Abstract
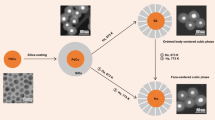
Disentangling the effects of nanoparticle proximity and size on thermal catalytic performance is challenging with traditional synthetic methods. Here we adapt a modular raspberry-colloid-templating approach to tune the average interparticle distance of PdAu alloy nanoparticles, while preserving all other physicochemical characteristics, including nanoparticle size. By controlling the metal loading and placement of pre-formed nanoparticles within a 3D macroporous SiO2 support and using the hydrogenation of benzaldehyde to benzyl alcohol and toluene as the probe reaction, we report that increasing the interparticle distance (from 12 to 21 nm) substantially enhances selectivity towards benzyl alcohol (from 54% to 99%) without compromising catalytic performance. Combining electron tomography, kinetic evaluation and simulations, we show that interparticle distance modulates the local benzyl alcohol concentration profile between active sites, consequently affecting benzyl alcohol readsorption, which promotes hydrogenolysis to toluene. Our results illustrate the relevance of proximity effects as a mesoscale tool to control the adsorption of intermediates and, hence, catalytic performance.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data that support the findings of this study are available within the paper, Supplementary Information and Source data files. Additional data are available from the authors upon reasonable request. Source data are provided with this paper.
Code availability
The MATLAB script used to extract the centre-to-centre interparticle distance from the processed electron tomographic reconstructions is provided in Supplementary Note 1.
References
Rothenberg, G. Catalysis: Concepts and Green Applications (Wiley-VCH, 2008).
Friend, C. M. & Xu, B. Heterogeneous catalysis: a central science for a sustainable future. Acc. Chem. Res. 50, 517–521 (2017).
Munnik, P., de Jongh, P. E. & de Jong, K. P. Recent developments in the synthesis of supported catalysts. Chem. Rev. 115, 6687–6718 (2015).
van Deelen, T. W., Hernández Mejía, C. & de Jong, K. P. Control of metal–support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2, 955–970 (2019).
Shi, Y. et al. Noble-metal nanocrystals with controlled shapes for catalytic and electrocatalytic applications. Chem. Rev. 121, 649–735 (2021).
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Wang, Y. et al. Recent advances in ordered meso/macroporous metal oxides for heterogeneous catalysis: a review. J. Mater. Chem. A 5, 8825–8846 (2017).
Yamada, Y. et al. Nanocrystal bilayer for tandem catalysis. Nat. Chem. 3, 372–376 (2011).
Holm, A. et al. Nanoscale spatial distribution of supported nanoparticles controls activity and stability in powder catalysts for CO oxidation and photocatalytic H2 evolution. J. Am. Chem. Soc. 142, 14481–14494 (2020).
Elias, R. C. & Linic, S. Elucidating the roles of local and nonlocal rate enhancement mechanisms in plasmonic catalysis. J. Am. Chem. Soc. 144, 19990–19998 (2022).
Keijzer, P. H., van den Reijen, J. E., Keijzer, C. J., de Jong, K. P. & de Jongh, P. E. Influence of atmosphere, interparticle distance and support on the stability of silver on α-alumina for ethylene epoxidation. J. Catal. 405, 534–544 (2022).
Goodman, E. D. et al. Size-controlled nanocrystals reveal spatial dependence and severity of nanoparticle coalescence and Ostwald ripening in sintering phenomena. Nanoscale 13, 930–938 (2021).
Yin, P. et al. Quantification of critical particle distance for mitigating catalyst sintering. Nat. Commun. 12, 4865 (2021).
Goodman, E. D. et al. Catalyst deactivation via decomposition into single atoms and the role of metal loading. Nat. Catal. 2, 748–755 (2019).
Aitbekova, A. et al. Templated encapsulation of platinum-based catalysts promotes high-temperature stability to 1,100 °C. Nat. Mater. 21, 1290–1297 (2022).
Prieto, G., Zečević, J., Friedrich, H., de Jong, K. P. & de Jongh, P. E. Towards stable catalysts by controlling collective properties of supported metal nanoparticles. Nat. Mater. 12, 34–39 (2013).
Kumar, S. & Zou, S. Electrooxidation of CO on uniform arrays of Au nanoparticles: effects of particle size and interparticle spacing. Langmuir 25, 574–581 (2009).
Inaba, M. et al. The oxygen reduction reaction on Pt: why particle size and interparticle distance matter. ACS Catal. 11, 7144–7153 (2021).
Nesselberger, M. et al. The effect of particle proximity on the oxygen reduction rate of size-selected platinum clusters. Nat. Mater. 12, 919–924 (2013).
