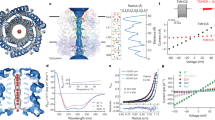
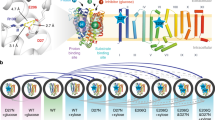
Abstract
Selective proton transport through proteins is essential for forming and using proton gradients in cells. Protons are conducted along hydrogen-bonded ‘wires’ of water molecules and polar side chains, which, somewhat surprisingly, are often interrupted by dry apolar stretches in the conduction pathways, inferred from static protein structures. Here we hypothesize that protons are conducted through such dry spots by forming transient water wires, often highly correlated with the presence of the excess protons in the water wire. To test this hypothesis, we performed molecular dynamics simulations to design transmembrane channels with stable water pockets interspersed by apolar segments capable of forming flickering water wires. The minimalist designed channels conduct protons at rates similar to viral proton channels, and they are at least 106-fold more selective for H+ over Na+. These studies inform the mechanisms of biological proton conduction and the principles for engineering proton-conductive materials.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Moriyama, Y. & Futai, M. H+-ATPase, a primary pump for accumulation of neurotransmitters, is a major constituent of brain synaptic vesicles. Biochem. Biophys. Res. Commun. 173, 443–448 (1990).
Nishi, T. & Forgac, M. The vacuolar (H+)-ATPases—nature’s most versatile proton pumps. Nat. Rev. Mol. Cell Biol. 3, 94–103 (2002).
Mitchell, P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature 191, 144–148 (1961).
Nicholls, D. G. Mitochondrial ion circuits. Essays Biochem. 47, 25–35 (2010).
Diering, G. H. & Numata, M. Endosomal pH in neuronal signaling and synaptic transmission: role of Na+/H+ exchanger NHE5. Front. Physiol. 4, 412 (2014).
Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 244, 456–462 (1995).
Calio, P. B., Li, C. & Voth, G. A. Resolving the structural debate for the hydrated excess proton in water. J. Am. Chem. Soc. 143, 18672–18683 (2021).
Li, C. & Voth, G. A. A quantitative paradigm for water-assisted proton transport through proteins and other confined spaces. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.2113141118 (2021).
Wraight, C. A. Chance and design—proton transfer in water, channels and bioenergetic proteins. Biochim. Biophys. Acta 1757, 886–912 (2006).
Decoursey, T. E. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. 83, 475–579 (2003).
Peng, Y., Swanson, J. M., Kang, S. G., Zhou, R. & Voth, G. A. Hydrated excess protons can create their own water wires. J. Phys. Chem. B 119, 9212–9218 (2015).
Banh, R. et al. Hydrophobic gasket mutation produces gating pore currents in closed human voltage-gated proton channels. Proc. Natl Acad. Sci. USA 116, 18951–18961 (2019).
Garczarek, F. & Gerwert, K. Functional waters in intraprotein proton transfer monitored by FTIR difference spectroscopy. Nature 439, 109–112 (2006).
Kaur, D., Khaniya, U., Zhang, Y. & Gunner, M. R. Protein motifs for proton transfers that build the transmembrane proton gradient. Front. Chem. 9, 660954 (2021).
Kalra, A., Garde, S. & Hummer, G. Osmotic water transport through carbon nanotube membranes. Proc. Natl Acad. Sci. USA 100, 10175–10180 (2003).
Ben-Abu, Y., Zhou, Y., Zilberberg, N. & Yifrach, O. Inverse coupling in leak and voltage-activated K+ channel gates underlies distinct roles in electrical signaling. Nat. Struct. Mol. Biol. 16, 71–79 (2009).
Jensen, M. O. et al. Principles of conduction and hydrophobic gating in K+ channels. Proc. Natl Acad. Sci. USA 107, 5833–5838 (2010).
Aryal, P., Sansom, M. S. & Tucker, S. J. Hydrophobic gating in ion channels. J. Mol. Biol. 427, 121–130 (2015).
Zhu, F. & Hummer, G. Drying transition in the hydrophobic gate of the GLIC channel blocks ion conduction. Biophys. J. 103, 219–227 (2012).
Rasaiah, J. C., Garde, S. & Hummer, G. Water in nonpolar confinement: from nanotubes to proteins and beyond. Annu. Rev. Phys. Chem. 59, 713–740 (2008).
Wang, T. et al. Deprotonation of D96 in bacteriorhodopsin opens the proton uptake pathway. Structure 21, 290–297 (2013).
