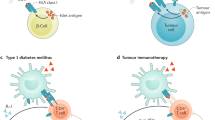
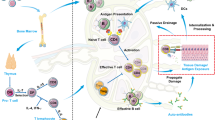
Abstract
Following the seminal discovery of insulin a century ago, treatment of individuals with type 1 diabetes (T1D) has been largely restricted to efforts to monitor and treat metabolic glucose dysregulation. The recent regulatory approval of the first immunotherapy that targets T cells as a means to delay the autoimmune destruction of pancreatic β-cells highlights the critical role of the immune system in disease pathogenesis and tends to pave the way for other immune-targeted interventions for T1D. Improving the efficacy of such interventions across the natural history of the disease will probably require a more detailed understanding of the immunobiology of T1D, as well as technologies to monitor residual β-cell mass and function. Here we provide an overview of the immune mechanisms that underpin the pathogenesis of T1D, with a particular emphasis on T cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gregory, G. A. et al. Global incidence, prevalence, and mortality of type 1 diabetes in 2021 with projection to 2040: a modelling study. Lancet Diabetes Endocrinol. 10, 741–760 (2022).
Evans-Molina, C. et al. β Cell dysfunction exists more than 5 years before type 1 diabetes diagnosis. JCI Insight 3, e120877 (2018).
Sims, E. K. et al. Teplizumab improves and stabilizes beta cell function in antibody-positive high-risk individuals. Sci. Transl. Med. 13, eabc8980 (2021).
Turtinen, M. et al. Characteristics of familial type 1 diabetes: effects of the relationship to the affected family member on phenotype and genotype at diagnosis. Diabetologia 62, 2025–2039 (2019).
Redondo, M. J., Steck, A. K. & Pugliese, A. Genetics of type 1 diabetes. Pediatr. Diabetes 19, 346–353 (2018).
Mrena, S. et al. Models for predicting type 1 diabetes in siblings of affected children. Diabetes Care 29, 662–667 (2006).
Dorman, J. S. et al. Type 1 diabetes in offspring of parents with type 1 diabetes: the tip of an autoimmune iceberg? Pediatr. Diabetes 1, 17–22 (2000).
Noble, J. A. & Valdes, A. M. Genetics of the HLA region in the prediction of type 1 diabetes. Curr. Diabetes Rep. 11, 533–542 (2011).
Aly, T. A. et al. Genetic prediction of autoimmunity: initial oligogenic prediction of anti-islet autoimmunity amongst DR3/DR4-DQ8 relatives of patients with type 1A diabetes. J. Autoimmun. 25, 40–45 (2005).
Astill, T. P., Ellis, R. J., Arif, S., Tree, T. I. & Peakman, M. Promiscuous binding of proinsulin peptides to type 1 diabetes-permissive and -protective HLA class II molecules. Diabetologia 46, 496–503 (2003).
Todd, J. A., Bell, J. I. & McDevitt, H. O. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature 329, 599–604 (1987). This study established that the amino acid sequence of the HLA-DQβ chain is linked to predisposition to T1D in a manner dependent on the identity of amino acid residue 57.
Gioia, L. et al. Position beta57 of I-A(g7) controls early anti-insulin responses in NOD mice, linking an MHC susceptibility allele to type 1 diabetes onset. Sci. Immunol. 4, eaaw6329 (2019).
Robertson, C. C. et al. Fine-mapping, trans-ancestral and genomic analyses identify causal variants, cells, genes and drug targets for type 1 diabetes. Nat. Genet. 53, 962–971 (2021).
Garg, G. et al. Type 1 diabetes-associated IL2RA variation lowers IL-2 signaling and contributes to diminished CD4+CD25+ regulatory T cell function. J. Immunol. 188, 4644–4653 (2012).
Pugliese, A. et al. The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat. Genet. 15, 293–297 (1997).
Sabater, L. et al. Insulin alleles and autoimmune regulator (AIRE) gene expression both influence insulin expression in the thymus. J. Autoimmun. 25, 312–318 (2005).
Vafiadis, P. et al. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat. Genet. 15, 289–292 (1997).
Oakey, H. et al. Protocol for a nested case-control study design for omics investigations in the Environmental Determinants of Islet Autoimmunity cohort. Ann. Med. 55, 2198255 (2023).
Ziegler, A. G. et al. Primary prevention of beta-cell autoimmunity and type 1 diabetes — the Global Platform for the Prevention of Autoimmune Diabetes (GPPAD) perspectives. Mol. Metab. 5, 255–262 (2016).
Krischer, J. P. et al. Genetic and environmental interactions modify the risk of diabetes-related autoimmunity by 6 years of age: the TEDDY study. Diabetes Care 40, 1194–1202 (2017).
Rewers, M. et al. Newborn screening for HLA markers associated with IDDM: Diabetes Autoimmunity Study in the Young (DAISY). Diabetologia 39, 807–812 (1996).
Bach, J. F. Autoimmune diseases as the loss of active “self-control”. Ann. N. Y. Acad. Sci. 998, 161–177 (2003).
Gepts, W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes 14, 619–633 (1965).
Krogvold, L. et al. Detection of a low-grade enteroviral infection in the islets of Langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes 64, 1682–1687 (2015).
Oikarinen, S. et al. Characterisation of enterovirus RNA detected in the pancreas and other specimens of live patients with newly diagnosed type 1 diabetes in the DiViD study. Diabetologia 64, 2491–2501 (2021).
Nekoua, M. P., Alidjinou, E. K. & Hober, D. Persistent coxsackievirus B infection and pathogenesis of type 1 diabetes mellitus. Nat. Rev. Endocrinol. 18, 503–516 (2022).
Rodriguez-Calvo, T. Enterovirus infection and type 1 diabetes: unraveling the crime scene. Clin. Exp. Immunol. 195, 15–24 (2019).
Krogvold, L. et al. Pleconaril and ribavirin in new-onset type 1 diabetes: a phase 2 randomized trial. Nat. Med. 29, 2902–2908 (2023).