Fortunato, G. V. et al. Impact of palladium loading and interparticle distance on the selectivity for the oxygen reduction reaction toward hydrogen peroxide. J. Phys. Chem. C 122, 15878–15885 (2018).
Lum, Y. & Ager, J. W. Sequential catalysis controls selectivity in electrochemical CO2 reduction on Cu. Energy Environ. Sci. 11, 2935–2944 (2018).
Mistry, H. et al. Tuning catalytic selectivity at the mesoscale via interparticle interactions. ACS Catal. 6, 1075–1080 (2016).
Zecevic, J., Vanbutsele, G., de Jong, K. P. & Martens, J. A. Nanoscale intimacy in bifunctional catalysts for selective conversion of hydrocarbons. Nature 528, 245–248 (2015).
Karim, W. et al. Catalyst support effects on hydrogen spillover. Nature 541, 68–71 (2017).
Dai, J. & Zhang, H. Recent advances in catalytic confinement effect within micro/meso‐porous crystalline materials. Small 17, 2005334 (2021).
Le, T. T. et al. Elemental zoning enhances mass transport in zeolite catalysts for methanol to hydrocarbons. Nat. Catal. 6, 254–265 (2023).
Liu, J. et al. MOF-enabled confinement and related effects for chemical catalyst presentation and utilization. Chem. Soc. Rev. 51, 1045–1097 (2022).
Zhang, J., Wang, B., Nikolla, E. & Medlin, J. W. Directing reaction pathways through controlled reactant binding at Pd–TiO2 interfaces. Angew. Chem. Int. Ed. 56, 6594–6598 (2017).
Durndell, L. J. et al. Selectivity control in Pt-catalyzed cinnamaldehyde hydrogenation. Sci. Rep. 5, 9425 (2015).
Parlett, C. M. A., Bruce, D. W., Hondow, N. S., Lee, A. F. & Wilson, K. Support-enhanced selective aerobic alcohol oxidation over Pd/mesoporous silicas. ACS Catal. 1, 636–640 (2011).
Cargnello, M. Colloidal nanocrystals as building blocks for well-defined heterogeneous catalysts. Chem. Mater. 31, 576–596 (2019).
Losch, P. et al. Colloidal nanocrystals for heterogeneous catalysis. Nano Today 24, 15–47 (2019).
Cargnello, M. et al. Efficient removal of organic ligands from supported nanocrystals by fast thermal annealing enables catalytic studies on well-defined active phases. J. Am. Chem. Soc. 137, 6906–6911 (2015).
Lu, L., Zou, S. & Fang, B. The critical impacts of ligands on heterogeneous nanocatalysis: a review. ACS Catal. 11, 6020–6058 (2021).
Collins, G., Davitt, F., O’Dwyer, C. & Holmes, J. D. Comparing thermal and chemical removal of nanoparticle stabilizing ligands: effect on catalytic activity and stability. ACS Appl. Nano Mater. 1, 7129–7138 (2018).
Eppler, A. S., Rupprechter, G., Guczi, L. & Somorjai, G. A. Model catalysts fabricated using electron beam lithography and pulsed laser deposition. J. Phys. Chem. B 101, 9973–9977 (1997).
Shirman, E. et al. Modular design of advanced catalytic materials using hybrid organic–inorganic raspberry particles. Adv. Funct. Mater. 28, 1704559 (2018).
Singh, N. et al. Aqueous phase catalytic and electrocatalytic hydrogenation of phenol and benzaldehyde over platinum group metals. J. Catal. 382, 372–384 (2020).
Bhanushali, J. T., Kainthla, I., Keri, R. S. & Nagaraja, B. M. Catalytic hydrogenation of benzaldehyde for selective synthesis of benzyl alcohol: a review. ChemistrySelect 1, 3839–3853 (2016).
Kaiser, S. K. et al. Identifying the optimal Pd ensemble size in dilute PdAu alloy nanomaterials for benzaldehyde hydrogenation. ACS Catal. 13, 12092–12103 (2023).
Song, Y. et al. Hydrogenation of benzaldehyde via electrocatalysis and thermal catalysis on carbon-supported metals. J. Catal. 359, 68–75 (2018).
Cheng, G. et al. Critical role of solvent-modulated hydrogen-binding strength in the catalytic hydrogenation of benzaldehyde on palladium. Nat. Catal. 4, 976–985 (2021).
Procházková, D., Zámostný, P., Bejblová, M., Červený, L. & Čejka, J. Hydrodeoxygenation of aldehydes catalyzed by supported palladium catalysts. Appl. Catal. A 332, 56–64 (2007).