Weinert, T. et al. Proton uptake mechanism in bacteriorhodopsin captured by serial synchrotron crystallography. Science 365, 61–65 (2019).
Freier, E., Wolf, S. & Gerwert, K. Proton transfer via a transient linear water-molecule chain in a membrane protein. Proc. Natl Acad. Sci. USA 108, 11435–11439 (2011).
Regan, L. & DeGrado, W. F. Characterization of a helical protein designed from first principles. Science 241, 976–978 (1988).
Walsh, S. T., Cheng, H., Bryson, J. W., Roder, H. & DeGrado, W. F. Solution structure and dynamics of a de novo designed three-helix bundle protein. Proc. Natl Acad. Sci. USA 96, 5486–5491 (1999).
Kuhlman, B. et al. Design of a novel globular protein fold with atomic-level accuracy. Science 302, 1364–1368 (2003).
Vorobieva, A. A. et al. De novo design of transmembrane β barrels. Science https://doi.org/10.1126/science.abc8182 (2021).
Yang, C. et al. Bottom-up de novo design of functional proteins with complex structural features. Nat. Chem. Biol. 17, 492–500 (2021).
Polizzi, N. F. & DeGrado, W. F. A defined structural unit enables de novo design of small-molecule-binding proteins. Science 369, 1227–1233 (2020).
Cao, L. et al. De novo design of picomolar SARS-CoV-2 miniprotein inhibitors. Science 370, 426–431 (2020).
Fleishman, S. J. et al. Computational design of proteins targeting the conserved stem region of influenza hemagglutinin. Science 332, 816–821 (2011).
Jiang, L. et al. De novo computational design of retro-aldol enzymes. Science 319, 1387–1391 (2008).
Lassila, J. K., Privett, H. K., Allen, B. D. & Mayo, S. L. Combinatorial methods for small-molecule placement in computational enzyme design. Proc. Natl Acad. Sci. USA 103, 16710–16715 (2006).
Polizzi, N. F. et al. De novo design of a hyperstable non-natural protein–ligand complex with sub-A accuracy. Nat. Chem. 9, 1157–1164 (2017).
Leaver-Fay, A. et al. ROSETTA3: an object-oriented software suite for the simulation and design of macromolecules. Methods Enzymol. 487, 545–574 (2011).
Koga, N. et al. Principles for designing ideal protein structures. Nature 491, 222–227 (2012).
Scott, A. J. et al. Constructing ion channels from water-soluble α-helical barrels. Nat. Chem. 13, 643–650 (2021).
Xu, C. et al. Computational design of transmembrane pores. Nature 585, 129–134 (2020).
Joh, N. H. et al. De novo design of a transmembrane Zn2+-transporting four-helix bundle. Science 346, 1520–1524 (2014).
Lu, P. et al. Accurate computational design of multipass transmembrane proteins. Science 359, 1042–1046 (2018).
Thomaston, J. L. et al. X-ray crystal structure of the influenza A M2 proton channel S31N mutant in two conformational states: an open and shut case. J. Am. Chem. Soc. 141, 11481–11488 (2019).
Saotome, K. et al. Structures of the otopetrin proton channels Otop1 and Otop3. Nat. Struct. Mol. Biol. 26, 518–525 (2019).
Mravic, M. et al. Packing of apolar side chains enables accurate design of highly stable membrane proteins. Science 363, 1418–1423 (2019).
Klesse, G., Rao, S., Sansom, M. S. P. & Tucker, S. J. CHAP: a versatile tool for the structural and functional annotation of ion channel pores. J. Mol. Biol. 431, 3353–3365 (2019).
Lee, S., Liang, R., Voth, G. A. & Swanson, J. M. Computationally efficient multiscale reactive molecular dynamics to describe amino acid deprotonation in proteins. J. Chem. Theory Comput. 12, 879–891 (2016).
Knight, C., Lindberg, G. E. & Voth, G. A. Multiscale reactive molecular dynamics. J. Chem. Phys. 137, 22A525 (2012).
Yamashita, T., Peng, Y., Knight, C. & Voth, G. A. Computationally efficient multiconfigurational reactive molecular dynamics. J. Chem. Theory Comput. 8, 4863–4875 (2012).
Moffat, J. C. et al. Proton transport through influenza A virus M2 protein reconstituted in vesicles. Biophys. J. 94, 434–445 (2008).
Ma, C. et al. Identification of the functional core of the influenza A virus A/M2 proton-selective ion channel. Proc. Natl Acad. Sci. USA 106, 12283–12288 (2009).