Kamrath, C. et al. Incidence of type 1 diabetes in children and adolescents during the COVID-19 pandemic in Germany: results from the DPV Registry. Diabetes Care 45, 1762–1771 (2022).
D’Souza, D. et al. Incidence of diabetes in children and adolescents during the COVID-19 pandemic: a systematic review and meta-analysis. JAMA Netw. Open 6, e2321281 (2023).
Hippich, M. et al. A public health antibody screening indicates a 6-fold higher SARS-CoV-2 exposure rate than reported cases in children. Med 2, 149–163 e144 (2021).
Rewers, M. et al. SARS-CoV-2 infections and presymptomatic type 1 diabetes autoimmunity in children and adolescents from Colorado, USA, and Bavaria, Germany. JAMA 328, 1252–1255 (2022).
Del Chierico, F. et al. Pathophysiology of type 1 diabetes and gut microbiota role. Int. J. Mol. Sci. 23, 14650 (2022).
Morse, Z. J., Simister, R. L., Crowe, S. A., Horwitz, M. S. & Osborne, L. C. Virus induced dysbiosis promotes type 1 diabetes onset. Front. Immunol. 14, 1096323 (2023).
Gavin, P. G., Kim, K. W., Craig, M. E., Hill, M. M. & Hamilton-Williams, E. E. Multi-omic interactions in the gut of children at the onset of islet autoimmunity. Microbiome 10, 230 (2022).
Paun, A. et al. Association of HLA-dependent islet autoimmunity with systemic antibody responses to intestinal commensal bacteria in children. Sci. Immunol. 4, eaau8125 (2019).
Rouxel, O. et al. Cytotoxic and regulatory roles of mucosal-associated invariant T cells in type 1 diabetes. Nat. Immunol. 18, 1321–1331 (2017).
Nel, I. et al. MAIT cell alterations in adults with recent-onset and long-term type 1 diabetes. Diabetologia 64, 2306–2321 (2021).
Gulden, E. et al. Microbiota control immune regulation in humanized mice. JCI Insight 2, e91709 (2017).
Sun, L. et al. Two to tango: dialogue between adaptive and innate immunity in type 1 diabetes. J. Diabetes Res. 2020, 4106518 (2020).
Ferris, S. T. et al. A minor subset of Batf3-dependent antigen-presenting cells in islets of Langerhans is essential for the development of autoimmune diabetes. Immunity 41, 657–669 (2014).
Zirpel, H. & Roep, B. O. Islet-resident dendritic cells and macrophages in type 1 diabetes: in search of bigfoot’s print. Front. Endocrinol. 12, 666795 (2021).
Citro, A., Campo, F., Dugnani, E. & Piemonti, L. Innate immunity mediated inflammation and beta cell function: neighbors or enemies? Front. Endocrinol. 11, 606332 (2020).
Calderon, B. et al. The pancreas anatomy conditions the origin and properties of resident macrophages. J. Exp. Med. 212, 1497–1512 (2015).
Zakharov, P. N., Hu, H., Wan, X. & Unanue, E. R. Single-cell RNA sequencing of murine islets shows high cellular complexity at all stages of autoimmune diabetes. J. Exp. Med. 217, e20192362 (2020).
Carrero, J. A. et al. Resident macrophages of pancreatic islets have a seminal role in the initiation of autoimmune diabetes of NOD mice. Proc. Natl Acad. Sci. USA 114, E10418–E10427 (2017). This paper demonstrates the essential role of islet-resident macrophages in the development of autoimmune diabetes in NOD mice.
Dror, E. et al. Postprandial macrophage-derived IL-1beta stimulates insulin, and both synergistically promote glucose disposal and inflammation. Nat. Immunol. 18, 283–292 (2017).
Alleva, D. G., Pavlovich, R. P., Grant, C., Kaser, S. B. & Beller, D. I. Aberrant macrophage cytokine production is a conserved feature among autoimmune-prone mouse strains: elevated interleukin (IL)-12 and an imbalance in tumor necrosis factor-alpha and IL-10 define a unique cytokine profile in macrophages from young nonobese diabetic mice. Diabetes 49, 1106–1115 (2000).
Calderon, B., Carrero, J. A., Miller, M. J. & Unanue, E. R. Entry of diabetogenic T cells into islets induces changes that lead to amplification of the cellular response. Proc. Natl Acad. Sci. USA 108, 1567–1572 (2011).
Hu, H., Zakharov, P. N., Peterson, O. J. & Unanue, E. R. Cytocidal macrophages in symbiosis with CD4 and CD8 T cells cause acute diabetes following checkpoint blockade of PD-1 in NOD mice. Proc. Natl Acad. Sci. USA 117, 31319–31330 (2020).
Vecchio, F. et al. Abnormal neutrophil signature in the blood and pancreas of presymptomatic and symptomatic type 1 diabetes. JCI Insight 3, e122146 (2018).
Huang, J. et al. Distinct neutrophil counts and functions in newly diagnosed type 1 diabetes, latent autoimmune diabetes in adults, and type 2 diabetes. Diabetes Metab. Res. Rev. 35, e3064 (2019).
Diana, J. et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat. Med. 19, 65–73 (2013). This study highlights the essential role of innate immune cells and their crosstalk in the initiation of autoimmune diabetes.
Diana, J. & Lehuen, A. Macrophages and beta-cells are responsible for CXCR2-mediated neutrophil infiltration of the pancreas during autoimmune diabetes. EMBO Mol. Med. 6, 1090–1104 (2014).
Battaglia, M., Petrelli, A. & Vecchio, F. Neutrophils and type 1 diabetes: current knowledge and suggested future directions. Curr. Opin. Endocrinol. Diabetes Obes. 26, 201–206 (2019).
Valle, A. et al. Reduction of circulating neutrophils precedes and accompanies type 1 diabetes. Diabetes 62, 2072–2077 (2013).
de Boer, P. et al. Large-scale electron microscopy database for human type 1 diabetes. Nat. Commun. 11, 2475 (2020).
