both substances (UFH and LMWH) reduced bone formation by decreasing the surface area of osteoblasts and osteoids, while bone resorption increased only by UFH by increasing the area of osteoclasts [27, 28]. These results were supported by biochemical markers of bone homeostasis, suggesting bone destruction and/or repair. Treatment with UFH and LMWH resulted in a dose-dependent decrease in serum alkaline phosphatase (a marker of bone formation); a short-term increase in the amount of type I collagen associated with pyridinoline (a marker of bone resorption) in urine was observed only in the case of UFH [27, 28] (Fig. 1).
Experiments in vitro have shown that heparin enhanced osteoclastic bone resorption by inhibiting the activity of osteoprotegerin (OPG) [19]. Heparin specifically binds to OPG and prevents its interaction with RANKL on the osteoblast membrane, thereby promoting RANK-RANKL interaction and osteoclast activation. LMWH preparations (of about 4000–6000 Da) produced a less pronounced osteoporotic effect than the standard UFH (approximately 7000–25 000 Da). More active inhibition of OPG by UFH is apparently due to the fact that its molecules are more bulky and sterically interfere with the OPG-RANKL interaction [19].
3 THE EFFECT OF HEPARIN ON ANGIOGENESIS
In porous type I collagen/HAP scaffolds low heparin concentration (30 mg/g collagen) promoted in vitro growth of the capillary network from human umbilical vein endothelial cells and its ingrowth into the pores of the composite material [18]. At a higher concentration (150 mg/g collagen), heparin inhibited this process. In addition, the anticoagulant inhibited the angiogenic effect of endothelial growth factor (VEGF) [18].
VEGF and fibroblast growth factors (FGF1, FGF2) are one of the main mediators of angiogenesis [29, 30]. A functional interaction between FGF and heparin was originally reported in 1983. In contrast to the data of Quade et al. [18], a low concentration of heparin enhanced the effect of the crude FGF1 preparation, thus maintaining the culture of endothelial cells of adult blood vessels [31]. The observation that, besides interaction with FGF, heparin could bind to a specific domain of the FGF receptor (FGFR) led to the elucidation of the molecular mechanism of the involvement of heparan sulfate in angiogenesis [32]. Complex formation between FGF, FGFR and heparan sulfate is required for the activation of the angiogenic FGF-dependent signaling pathway. This conceptual framework influenced subsequent attempts to identify specific modulators of the process and to develop anti-angiogenic agents [33] required to limit the development of tumors. Heparin or some of its derivatives can act as such agents, which in high concentrations in biological body fluids are able to bind an angiogenic factor, competing with heparan sulfate on the cell membrane [33].
4 HEPARIN AS A DELIVERY SYSTEM OF OSTEOGENIC AND ANGIOGENIC MOLECULES
Natural biomacromolecules, including GAGs and their derivatives, exhibit good compatibility, controlled biodegradability, and long circulation time; they are non-toxic and non-immunogenic and therefore, they are considered as promising carriers for therapeutic delivery of drugs, biomolecules, genes and probes for imaging in various pathologies, as well as for tissue engineering [34, 35]. Currently, protocols for the use of heparin for the expansion and differentiation of stem cells and for improving the targeted delivery of growth factors are actively developed. The PubMed search by the keyword “heparin as a drug delivery system” gives 1290 results, while the query “heparin and bone bioengineering” yields only 206 references. The latter may be attributed to certain concerns of researchers to variability of the results and side effects of the heparin use as a delivery system for biomolecules in the bioengineering of the musculoskeletal system [36].
Among 40 cytokines of the TGF-β superfamily, about one-third may bind to heparin; these include TGF-β1, TGF-β2, some BMPs (e.g. BMP-2; BMP-6), growth and differentiation factors (GDFs), and GDNF (Glial cell-derived neurotrophic factor) and its two close homologues [37].
Heparin has a high affinity for growth factors such as VEGF, basic FGF, and bone BMP-2 [38], which makes heparin a potential carrier for the delivery and local targeting of these biomolecules [39].
For example, the clinical application of BMP-2, one of the most active osteoinductive molecules, requires the use of supraphysiological doses due to its instability and rapid enzymatic degradation, which cause systemic toxicity and side effects [40]. Lee et al. [41] developed a heparin-conjugated fibrin (HCF) system for the delivery of recombinant human BMP-2 for bone bioengineering. Later, a heparin-polyelectrolyte (PEC)-based carrier was developed for BMP-2 delivery [42].