Leiding, T., Wang, J., Martinsson, J., DeGrado, W. F. & Arskold, S. P. Proton and cation transport activity of the M2 proton channel from influenza A virus. Proc. Natl Acad. Sci. USA 107, 15409–15414 (2010).
Slope, L. N. & Peacock, A. F. De novo design of xeno-metallo coiled coils. Chem. Asian J. 11, 660–666 (2016).
Pinter, T. B. J., Koebke, K. J. & Pecoraro, V. L. Catalysis and electron transfer in de novo designed helical scaffolds. Angew. Chem. Int. Ed. 59, 7678–7699 (2020).
Khurana, E. et al. Molecular dynamics calculations suggest a conduction mechanism for the M2 proton channel from influenza A virus. Proc. Natl Acad. Sci. USA 106, 1069–1074 (2009).
Yi, M., Cross, T. A. & Zhou, H. X. A secondary gate as a mechanism for inhibition of the M2 proton channel by amantadine. J. Phys. Chem. B 112, 7977–7979 (2008).
Ramsey, I. S. et al. An aqueous H+ permeation pathway in the voltage-gated proton channel Hv1. Nat. Struct. Mol. Biol. 17, 869–875 (2010).
Chamberlin, A. et al. Hydrophobic plug functions as a gate in voltage-gated proton channels. Proc. Natl Acad. Sci. USA 111, E273–E282 (2014).
Takeshita, K. et al. X-ray crystal structure of voltage-gated proton channel. Nat. Struct. Mol. Biol. 21, 352–357 (2014).
Wikstrom, M., Krab, K. & Sharma, V. Oxygen activation and energy conservation by cytochrome c oxidase. Chem. Rev. 118, 2469–2490 (2018).
Hofacker, I. & Schulten, K. Oxygen and proton pathways in cytochrome c oxidase. Proteins 30, 100–107 (1998).
Wikström, M., Verkhovsky, M. I. & Hummer, G. Water-gated mechanism of proton translocation by cytochrome c oxidase. Biochim. Biophys. Acta Bioenerg. 1604, 61–65 (2003).
Tashiro, M. & Stuchebrukhov, A. A. Thermodynamic properties of internal water molecules in the hydrophobic cavity around the catalytic center of cytochrome c oxidase. J. Phys. Chem. B 109, 1015–1022 (2005).
Goyal, P., Lu, J., Yang, S., Gunner, M. R. & Cui, Q. Changing hydration level in an internal cavity modulates the proton affinity of a key glutamate in cytochrome c oxidase. Proc. Natl Acad. Sci. USA 110, 18886–18891 (2013).
Liang, R., Swanson, J. M. J., Wikstrom, M. & Voth, G. A. Understanding the essential proton-pumping kinetic gates and decoupling mutations in cytochrome c oxidase. Proc. Natl Acad. Sci. USA 114, 5924–5929 (2017).
Liang, R., Swanson, J. M., Peng, Y., Wikstrom, M. & Voth, G. A. Multiscale simulations reveal key features of the proton-pumping mechanism in cytochrome c oxidase. Proc. Natl Acad. Sci. USA 113, 7420–7425 (2016).
Lynch, C. I., Rao, S. & Sansom, M. S. P. Water in nanopores and biological channels: a molecular simulation perspective. Chem. Rev. https://doi.org/10.1021/acs.chemrev.9b00830 (2020).
Chen, H. et al. Charge delocalization in proton channels, I: the aquaporin channels and proton blockage. Biophys. J. 92, 46–60 (2007).
Murata, K. et al. Structural determinants of water permeation through aquaporin-1. Nature 407, 599–605 (2000).
Mondal, D., Kolev, V. & Warshel, A. Combinatorial approach for exploring conformational space and activation barriers in computer-aided enzyme design. ACS Catal. 10, 6002–6012 (2020).
Tunuguntla, R. H., Allen, F. I., Kim, K., Belliveau, A. & Noy, A. Ultrafast proton transport in sub-1-nm diameter carbon nanotube porins. Nat. Nanotechnol. 11, 639–644 (2016).
Geng, J. et al. Stochastic transport through carbon nanotubes in lipid bilayers and live cell membranes. Nature 514, 612–615 (2014).
Jiang, T. et al. Single-chain heteropolymers transport protons selectively and rapidly. Nature 577, 216–220 (2020).