Gardner, G. & Fraker, C. A. Natural killer cells as key mediators in type I diabetes immunopathology. Front. Immunol. 12, 722979 (2021).
Poirot, L., Benoist, C. & Mathis, D. Natural killer cells distinguish innocuous and destructive forms of pancreatic islet autoimmunity. Proc. Natl Acad. Sci. USA 101, 8102–8107 (2004).
Hussain, M. J. et al. Elevated serum levels of macrophage-derived cytokines precede and accompany the onset of IDDM. Diabetologia 39, 60–69 (1996).
Bradshaw, E. M. et al. Monocytes from patients with type 1 diabetes spontaneously secrete proinflammatory cytokines inducing Th17 cells. J. Immunol. 183, 4432–4439 (2009).
Apaolaza, P. S. et al. Islet expression of type I interferon response sensors is associated with immune infiltration and viral infection in type 1 diabetes. Sci. Adv. 7, eabd6527 (2021).
Li, Q. et al. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc. Natl Acad. Sci. USA 105, 12439–12444 (2008). This study is one of the seminal papers demonstrating the role of IFNα as an initiator of T1D.
Rodrigues, K. B. et al. Innate immune stimulation of whole blood reveals IFN-1 hyper-responsiveness in type 1 diabetes. Diabetologia 63, 1576–1587 (2020).
Kallionpaa, H. et al. Innate immune activity is detected prior to seroconversion in children with HLA-conferred type 1 diabetes susceptibility. Diabetes 63, 2402–2414 (2014).
Ferreira, R. C. et al. A type I interferon transcriptional signature precedes autoimmunity in children genetically at risk for type 1 diabetes. Diabetes 63, 2538–2550 (2014).
Lombardi, A., Tsomos, E., Hammerstad, S. S. & Tomer, Y. Interferon alpha: the key trigger of type 1 diabetes. J. Autoimmun. 94, 7–15 (2018).
Lundberg, M., Krogvold, L., Kuric, E., Dahl-Jorgensen, K. & Skog, O. Expression of interferon-stimulated genes in insulitic pancreatic islets of patients recently diagnosed with type 1 diabetes. Diabetes 65, 3104–3110 (2016).
Marroqui, L. et al. Interferon-alpha mediates human beta cell HLA class I overexpression, endoplasmic reticulum stress and apoptosis, three hallmarks of early human type 1 diabetes. Diabetologia 60, 656–667 (2017).
Lombardi, A. & Tomer, Y. Interferon alpha impairs insulin production in human beta cells via endoplasmic reticulum stress. J. Autoimmun. 80, 48–55 (2017).
Coomans de Brachene, A. et al. IFN-alpha induces a preferential long-lasting expression of MHC class I in human pancreatic beta cells. Diabetologia 61, 636–640 (2018).
Chandra, V. et al. The type 1 diabetes gene TYK2 regulates β-cell development and its responses to interferon-α. Nat. Commun. 13, 6363 (2022).
Ziegler, A. G. et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 309, 2473–2479 (2013). This is a landmark study establishing the link between multiple islet autoantibody positivity and risk of progressing to stage 3 diabetes.
Krischer, J. P. et al. Predicting islet cell autoimmunity and type 1 diabetes: an 8-year TEDDY study progress report. Diabetes Care 41, 1051–1060 (2019).
Kwon, B. C. et al. Progression of type 1 diabetes from latency to symptomatic disease is predicted by distinct autoimmune trajectories. Nat. Commun. 13, 1514 (2022).
Redondo, M. J. et al. A type 1 diabetes genetic risk score predicts progression of islet autoimmunity and development of type 1 diabetes in individuals at risk. Diabetes Care 41, 1887–1894 (2018). This representative study capitalizes on the ability to create polygenic risk scores for population screening efforts to identify at-risk subjects and those with distinct HLA genotypes or risk variants for pathway targeted therapies.
Warncke, K. et al. Elevations in blood glucose before and after the appearance of islet autoantibodies in children. J. Clin. Invest. 132, e162123 (2022).
Wong, F. S. et al. Investigation of the role of B-cells in type 1 diabetes in the NOD mouse. Diabetes 53, 2581–2587 (2004).
Hu, C. Y. et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J. Clin. Invest. 117, 3857–3867 (2007).
Silveira, P. A. et al. The preferential ability of B lymphocytes to act as diabetogenic APC in NOD mice depends on expression of self-antigen-specific immunoglobulin receptors. Eur. J. Immunol. 32, 3657–3666 (2002).
Hulbert, C., Riseili, B., Rojas, M. & Thomas, J. W. B cell specificity contributes to the outcome of diabetes in nonobese diabetic mice. J. Immunol. 167, 5535–5538 (2001).
Smith, M. J. et al. Loss of anergic B cells in prediabetic and new-onset type 1 diabetic patients. Diabetes 64, 1703–1712 (2015).
Leete, P. et al. Studies of insulin and proinsulin in pancreas and serum support the existence of aetiopathological endotypes of type 1 diabetes associated with age at diagnosis. Diabetologia 63, 1258–1267 (2020).
Campbell-Thompson, M. L. et al. The diagnosis of insulitis in human type 1 diabetes. Diabetologia 56, 2541–2543 (2013).
Damond, N. et al. A map of human type 1 diabetes progression by imaging mass cytometry. Cell Metab. 29, 755–768 e755 (2019).
Willcox, A., Richardson, S. J., Bone, A. J., Foulis, A. K. & Morgan, N. G. Analysis of islet inflammation in human type 1 diabetes. Clin. Exp. Immunol. 155, 173–181 (2009).
Campbell-Thompson, M. et al. Insulitis and beta-cell mass in the natural history of type 1. Diabetes 65, 719–731 (2016).
Babon, J. A. et al. Analysis of self-antigen specificity of islet-infiltrating T cells from human donors with type 1 diabetes. Nat. Med. 22, 1482–1487 (2016). This study identified several autoreactive T cells directly isolated from organ donors with T1D.