Hoeven, J. E. S. et al. On the origin of sinter‐resistance and catalyst accessibility in raspberry‐colloid‐templated catalyst design. Adv. Funct. Mater. 31, 2106876 (2021).
Batista, A. T. F. et al. Atomic scale insight into the formation, size, and location of platinum nanoparticles supported on γ-alumina. ACS Catal. 10, 4193–4204 (2020).
Meier, J. C. et al. Design criteria for stable Pt/C fuel cell catalysts. Beilstein J. Nanotechnol. 5, 44–67 (2014).
Tournus, F. Random nanoparticle deposition: inter-particle distances in 2D, 3D, and multilayer samples. J. Nanopart. Res. 13, 5211–5223 (2011).
Okamoto, M., Hirao, T. & Yamaai, T. Polymers as novel modifiers for supported metal catalyst in hydrogenation of benzaldehydes. J. Catal. 276, 423–428 (2010).
van der Hoeven, J. E. S. et al. Entropic control of HD exchange rates over dilute Pd-in-Au alloy nanoparticle catalysts. ACS Catal. 11, 6971–6981 (2021).
Luneau, M. et al. Achieving high selectivity for alkyne hydrogenation at high conversions with compositionally optimized PdAu nanoparticle catalysts in raspberry colloid-templated SiO2. ACS Catal. 10, 441–450 (2020).
Hao, Y., Pischetola, C., Cárdenas-Lizana, F. & Keane, M. A. Selective liquid phase hydrogenation of benzaldehyde to benzyl alcohol over alumina supported gold. Catal. Lett. 150, 881–887 (2020).
Mohamed‐Ibrahim, N. A. B. et al. Applying magnetic‐responsive nanocatalyst–liquid interface for active molecule manipulation to boost catalysis beyond diffusion limit. ChemCatChem 14, e202200036 (2022).
Cui, T. et al. Encapsulating palladium nanoparticles inside mesoporous MFI zeolite nanocrystals for shape‐selective catalysis. Angew. Chem. Int. Ed. 55, 9178–9182 (2016).
Shirman, E., Shirman, T., Aizenberg, M. & Aizenberg, J. Enhanced catalytic materials with partially embedded catalytic nanoparticles. US patent 11,590,483 B2 (2023).
Kolle, M., Shirman, T., Aizenberg, M., Vogel, N. & Aizenberg, J. High-surface area functional material coated structures. US patent 11,325,114 B2 (2022).
Kao, K. et al. A general approach for monolayer adsorption of high weight loadings of uniform nanocrystals on oxide supports. Angew. Chem. Int. Ed. 60, 7971–7979 (2021).
Casavola, M., Hermannsdörfer, J., De Jonge, N., Dugulan, A. I. & De Jong, K. P. Fabrication of Fischer–Tropsch catalysts by deposition of iron nanocrystals on carbon nanotubes. Adv. Funct. Mater. 25, 5309–5319 (2015).
Santos, H. & Costa, M. Analysis of the mass transfer controlled regime in automotive catalytic converters. Int. J. Heat. Mass Transf. 51, 41–51 (2008).
Lohr, T. L. & Marks, T. J. Orthogonal tandem catalysis. Nat. Chem. 7, 477–482 (2015).
Climent, M. J., Corma, A., Iborra, S. & Sabater, M. J. Heterogeneous catalysis for tandem reactions. ACS Catal. 4, 870–891 (2014).
Isaacs, M. A. et al. A spatially orthogonal hierarchically porous acid–base catalyst for cascade and antagonistic reactions. Nat. Catal. 3, 921–931 (2020).
Wheeldon, I. et al. Substrate channelling as an approach to cascade reactions. Nat. Chem. 8, 299–309 (2016).
Zou, H. et al. Dual metal nanoparticles within multicompartmentalized mesoporous organosilicas for efficient sequential hydrogenation. Nat. Commun. 12, 4968 (2021).
Zou, N. et al. Cooperative communication within and between single nanocatalysts. Nat. Chem. 10, 607–614 (2018).
Cheng, K. et al. Maximizing noble metal utilization in solid catalysts by control of nanoparticle location. Science 377, 204–208 (2022).
Xiao, J. et al. Tandem catalysis with double-shelled hollow spheres. Nat. Mater. 21, 572–579 (2022).
Parlett, C. M. A. et al. Spatially orthogonal chemical functionalization of a hierarchical pore network for catalytic cascade reactions. Nat. Mater. 15, 178–182 (2016).
Piella, J., Bastús, N. G. & Puntes, V. Size-controlled synthesis of sub-10-nanometer citrate-stabilized gold nanoparticles and related optical properties. Chem. Mater. 28, 1066–1075 (2016).
