Abstract
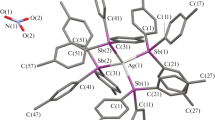
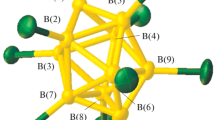
The reactivity of sulfonyl-substituted closo-decaborate derivatives (the [2-B10H9SH]2– and [2-B10H9S(CН2C(О)NН2)2]– anions) in the complexation of Ag(I) and Pb(II), Pearson soft acids, in the presence of competitive organic ligands has been studied. The substituted derivatives act as bridging ligands to form silver(I) binuclear complexes [(Ag(bipy)2)2(2-B10H9SH)] and [(Ag(bipy)2)2(2-B10H9S(CН2C(О)NН2)2]NO3; the [2-B10H9SH]2– anion is involved in the lead(II) coordination polyhedron in the [Pb(2-B10H9SH)] and [Pb(bipy)2(2-B10H9SH)] complexes; in [Pb(bipy)2][2-B10H9S(CН2C(О)NН2)2]2, the substituted decaborate derivative acts as a counterion. The solubility of the mixed-ligand complexes is different due to the variety of their structures. The synthesized complexes are the first water-soluble lead and silver compounds with the closo-decaborate anion.
Similar content being viewed by others
Notes
Elemental analysis on carbon, hydrogen, and nitrogen was performed on a Carbo Erba CHNS-3 FA 1108 Elemental Analyzer. Boron was quantified by atomic absorption spectroscopy on a Perkin-Elmer Model 2100 spectrometer with an HGA-700 electrothermal atomizer [9]; Ag was determined on an AAS-303 in an acetylene–air flame.
IR spectra were recorded on a Lumex Infralum FT-02 spectrophotometer as Nujol mulls, NaCl plates, 4000–400 cm–1, resolution 1 cm–1.
REFERENCES
Sivaev, I.B. and Bregadze, V.V., Eur. J. Inorg. Chem., 2009, vol. 11, p. 1433.
Ivanov, S.V., Ivanova, S.M., Miller, S.M., Anderson, O.P., Solntsev, K.A., and Strauss, S.H., Inorg. Chem., 1996, vol. 35, p. 6914.
Avdeeva, V.V., Polyakova, I.N., Vologzhanina, A.V., Malinina, E.A., Zhizhin, K.Yu., and Kuznetsov, N.T., Polyhedron, 2017, vol. 123, p. 396.
Zhizhin, K.Yu., Vovk, O.O., Malinina, E.A., Mustyatsa, L.V. Goeva, L.V., Polyakova, I.N., and Kuznetsov, N.T., Russ. J. Coord. Chem., 2011, vol. 27, no. 9, pp. 653–658.
Zhizhin, K.Yu., Malinina, E.A., Polyakova, I.N., Lisovskii, M.V., et al., Russ. J. Inorg. Chem., 2002, vol. 47, no. 8, pp. 1158–1167.
Zhizhin, K.Yu., Mustyatsa, V.N., Malinina, E.A., Matveev, E.Yu., et al., Russ. J. Inorg. Chem., 2005, vol. 50, no. 2, pp. 203—209.
Zhizhin, K.Yu., Malinina, E.A., Goeva, L.V., Chernyavskii, S.A., Ivanov, S.V., Luk’yanets, E.A., Solntsev, K.A., and Kuznetsov, N.T., Dokl. Akad. Nauk, 1997, vol. 357, no. 2, p. 206.
Orlova, A.M., Sivaev, I.B., Lagun, V.L., Solntsev, K.A., and Kuznetsov, N.T., Koord. Khim., 1993, vol. 19, p. 116.
Ochertyanova, L.I., Mustyatsa, V.N., Belousova, O.N., Zhizhin, K.Yu., and Kuznetsov, N.T., Inorg. Mater., 2004, vol. 40, no. 2, pp. 144—146.
ACKNOWLEDGMENTS
This work was supported by the Council of the President of the Russian Federation for State Support of Leading Scientific Schools (project no. NSh-2845.2018.3).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by G. Kirakosyan
Rights and permissions
About this article
Cite this article
Malinina, E.A., Korolenko, S.E., Goeva, L.V. et al. Synthesis and Structure of New Water-Soluble Ag(I) and Pb(II) Complexes with Sulfonyl-Substituted Derivatives of the closo-Decaborate Anion. Dokl Chem 483, 297–300 (2018). https://doi.org/10.1134/S0012500818120042
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0012500818120042