Landry, L. G. et al. Proinsulin-reactive CD4 T cells in the islets of type 1 diabetes organ donors. Front. Endocrinol. 12, 622647 (2021).
Pathiraja, V. et al. Proinsulin-specific, HLA-DQ8, and HLA-DQ8-transdimer-restricted CD4+ T cells infiltrate islets in type 1 diabetes. Diabetes 64, 172–182 (2015).
Nakayama, M. et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 435, 220–223 (2005).
Yang, J. et al. Antigen-specific T cell analysis reveals that active immune responses to beta cell antigens are focused on a unique set of epitopes. J. Immunol. 199, 91–96 (2017).
Walker, L. S. & von Herrath, M. CD4 T cell differentiation in type 1 diabetes. Clin. Exp. Immunol. 183, 16–29 (2016).
Ferraro, A. et al. Expansion of Th17 cells and functional defects in T regulatory cells are key features of the pancreatic lymph nodes in patients with type 1 diabetes. Diabetes 60, 2903–2913 (2011).
Arif, S. et al. Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated beta-cell death. Diabetes 60, 2112–2119 (2011).
Kenefeck, R. et al. Follicular helper T cell signature in type 1 diabetes. J. Clin. Invest. 125, 292–303 (2015). This study demonstrated that TFH cells and IL-21 are overproduced in T1D, challenging the established TH1 paradigm.
Ferreira, R. C. et al. IL-21 production by CD4+ effector T cells and frequency of circulating follicular helper T cells are increased in type 1 diabetes patients. Diabetologia 58, 781–790 (2015).
Viisanen, T. et al. Circulating CXCR5+PD-1+ICOS+ follicular T helper cells are increased close to the diagnosis of type 1 diabetes in children with multiple autoantibodies. Diabetes 66, 437–447 (2017).
Pinto, A. I., Smith, J., Kissack, M. R., Hogg, K. G. & Green, E. A. Thymic B cell-mediated attack of thymic stroma precedes type 1 diabetes development. Front. Immunol. 9, 1281 (2018).
Ekman, I. et al. Circulating CXCR5−PD-1hi peripheral T helper cells are associated with progression to type 1 diabetes. Diabetologia 62, 1681–1688 (2019).
Edner, N. M. et al. Follicular helper T cell profiles predict response to costimulation blockade in type 1 diabetes. Nat. Immunol. 21, 1244–1255 (2020).
Kendall, P. L., Yu, G., Woodward, E. J. & Thomas, J. W. Tertiary lymphoid structures in the pancreas promote selection of B lymphocytes in autoimmune diabetes. J. Immunol. 178, 5643–5651 (2007).
Penaranda, C., Tang, Q., Ruddle, N. H. & Bluestone, J. A. Prevention of diabetes by FTY720-mediated stabilization of peri-islet tertiary lymphoid organs. Diabetes 59, 1461–1468 (2010).
Korpos, E. et al. Identification and characterisation of tertiary lymphoid organs in human type 1 diabetes. Diabetologia 64, 1626–1641 (2021).
Mallone, R. et al. CD8+ T-cell responses identify beta-cell autoimmunity in human type 1 diabetes. Diabetes 56, 613–621 (2007).
Cerosaletti, K. et al. Single-cell RNA sequencing reveals expanded clones of islet antigen-reactive CD4+ T cells in peripheral blood of subjects with type 1 diabetes. J. Immunol. 199, 323–335 (2017).
Anderson, A. M. et al. Human islet T cells are highly reactive to preproinsulin in type 1 diabetes. Proc. Natl Acad. Sci. USA 118, e2107208118 (2021).
Culina, S. et al. Islet-reactive CD8+ T cell frequencies in the pancreas, but not in blood, distinguish type 1 diabetic patients from healthy donors. Sci. Immunol. 3, eaao4013 (2018).
Bender, C., Rodriguez-Calvo, T., Amirian, N., Coppieters, K. T. & von Herrath, M. G. The healthy exocrine pancreas contains preproinsulin-specific CD8 T cells that attack islets in type 1 diabetes. Sci. Adv. 6, eabc5586 (2020).
Skowera, A. et al. CTLs are targeted to kill beta cells in patients with type 1 diabetes through recognition of a glucose-regulated preproinsulin epitope. J. Clin. Invest. 118, 3390–3402 (2008). In this study, preproinsulin-specific CTLs that were capable of killing β-cells were isolated and characterized from peripheral blood of patients. The presence of these cells with a direct mechanism for β-cell killing suggests a pathological mechanism.
Coppieters, K. T. et al. Demonstration of islet-autoreactive CD8 T cells in insulitic lesions from recent onset and long-term type 1 diabetes patients. J. Exp. Med. 209, 51–60 (2012). This is a seminal paper showing direct HLA multimer staining of in situ autoreactive T cells.
Yeo, L. et al. Autoreactive T effector memory differentiation mirrors beta cell function in type 1 diabetes. J. Clin. Invest. 128, 3460–3474 (2018).
Wiedeman, A. E. et al. Autoreactive CD8+ T cell exhaustion distinguishes subjects with slow type 1 diabetes progression. J. Clin. Invest. 130, 480–490 (2020).
Abdelsamed, H. A. et al. Beta cell-specific CD8+ T cells maintain stem cell memory-associated epigenetic programs during type 1 diabetes. Nat. Immunol. 21, 578–587 (2020). In this study, tetramer-sorted antigen specific CD8+ T cells from individualswith T1D were found to have an epigenetic signature suggesting stem cell memory features. Together with Bender et al. (2020), this observation suggests novel features of autoreactive CD8+ T cells.
Gearty, S. V. et al. An autoimmune stem-like CD8 T cell population drives type 1 diabetes. Nature 602, 156–161 (2021).
Skowera, A. et al. β-Cell-specific CD8 T cell phenotype in type 1 diabetes reflects chronic autoantigen exposure. Diabetes 64, 916–925 (2015).
Tai, N. et al. Microbial antigen mimics activate diabetogenic CD8 T cells in NOD mice. J. Exp. Med. 213, 2129–2146 (2016).