Decellularized heparinized mineralized small intestinal submucosa (SIS) membranes loaded with BMP-2 had a controlled long-term 40-day release of the bone protein, promoted in vitro expression of osteogenic genes in bone marrow MSCs, and healed a jaw bone defect in osteoporotic rats. In this case, heparin was used to improve immobilization and controlled release of BMP-2 [43].
Recently, a synthetic hydrogel based on heparin mimetic molecules, polyvinylsulfonic acid or poly-4-styrenesulfonic acid, was developed for local concentration and prolongation of the BMP-2 effect [40]. The heparin-mimetic sulfonated gel demonstrating effective binding of BMP-2, stabilized its concentration, and prolonged the increase in osteogenic differentiation of encapsulated bone marrow MSCs without adding an exogenous growth factor to the culture. Moreover, the gel increased its osteogenic activity by localizing new BMP-2 molecules produced by MSCs. This indicates that heparin-associated systems for the delivery and release of biomolecules are promising for enhancing bone tissue regeneration [40].
At the same time, differentiation factor 5 (GDF5) binds to heparin; however, clinical heparin concentrations (>10 nM) suppress the activity of GDF5 towards chondrogenic differentiation of human MSCs and muscle cells of the ATDC5 line [36].
CONCLUSIONS
Surgical treatment of bone fractures by means of osteosynthesis and endoprosthetics may cause a shift in the dynamic balance of the hemostasis system towards increased blood coagulability. The presence of hematomas is a necessary stage in the reparative regeneration of bone tissue. At the same time, the risk of thrombosis and embolism, especially under conditions of implantation of artificial materials, inevitably leads to the prophylactic or therapeutic prescription of heparin and its derivatives, which (according to various authors) have an controversial and individual effects on the bone and circulatory systems, depending on the dosage, the state of the cells and tissues of the recipient.
The ability of heparin to bind osteogenic and angiogenic biomolecules is a promising feature for the development of systems for their delivery and controlled sustained release. At the same time, the problem is the intrinsic potential of heparin as a modulator of osteo- and angiogenesis. In conditions of progressive osteoporosis in a population (the expected prognosis is up to 30% of hospital beds) [44], the use of heparin alone or in combination with biomolecules requires a personalized approach and careful monitoring, primarily in patients with a burdened history and concomitant somatic diseases.
REFERENCES
Marsell, R. and Einhorn, T.A., Injury, 2011, vol. 42, no. 6, pp. 551–555. https://doi.org/10.1016/j.injury.2011.03.031
Muire, P.J., Mangum, L.H., and Wenke, J.C., Front. Immunol., 2020, vol. 11, 1056. https://doi.org/10.3389/fimmu.2020.01056
Zhao, W., Jin, K., Li, J., Qiu, X., and Li, S., J. Biol. Eng., 2017, vol. 11, 22. https://doi.org/10.1186/s13036-017-0058-3
Yee, J. and Kaide, C.G., West. J. Emerg. Med., 2019, vol. 20, no. 5, pp. 770–783. https://doi.org/10.5811/westjem.2018.5.38235
Ling, L., Camilleri, E.T., Helledie, N., Samsonraj, R., Titmarsh, D.M., Chua, R.J., Dreesen, O., Dombrowski, C., Rider, D.A., Galindo, M., Lee, I., Hong, W., Hui, J.H., Nurcombe, V., van Wijnen, A.J., and Cool, S.M., Gene, 2016, vol. 576, no. 1, pt 2, pp. 292–303. https://doi.org/10.1016/j.gene.2015.10.039
Shore-Lesserson, L., Baker, R.A., Ferraris, V., Greilich, P.E., Fitzgerald, D., Roman, P., and Hammon, J., J. Extra Corpor. Technol., 2018, vol. 50, no. 1, pp. 5–18.