Vogel, N., de Viguerie, L., Jonas, U., Weiss, C. K. & Landfester, K. Wafer-scale fabrication of ordered binary colloidal monolayers with adjustable stoichiometries. Adv. Funct. Mater. 21, 3064–3073 (2011).
Shi, W., Sahoo, Y., Swihart, M. T. & Prasad, P. N. Gold nanoshells on polystyrene cores for control of surface plasmon resonance. Langmuir 21, 1610–1617 (2005).
van der Hoeven, J. E. S. et al. Structural control over bimetallic core–shell nanorods for surface-enhanced raman spectroscopy. ACS Omega 6, 7034–7046 (2021).
Kremer, J. R., Mastronarde, D. N. & McIntosh, J. R. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 116, 71–76 (1996).
Bolte, S. & Cordelières, F. P. A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224, 213–232 (2006).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Sankar, M. et al. The benzaldehyde oxidation paradox explained by the interception of peroxy radical by benzyl alcohol. Nat. Commun. 5, 3332 (2014).
Acknowledgements
This work was supported by the Integrated Mesoscale Architectures for Sustainable Catalysis (IMASC), an Energy Frontier Research Center funded by the US Department of Energy, Office of Science, Basic Energy Sciences under award number DE-SC0012573 (S.K.K. and J.A.), by the US Defense Threat Reduction Agency (DTRA) under award number HDTR1211001612 (K.R.G.L., H.W., S.G. and M.A.), and by the Starting PI Fund of the Electron Microscopy Center at Utrecht University (M.P.P. and J.E.S.v.d.H.). Electron microscopy, TGA, zeta potential measurements, infrared spectroscopy, XPS and CO chemisorption measurements were performed at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Coordinated Infrastructure Network (NNCI), which is supported by the National Science Foundation under NSF ECCS award number 1541959. K.R.G.L., M.A. and J.A. thank C. Friend for the use of her laboratory facilities to conduct the batch hydrogenation catalytic reactions. K.R.G.L. acknowledges financial support from the Agency for Science, Technology and Research (A*STAR) Singapore National Science Scholarship (PhD). S.K.K. acknowledges the Swiss National Science Foundation for the award of an Early Postdoc. Mobility fellowship (SNSF grant number P2EZP2_199972). K.R.G.L. thanks R. J. Madix, J. Gardener, S. Li, H. G. Lin and J. Hungerford for the helpful discussions on the analysis of catalytic, STEM–EDX, small-angle X-ray scattering, XPS and CO chemisorption data, respectively.
Author information
Authors and Affiliations
Contributions
K.R.G.L. and S.K.K. contributed equally to this work. K.R.G.L. conceived the research idea and coordinated the work. K.R.G.L., S.K.K., M.A. and J.A. designed the experiments. K.R.G.L., supported by S.G., designed, synthesized, characterized and analysed the materials. K.R.G.L., guided by S.K.K., conducted catalysis testing and kinetic modelling and analysed the results. M.P.P., supervised by J.E.S.v.d.H., performed the electron tomography experiments and analysed the data. K.R.G.L., S.G. and M.P.P. calculated the interparticle distances. H.W. performed the COMSOL modelling. H.W. and K.R.G.L. analysed the COMSOL modelling results. K.R.G.L. and S.K.K. wrote the manuscript with input from all the authors. J.A. supervised the entire work. All authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Catalysis thanks Yue Liu, Xin Gao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Notes 1–8, Figs. 1–19, Tables 1–6 and references.
Supplementary Video 1
Reconstructed dark-field STEM tomogram of 5PdAu/SiO2 RCT catalyst. NPs are shown as bright spots on the grey SiO2 support.
Supplementary Video 2
Reconstructed dark-field STEM tomogram of 20PdAu/SiO2 RCT catalyst. NPs are shown as bright spots on the grey SiO2 support.
Supplementary Video 3
STEM tilt series of 5PdAu/SiO2 RCT catalyst used for tomographic reconstruction. NPs are shown as bright spots on the grey SiO2 support.
Supplementary Video 4
STEM tilt series of 20PdAu/SiO2 RCT catalyst used for tomographic reconstruction. NPs are shown as bright spots on the grey SiO2 support.
Source data
Source Data Fig. 2
Quantitative numerical data shown in Fig. 2.
Source Data Fig. 3
Quantitative numerical data shown in Fig. 3.
Source Data Fig. 4
Quantitative numerical data shown in Fig. 4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lim, K.R.G., Kaiser, S.K., Wu, H. et al. Nanoparticle proximity controls selectivity in benzaldehyde hydrogenation. Nat Catal 7, 172–184 (2024). https://doi.org/10.1038/s41929-023-01104-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41929-023-01104-1