Hebbandi Nanjundappa, R. et al. A gut microbial mimic that hijacks diabetogenic autoreactivity to suppress colitis. Cell 171, 655–667.17 (2017).
Okada, M. et al. Islet-specific CD8+ T cells gain effector function in the gut lymphoid tissues via bystander activation not molecular mimicry. Immunol. Cell Biol. 101, 36–48 (2023).
Wan, X. et al. Pancreatic islets communicate with lymphoid tissues via exocytosis of insulin peptides. Nature 560, 107–111 (2018).
Quesada-Masachs, E. et al. Upregulation of HLA class II in pancreatic beta cells from organ donors with type 1 diabetes. Diabetologia 65, 387–401 (2022).
Bottazzo, G. F. et al. In situ characterization of autoimmune phenomena and expression of HLA molecules in the pancreas in diabetic insulitis. N. Engl. J. Med. 313, 353–360 (1985).
Foulis, A. K., Farquharson, M. A. & Hardman, R. Aberrant expression of class II major histocompatibility complex molecules by B cells and hyperexpression of class I major histocompatibility complex molecules by insulin containing islets in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 30, 333–343 (1987). Together with Bottazo et al. (1985), this study revealed that HLA molecules can be aberrantly expressed in the pancreatic islets of donors with T1D, raising the possibility that this contributes to immune-mediated destruction.
Marroqui, L. et al. TYK2, a candidate gene for type 1 diabetes, modulates apoptosis and the innate immune response in human pancreatic beta-cells. Diabetes 64, 3808–3817 (2015).
Thompson, P. J. et al. Targeted elimination of senescent beta cells prevents type 1 diabetes. Cell Metab. 29, 1045–1060.e10 (2019).
Rui, J. et al. Tet2 controls the responses of beta cells to inflammation in autoimmune diabetes. Nat. Commun. 12, 5074 (2021).
Shalev, A. Minireview: Thioredoxin-interacting protein: regulation and function in the pancreatic beta-cell. Mol. Endocrinol. 28, 1211–1220 (2014).
Forlenza, G. P. et al. Effect of verapamil on pancreatic beta cell function in newly diagnosed pediatric type 1 diabetes: a randomized clinical trial. JAMA 329, 990–999 (2023).
Xu, G. et al. Exploratory study reveals far reaching systemic and cellular effects of verapamil treatment in subjects with type 1 diabetes. Nat. Commun. 13, 1159 (2022).
Purcell, A. W., Sechi, S. & DiLorenzo, T. P. The evolving landscape of autoantigen discovery and characterization in type 1 diabetes. Diabetes 68, 879–886 (2019).
Rodriguez-Calvo, T., Johnson, J. D., Overbergh, L. & Dunne, J. L. Neoepitopes in type 1 diabetes: etiological insights, biomarkers and therapeutic targets. Front. Immunol. 12, 667989 (2021).
Mannering, S. I. et al. The insulin A-chain epitope recognized by human T cells is posttranslationally modified. J. Exp. Med. 202, 1191–1197 (2005).
Wiles, T. A. et al. Identification of hybrid insulin peptides (HIPs) in mouse and human islets by mass spectrometry. J. Proteome Res. 18, 814–825 (2019).
Wiles, T. A., Saba, L. M. & Delong, T. Peptide-spectrum match validation with internal standards (p-vis): internally-controlled validation of mass spectrometry-based peptide identifications. J. Proteome Res. 20, 236–249 (2021).
Wiles, T. A. et al. Characterization of human CD4 T cells specific for a C-Peptide/C-peptide hybrid insulin peptide. Front. Immunol. 12, 668680 (2021).
Baker, R. L. et al. Hybrid insulin peptides are autoantigens in type 1 diabetes. Diabetes 68, 1830–1840 (2019).
Mitchell, A. M. et al. T-cell responses to hybrid insulin peptides prior to type 1 diabetes development. Proc. Natl Acad. Sci. USA 118, e2019129118 (2021).
Delong, T. et al. Pathogenic CD4 T cells in type 1 diabetes recognize epitopes formed by peptide fusion. Science 351, 711–714 (2016). This study in diabetic mice and human pancreatic samples suggests a role for hybrid peptides in breaking immune tolerance.
Wiles, T. A. et al. An insulin-IAPP hybrid peptide is an endogenous antigen for CD4 T cells in the non-obese diabetic mouse. J. Autoimmun. 78, 11–18 (2017).
Parras, D., Sole, P., Delong, T., Santamaria, P. & Serra, P. Recognition of multiple hybrid insulin peptides by a single highly diabetogenic T-cell receptor. Front. Immunol. 12, 737428 (2021).
Burton, A. R. et al. On the pathogenicity of autoantigen-specific T-cell receptors. Diabetes 57, 1321–1330 (2008).
Crawford, S. A. et al. Cathepsin D drives the formation of hybrid insulin peptides relevant to the pathogenesis of type 1 diabetes. Diabetes 71, 2793–2803 (2022). This study shows that HIPs, formed by peptide bond cross-linking between proinsulin fragments and other peptides in pancreatic β-cells, are identified in the CD4 T cell targets of indiviuals with T1D; cathepsin D is revealed as the key protease driving HIP formation.
Daniel, D., Gill, R. G., Schloot, N. & Wegmann, D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur. J. Immunol. 25, 1056–1062 (1995).
Strollo, R. et al. Autoantibody and T cell responses to oxidative post-translationally modified insulin neoantigenic peptides in type 1 diabetes. Diabetologia 66, 132–146 (2023).
Mallone, R., Brezar, V. & Boitard, C. T cell recognition of autoantigens in human type 1 diabetes: clinical perspectives. Clin. Dev. Immunol. 2011, 513210 (2011).
Yang, M. L. et al. Carbonyl posttranslational modification associated with early-onset type 1 diabetes autoimmunity. Diabetes 71, 1979–1993 (2022).