Hadid, T., Kafri, Z., and Al-Katib, A., Blood Rev., 2020, vol. 2020, 100761. https://doi.org/10.1016/j.blre.2020.100761
Alban, S., Handb. Exp. Pharmacol., 2012, vol. 207, pp. 211–263. https://doi.org/10.1007/978-3-642-23056-1_10
Signorelli, S.S., Scuto, S., Marino, E., Giusti, M., Xourafa, A., and Gaudio, A., Int. J. Mol. Sci., 2019, vol. 20, no. 21, 5275. https://doi.org/10.3390/ijms20215275
Rajgopal, R., Bear, M., Butcher, M.K., and Shaughnessy, S.G., Thromb. Res., 2008, vol. 122, pp. 293–298. https://doi.org/10.1016/j.thromres.2006.10.025
Galambosi, P., Hiilesmaa, V., Ulander, V.M., Laitinen, L., Tiitinen, A., and Kaaja, R., Thromb. Res., 2016, vol. 143, pp. 122–126. https://doi.org/10.1016/j.thromres.2016.05.016
Nader, H.B., Chavante, S.F., dos Santos, E.A., Oliveira, T.W., de Paiva J.F., Jerônimo, S.M., Medeiros, G.F., de Abreu, L.R., Leite E.L., de Sousa-Filho, J.F., Castro, R.A., Toma, L., Tersariol, I.L., Porcionatto, M.A., and Dietrich, C.P., Braz. J. Med. Biol. Res., 1999, vol. 32, no. 5, pp. 529–538. https://doi.org/10.1590/s0100-879x1999000500005
Zhang, F., Zheng, L., Cheng, S., Peng, Y., Fu, L., Zhang, X., and Linhardt, R.J., Molecules, 2019, vol. 24, no. 18, 3360. https://doi.org/10.3390/molecules24183360
Susanto, A., Putera, B.W., Wijaya, A., and Muh-tadi, A., Cytotherapy, 2019, vol. 21, no. 5, pp. 87–88. https://doi.org/10.1016/j.jcyt.2019.03.515
Khan, S.A., Nelson, M.S., Pan, C., Gaffney, P.M., and Gupta, P., Am. J. Physiol. Cell Physiol., 2008, vol. 294, no. 6, pp. 1387–1397. https://doi.org/10.1152/ajpcell.00346.2007
Brkljacic, J., Pauk, M., Erjavec, I., Cipcic, A., G-rgu-revic, L., Zadro, R., Inman, G.J., and Vulicevic, S., Int. Orthop., 2013, vol. 37, no. 3, pp. 529–541. https://doi.org/10.1007/s00264-012-1714-3
Rodriges, E.M., Cornelio, A.L.G., Godoi, P.H., da Costa, P.I., Rossa-Junior, C., Faria, G., Guerreiro Tanomaru, J.M., and Tanomaru-Filho, M., Int. Endod. J., 2019, vol. 52, no. 6, pp. 829–837. https://doi.org/10.1111/iej.13061
Quade, M., Knaack, S., Weber, D., Konig, U., Paul, B., Simon, P., Rossen-Wolf, A., Schwartz-Albiez, R., Gtlinsky, M., and Lode, A., Eur. Cell Mater., 2017, vol. 33, pp. 105–120. https://doi.org/10.22203/eCM.v033a08
Irie, A., Takami, M., Kubo, H., Sekino-Suzuki, N., Kasahara, K., and Sanai, Y., Bone, 2007, vol. 41, pp. 165–174. https://doi.org/10.1016/j.bone.2007.04.190
Vik, A., Brodin, E., Sveinbjornsson, B., and Hansen, J.B., Thromb. Haemost., 2007, vol. 98, pp. 148–154. https://doi.org/10.1160/TH06-11-0671
Greer, I.A. and Nelson-Piercy, C., Blood, 2005, vol. 106, pp. 401–407. https://doi.org/10.1182/blood-2005-02-0626
Sonawane, S., Kasbekar, N., and Berns, J.S., Semin. Dial., 2006, vol. 19, pp. 305–310. https://doi.org/10.1111/j.1525-139X.2006.00177.x
Compston, J., Curr. Osteoporos. Rep., 2013, vol. 11, pp. 30–35. https://doi.org/10.1007/s11914-012-0127-y
Coleman, R.E., Rathbone, E., and Brown, J.E., Nat. Rev. Rheumatol., 2013, vol. 9, pp. 365–374. https://doi.org/10.1038/nrrheum.2013.36
Gajic-Veljanoski, O., Chai, W., Prakesh, S., and Cheung, A.M., J. Gen. Intern. Med., 2016, vol. 31, pp. 947–957. https://doi.org/10.1007/s11606-016-3603-8
Hull, R.D., Pineo, G.F., Brant, R., Liang, J., Cook, R., Solymoss, S., Poon, M.-C., and Raskob, G., Am. J. Med., 2009, vol. 122, pp. 762–769. https://doi.org/10.1016/j.amjmed.2008.12.023