Kracht, M. J. et al. Autoimmunity against a defective ribosomal insulin gene product in type 1 diabetes. Nat. Med. 23, 501–507 (2017). This study explores a new source of immunogenic polypeptides originating from a nonconventional open reading frame within human insulin mRNA, which can be targeted by cytotoxic CD8+ T cells present in individuals with T1D, potentially contributing to β-cell destruction in the disease.
Schneider, A. et al. The effector T cells of diabetic subjects are resistant to regulation via CD4+ FOXP3+ regulatory T cells. J. Immunol. 181, 7350–7355 (2008).
Hulme, M. A., Wasserfall, C. H., Atkinson, M. A. & Brusko, T. M. Central role for interleukin-2 in type 1 diabetes. Diabetes 61, 14–22 (2012).
McClymont, S. A. et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J. Immunol. 186, 3918–3926 (2011).
Dean, J. W. et al. Innate inflammation drives NK cell activation to impair Treg activity. J. Autoimmun. 108, 102417 (2020).
Vecchione, A. et al. Reduced follicular regulatory T cells in spleen and pancreatic lymph nodes of patients with type 1 diabetes. Diabetes 70, 2892–2902 (2021).
Battaglia, M. et al. Introducing the endotype concept to address the challenge of disease heterogeneity in type 1 diabetes. Diabetes Care 43, 5–12 (2020). This study shows that demographic and immunological factors may be used to identify individuals with T1D in whom the mechanisms of disease and responses to therapies differ. Endotypes may be defined by these parameters.
Bluestone, J. A., Buckner, J. H. & Herold, K. C. Immunotherapy: building a bridge to a cure for type 1 diabetes. Science 373, 510–516 (2021).
Dayan, C. M. et al. Preventing type 1 diabetes in childhood. Science 373, 506–510 (2021).
Pearson, J. A., McKinney, E. F. & Walker, L. S. K. 100 years post-insulin: immunotherapy as the next frontier in type 1 diabetes. Immunother. Adv. 1, ltab024 (2021).
Mastrandrea, L. et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care 32, 1244–1249 (2009).
Quattrin, T. et al. Golimumab and beta-cell function in youth with new-onset type 1 diabetes. N. Engl. J. Med. 383, 2007–2017 (2020).
Moran, A. et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet 381, 1905–1915 (2013).
Greenbaum, C. J. et al. IL-6 receptor blockade does not slow beta cell loss in new-onset type 1 diabetes. JCI Insight 6, e150074 (2021).
Piemonti, L. et al. Ladarixin, an inhibitor of the interleukin-8 receptors CXCR1 and CXCR2, in new-onset type 1 diabetes: a multicentre, randomized, double-blind, placebo-controlled trial. Diabetes Obes. Metab. 24, 1840–1849 (2022).
Citro, A. et al. CXCR1/2 inhibition blocks and reverses type 1 diabetes in mice. Diabetes 64, 1329–1340 (2015).
Gitelman, S. E. et al. Imatinib therapy for patients with recent-onset type 1 diabetes: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 9, 502–514 (2021).
Waibel, M. et al. Baricitinib and beta-cell function in patients with new-onset type 1 diabetes. N. Engl. J. Med. 389, 2140–2150 (2023).
Ludvigsson, J. et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N. Engl. J. Med. 359, 1909–1920 (2008).
Ludvigsson, J. et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N. Engl. J. Med. 366, 433–442 (2012).
Wherrett, D. K. et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: a randomised double-blind trial. Lancet 378, 319–327 (2011).
Nowak, C. et al. Intralymphatic GAD-alum (Diamyd®) improves glycemic control in type 1 diabetes with HLA DR3-DQ2. J. Clin. Endocrinol. Metab. 107, 2644–2651 (2022).
Diabetes Prevention Trial — Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N. Engl. J. Med. 346, 1685–1691 (2002).
Skyler, J. S. et al. Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial — Type 1. Diabetes Care 28, 1068–1076 (2005).
Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study Group. et al. Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA 318, 1891–1902 (2017).
Bonifacio, E. et al. Effects of high-dose oral insulin on immune responses in children at high risk for type 1 diabetes: the Pre-POINT randomized clinical trial. JAMA 313, 1541–1549 (2015).
Pescovitz, M. D. et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl. J. Med. 361, 2143–2152 (2009). This study highlights a role for B cells in the pathogenesis in T1D and demonstrates that treatment with rituximab can preserve β-cell function in individuals with T1D.
Pescovitz, M. D. et al. B-lymphocyte depletion with rituximab and beta-cell function: two-year results. Diabetes Care 37, 453–459 (2014).
Chamberlain, N. et al. Rituximab does not reset defective early B cell tolerance checkpoints. J. Clin. Invest. 126, 282–287 (2016).
Linsley, P. S. et al. Elevated T cell levels in peripheral blood predict poor clinical response following rituximab treatment in new-onset type 1 diabetes. Genes Immun. 20, 293–307 (2019).
Lenschow, D. J. et al. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the nonobese diabetic mouse. J. Exp. Med. 181, 1145–1155 (1995).
Orban, T. et al. Costimulation modulation with abatacept in patients with recent-onset type 1 diabetes: follow-up 1 year after cessation of treatment. Diabetes Care 37, 1069–1075 (2014).
Orban, T. et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 378, 412–419 (2011). This randomized placebo-controlled clinical trial shows that treatment with CTLA4Ig over 2 years attenuated loss of β-cell function.
Russell, W. E. et al. Abatacept for delay of type 1 diabetes progression in stage 1 relatives at risk: a randomized, double-masked, controlled trial. Diabetes Care 46, 1005–1013 (2023).
Orban, T. et al. Reduction in CD4 central memory T-cell subset in costimulation modulator abatacept-treated patients with recent-onset type 1 diabetes is associated with slower C-peptide decline. Diabetes 63, 3449–3457 (2014).
Wang, C. J. et al. Costimulation blockade in combination with IL-2 permits regulatory T cell sparing immunomodulation that inhibits autoimmunity. Nat. Commun. 13, 6757 (2022).
Haller, M. J. et al. Anti-thymocyte globulin/G-CSF treatment preserves beta cell function in patients with established type 1 diabetes. J. Clin. Invest. 125, 448–455 (2015).
Haller, M. J. et al. Antithymocyte globulin plus G-CSF combination therapy leads to sustained immunomodulatory and metabolic effects in a subset of responders with established type 1 diabetes. Diabetes 65, 3765–3775 (2016).
Haller, M. J. et al. Low-dose anti-thymocyte globulin preserves C-peptide, reduces HbA1c, and increases regulatory to conventional T-cell ratios in new-onset type 1 diabetes: two-year clinical trial data. Diabetes 68, 1267–1276 (2019).
Haller, M. J. et al. Low-dose anti-thymocyte globulin (ATG) preserves beta-cell function and improves HbA1c in new-onset type 1 diabetes. Diabetes Care 41, 1917–1925 (2018). This randomized placebo-controlled clinical trial shows that ATG treatment reduced the decline in β-cell function in patients with new-onset T1D.
Jacobsen, L. M. et al. Responders to low-dose ATG induce CD4 T cell exhaustion in type 1 diabetes. JCI Insight 8, e161812 (2023).
Rigby, M. R. et al. Alefacept provides sustained clinical and immunological effects in new-onset type 1 diabetes patients. J. Clin. Invest. 125, 3285–3296 (2015).
Charpentier, B. et al. Evidence that antihuman tumor necrosis factor monoclonal antibody prevents OKT3-induced acute syndrome. Transplantation 54, 997–1002 (1992).
Chatenoud, L. OKT3-induced cytokine-release syndrome: prevention effect of anti-tumor necrosis factor monoclonal antibody. Transplant. Proc. 25, 47–51 (1993).
Norman, D. J., Chatenoud, L., Cohen, D., Goldman, M. & Shield, C. F. III Consensus statement regarding OKT3-induced cytokine-release syndrome and human antimouse antibodies. Transplant. Proc. 25, 89–92 (1993).
Herold, K. C. et al. Prevention of autoimmune diabetes with nonactivating anti-CD3 monoclonal antibody. Diabetes 41, 385–391 (1992).
Chatenoud, L., Primo, J. & Bach, J. F. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J. Immunol. 158, 2947–2954 (1997).
Chatenoud, L., Thervet, E., Primo, J. & Bach, J. F. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc. Natl Acad. Sci. USA 91, 123–127 (1994). This study shows, in the NOD model, how brief treatment with monolonal CD3 antibody could reverse spontaneous diabetes, after its appearance, and induce specific immune tolerance.
Sherry, N. A. et al. Effects of autoimmunity and immune therapy on beta-cell turnover in type 1 diabetes. Diabetes 55, 3238–3245 (2006).
Bach, J. F. Anti-CD3 antibodies for type 1 diabetes: beyond expectations. Lancet 378, 459–460 (2011).
Herold, K. C. et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N. Engl. J. Med. 346, 1692–1698 (2002). This clinical trial of teplizumab in patients with new-onset T1D shows that a single 14-day course would attenuate the loss of β-cell function.
Herold, K. C. et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 54, 1763–1769 (2005).
Keymeulen, B. et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N. Engl. J. Med. 352, 2598–2608 (2005).
Herold, K. C. et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 62, 3766–3774 (2013).
Herold, K. C. et al. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia 56, 391–400 (2013).
Herold, K. C. et al. Teplizumab: a disease-modifying therapy for type 1 diabetes that preserves beta-cell function. Diabetes Care 46, 1848–1856 (2023).
Ramos, E. L. et al. Teplizumab and beta-cell function in newly diagnosed type 1 diabetes. N. Engl. J. Med. 389, 2151–2161 (2023).
Herold, K. C. et al. An anti-CD3 antibody, teplizumab, in relatives at risk for type 1 diabetes. N. Engl. J. Med. 381, 603–613 (2019). This randomized placebo-controlled clinical trial shows that a single 14-day course of the CD3 antibody teplizumab would delay the onset of T1D in relatives at high risk.
Sosenko, J. M. et al. Phenotypes associated with zones defined by area under the curve glucose and C-peptide in a population with islet autoantibodies. Diabetes Care 46, 1098–1105 (2023).
McKinney, E. F., Lee, J. C., Jayne, D. R., Lyons, P. A. & Smith, K. G. T-cell exhaustion, co-stimulation and clinical outcome in autoimmunity and infection. Nature 523, 612–616 (2015).
Long, S. A. et al. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes 61, 2340–2348 (2012).
Seelig, E. et al. The DILfrequency study is an adaptive trial to identify optimal IL-2 dosing in patients with type 1 diabetes. JCI Insight 3, e99306 (2018).
Zhang, J. Y. et al. Low-dose IL-2 reduces IL-21+ T cell frequency and induces anti-inflammatory gene expression in type 1 diabetes. Nat. Commun. 13, 7324 (2022).
Rosenzwajg, M. et al. Low-dose IL-2 in children with recently diagnosed type 1 diabetes: a phase I/II randomised, double-blind, placebo-controlled, dose-finding study. Diabetologia 63, 1808–1821 (2020).
Putnam, A. L. et al. Expansion of human regulatory T-cells from patients with type 1 diabetes. Diabetes 58, 652–662 (2009).
Bluestone, J. A. et al. Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci. Transl. Med. 7, 315ra189 (2015). This phase Ib clinical study evaluates the safety, pharmacodynamics and limited information on the efficacy of autologous Treg cells that were expanded ex vivo and administered to indivduals with recent-onset T1D.
Dong, S. et al. The effect of low-dose IL-2 and Treg adoptive cell therapy in patients with type 1 diabetes. JCI Insight 6, e147474 (2021).
Ferreira, L. M. R., Muller, Y. D., Bluestone, J. A. & Tang, Q. Next-generation regulatory T cell therapy. Nat. Rev. Drug Discov. 18, 749–769 (2019).