Muir, J.M., Andrew, M., Hirsh, J., Weitz, J.I., Young, E., Deschamps, P., and Shaughnessy, S.G., Blood, 1996, vol. 88, pp. 1314–1320. https://doi.org/10.1182/blood.V88.4.1314.bloodjournal8841314
Muir, J.M., Hirsh, J., Weitz, J.I., Andrew, M., Young, E., and Shaughnessy, S.G., Blood, 1997, vol. 89, pp. 3236–3242. https://doi.org/10.1182/blood.V89.9.3236
Chiodelli, P., Bugatti, A., Urbinati, C., and Rusnati, M., Molecules, 2015, vol. 20, pp. 6342–6388. https://doi.org/10.3390/molecules20046342
Li, J.P. and Kusche-Gullberg, M., Int. Rev. Cell Mol. Biol., 2016, vol. 325, pp. 215–273. https://doi.org/10.1016/bs.ircmb.2016.02.009
Thornton, S.C., Mueller, S.N., and Levine, E.M., Science, 1983, vol. 222, pp. 623–625. https://doi.org/10.1126/science.6635659
Kan, M., Wang, F., Xu, J., Crabb, J.W., Hou, J., and McKeehan, W.L., Science, 1993, vol. 259, pp. 1918–1921. https://doi.org/10.1126/science.8456318
Ghiselli, G., Medicines, 2019, vol. 6, no. 3, 80, https://doi.org/10.3390/medicines6030080
Cassinelli, G. and Naggi, A., Int. J. Cardiol., 2016, vol. 212, suppl. 1, pp. S14–S21. https://doi.org/10.1016/S0167-5273(16)12004-2
Zhang, Y., Sun, T., and Jiang, C., Acta Pharm. Sin. B., 2018, vol. 8, no. 1, pp. 34–50. https://doi.org/10.1016/j.apsb.2017.11.005
Ayerst, B.I., Smith, R.A.A., Nurcombe, V., Day, A.J., Merry, C.L.R., and Cool, S.M., Tissue Eng. Part A, 2017, vol. 23, nos. 7–8, pp. 275–292. https://doi.org/10.1089/ten.TEA.2016.0364
Rider, C.C. and Mulloy, B., Molecules, 2017, vol. 22, no. 5, pii: E713. https://doi.org/10.3390/molecules22050713
Leppänen, V.M., Tvorogov, D., Kisko, K., Prota, A.E., Jeltsch, M., and Anisimov, A., Proc. Natl. Acad. Sci. USA, 2013, vol. 110, pp. 12960–12965. https://doi.org/10.1073/pnas.1301415110
Kim, J.Y., Al-Hilal, T.A., Chung, S.W., Kim, S.Y., Ryu, G.H., and Son, W.C., J. Control Release, 2015, vol. 199, pp. 122–131. https://doi.org/10.1016/j.jconrel.2014.12.015
Kim, S., Cui, Z.-K., Kim, P.J., Jung, L.Y., and Lee, M., Acta Biomater., 2018, vol. 72, pp. 45–54. https://doi.org/10.1016/j.actbio.2018.03.034
Lee, J.S., Lee, S.K., Kim, B.S., Im, G.I., Cho, K.S., and Kim, C.S., J. Biomed. Mater. Res. A, 2015, vol. 103, pp. 545–554. https://doi.org/10.1002/jbm.a.35207
Wang, M., Lam, R.W., Abbah, S.A., Hu, T., Toh, S.Y., and Cool, S., Spine, 2016, vol. 41, pp. 1199–1207. https://doi.org/10.1097/BRS.0000000000001543
Sun, T., Liu, M., Yao, S., Ji, Y., Shi, L., Tang, K., Xiong, Z., Yang, F., Chen, K., and Guo, X., Int. J. Nanomedicine, 2018, vol. 13, pp. 791–804. https://doi.org/10.2147/IJN.S152698
Habraken, W., Habibovic, P., Epple, M., and Bohner, M., Materials Today, 2016, vol. 19, no. 2, pp. 69–87. https://doi.org/10.1016/j.mattod.2015.10.008
Funding
This work was supported by the Russian Science Foundation (project no. 16-15-10031).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This work does not contain any research using humans and animals as research objects.
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Medvedev
Rights and permissions
About this article
Cite this article
Litvinova, L.S., Yurova, K.A., Khaziakhmatova, O.G. et al. Osteogenic and Angiogenic Properties of Heparin as a System for Delivery of Biomolecules for Bone Bioengineering: a Brief Critical Review. Biochem. Moscow Suppl. Ser. B 15, 147–152 (2021). https://doi.org/10.1134/S1990750821020050
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990750821020050