Cui, C., Craft, J. & Joshi, N. S. T follicular helper cells in cancer, tertiary lymphoid structures, and beyond. Semin. Immunol. 69, 101797 (2023).
Linsley, P. S., Greenbaum, C. J., Speake, C., Long, S. A. & Dufort, M. J. B lymphocyte alterations accompany abatacept resistance in new-onset type 1 diabetes. JCI Insight 4, e126136 (2019).
Thomas, H. E. et al. Interferon signalling in pancreatic beta cells. Front. Biosci. 14, 644–656 (2009).
Mayr, A. et al. GAD autoantibody affinity and epitope specificity identify distinct immunization profiles in children at risk for type 1 diabetes. Diabetes 56, 1527–1533 (2007).
Endesfelder, D. et al. Time-resolved autoantibody profiling facilitates stratification of preclinical type 1 diabetes in children. Diabetes 68, 119–130 (2019).
Achenbach, P. et al. Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes 53, 384–392 (2004).
Achenbach, P. et al. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J. Clin. Invest. 114, 589–597 (2004).
Schlosser, M. et al. In insulin-autoantibody-positive children from the general population, antibody affinity identifies those at high and low risk. Diabetologia 48, 1830–1832 (2005).
Yang, M. L. et al. Citrullination of glucokinase is linked to autoimmune diabetes. Nat. Commun. 13, 1870 (2022).
Schloot, N. C. et al. Comparison of cytokine ELISpot assay formats for the detection of islet antigen autoreactive T cells. Report of the Third Immunology of Diabetes Society T-cell Workshop. J. Autoimmun. 21, 365–376 (2003).
Herold, K. C. et al. Validity and reproducibility of measurement of islet autoreactivity by T-cell assays in subjects with early type 1 diabetes. Diabetes 58, 2588–2595 (2009).
James, E. A. et al. Combinatorial detection of autoreactive CD8+ T cells with HLA-A2 multimers: a multi-centre study by the Immunology of Diabetes Society T Cell Workshop. Diabetologia 61, 658–670 (2018).
Ogura, H. et al. Identification and analysis of islet antigen-specific CD8+ T cells with T cell libraries. J. Immunol. 201, 1662–1670 (2018).
Herold, K. C. et al. Increased T cell proliferative responses to islet antigens identify clinical responders to anti-CD20 monoclonal antibody (rituximab) therapy in type 1 diabetes. J. Immunol. 187, 1998–2005 (2011).
Brown, C. T. et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE 6, e25792 (2011).
Vatanen, T. et al. Variation in microbiome LPS immunogenicity contributes to autoimmunity in humans. Cell 165, 842–853 (2016).
Martinov, T. et al. Programmed death-1 restrains the germinal center in type 1 diabetes. J. Immunol. 203, 844–852 (2019).
Serr, I. et al. miRNA92a targets KLF2 and the phosphatase PTEN signaling to promote human T follicular helper precursors in T1D islet autoimmunity. Proc. Natl Acad. Sci. USA 113, E6659–E6668 (2016).
Smith, J. A., Tso, J. Y., Clark, M. R., Cole, M. S. & Bluestone, J. A. Nonmitogenic anti-CD3 monoclonal antibodies deliver a partial T cell receptor signal and induce clonal anergy. J. Exp. Med. 185, 1413–1422 (1997).
Esplugues, E. et al. Control of TH17 cells occurs in the small intestine. Nature 475, 514–518 (2011).
Waldron-Lynch, F. et al. Teplizumab induces human gut-tropic regulatory cells in humanized mice and patients. Sci. Transl. Med. 4, 118ra112 (2012).
Penaranda, C., Tang, Q. & Bluestone, J. A. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J. Immunol. 187, 2015–2022 (2011).
Belghith, M. et al. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat. Med. 9, 1202–1208 (2003).
Long, S. A. et al. Partial exhaustion of CD8 T cells and clinical response to teplizumab in new-onset type 1 diabetes. Sci. Immunol. 1, eaai7793 (2016).
Perdigoto, A. L. et al. Treatment of type 1 diabetes with teplizumab: clinical and immunological follow-up after 7 years from diagnosis. Diabetologia 62, 655–664 (2019).
Campbell-Thompson, M. et al. Network for Pancreatic Ogan Donors with Diabetes (nPOD): developing a tissue biobank for type 1 diabetes. Diabetes Metab. Res. Rev. 28, 608–617 (2012).
Acknowledgements
K.C.H. acknowledges support from the US National Institutes of Health (NIH) through grant numbers DK057846, DK129523, DK045735, AI66387, DK106993 (Type 1 Diabetes TrialNet) and CA2277473. A.L.P. also acknowledges support from NIH through grant numbers DK106993 (Type 1 Diabetes TrialNet) and CA215110. T.M.B. acknowledges support from the National Institutes of Allergy and Infectious Diseases (NIAID) through grant number P01AI042288 and National Institute of Diabetes Digestive and Kidney Diseases through grant numbers DK106191 and DK122638. L.S.K.W. acknowledges support from the Medical Research Council (MR/N001435/1), Wellcome Trust (220772/Z/20/Z), Diabetes UK (20/0006172) and Connect Immune Research (22931). We are grateful to Natalie Edner for the assistance with figure preparation.
Author information
Authors and Affiliations
Contributions
All authors researched data for the article and wrote and edited the article. All authors reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
K.C.H. is a co-inventor on a patent for the use of teplizumab to delay type 1 diabetes and has consulted for Sanofi, Provention Bio, GSK, Abata, Gentibio and Idorsia. He is on the scientific advisory board for Sonoma and NexImmune.
Peer review
Peer review information
Nature Reviews Immunology thanks Agnes Lehuen, Matthias von Herrath and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Herold, K.C., Delong, T., Perdigoto, A.L. et al. The immunology of type 1 diabetes. Nat Rev Immunol (2024). https://doi.org/10.1038/s41577-023-00985-4
Accepted:
Published:
DOI: https://doi.org/10.1038/s41577-023-00985-4