Caffrey, M. & Cherezov, V. Crystallizing membrane proteins using lipidic mesophases. Nat. Protoc. 4, 706–731 (2009).
Caffrey, M. Crystallizing membrane proteins for structure determination: use of lipidic mesophases. Annu. Rev. Biophys. 38, 29–51 (2009).
Kabsch, W. XDS. Acta Crystallogr. D 66, 125–132 (2010).
Winn, M. D. et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. D 67, 235–242 (2011).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D 68, 352–367 (2012).
Böckmann, A. et al. Characterization of different water pools in solid-state NMR protein samples. J. Biomol. NMR 45, 319–327 (2009).
Luo, W. & Hong, M. Conformational changes of an ion channel detected through water–protein interactions using solid-state NMR spectroscopy. J. Am. Chem. Soc. 132, 2378–2384 (2010).
Williams, J. K. & Hong, M. Probing membrane protein structure using water polarization transfer solid-state NMR. J. Magn. Reson. 247, 118–127 (2014).
Mandala, V. S. et al. Structure and drug binding of the SARS-CoV-2 envelope protein transmembrane domain in lipid bilayers. Nat. Struct. Mol. Biol. 27, 1202–1208 (2020).
Gelenter, M. D. et al. Water orientation and dynamics in the closed and open influenza B virus M2 proton channels. Commun. Biol. 4, 338 (2021).
Hong, M. et al. Coupling amplification in 2D MAS NMR and its application to torsion angle determination in peptides. J. Magn. Reson. 129, 85–92 (1997).
Krivov, G. G., Shapovalov, M. V. & Dunbrack, R. L. Jr. Improved prediction of protein side-chain conformations with SCWRL4. Proteins 77, 778–795 (2009).
Lomize, M. A., Pogozheva, I. D., Joo, H., Mosberg, H. I. & Lomize, A. L. OPM database and PPM web server: resources for positioning of proteins in membranes. Nucleic Acids Res. 40, D370–D376 (2012).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Van Der Spoel, D. et al. GROMACS: fast, flexible, and free. J. Comput. Chem. 26, 1701–1718 (2005).
Huang, J. & MacKerell, A. D. Jr. CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J. Comput. Chem. 34, 2135–2145 (2013).
Jo, S., Kim, T., Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008).
Jo, S., Kim, T. & Im, W. Automated builder and database of protein/membrane complexes for molecular dynamics simulations. PLoS ONE 2, e880 (2007).
Wu, E. L. et al. CHARMM-GUI Membrane Builder toward realistic biological membrane simulations. J. Comput. Chem. 35, 1997–2004 (2014).
Lee, J. et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 12, 405–413 (2016).
Best, R. B. et al. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone ϕ, ψ and side-chain χ1 and χ2 dihedral angles. J. Chem. Theory Comput. 8, 3257–3273 (2012).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015).
Nelson, J. G., Peng, Y., Silverstein, D. W. & Swanson, J. M. Multiscale reactive molecular dynamics for absolute pKa predictions and amino acid deprotonation. J. Chem. Theory Comput. 10, 2729–2737 (2014).
Biswas, R., Tse, Y. L., Tokmakoff, A. & Voth, G. A. Role of presolvation and anharmonicity in aqueous phase hydrated proton solvation and transport. J. Phys. Chem. B 120, 1793–1804 (2016).
Day, T. J. F., Soudackov, A. V., Čuma, M., Schmitt, U. W. & Voth, G. A. A second generation multistate empirical valence bond model for proton transport in aqueous systems. J. Chem. Phys. 117, 5839–5849 (2002).
Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 117, 1–19 (1995).
Bonomi, M. B. et al. Promoting transparency and reproducibility in enhanced molecular simulations. Nat. Methods 16, 670–673 (2019).
Tribello, G. A., Bonomi, M., Branduardi, D., Camilloni, C. & Bussi, G. PLUMED 2: new feathers for an old bird. Comput. Phys. Commun. 185, 604–613 (2014).
Grossfield, A. WHAM: the weighted histogram analysis method, v 2.0.9 (University of Rochester, 2002); http://membrane.urmc.rochester.edu/wordpress/?page_id=126
Hunter, J. D. Matplotlib: a 2D graphics environment. Comput. Sci. Eng. 9, 90–95 (2007).
Hodel, A., Kim, S.-H. & Brünger, A. T. Acta Crystallogr. A 48, 851–858 (1992).
Acknowledgements
Diffraction data were collected at the GM/CA@APS and ALS BL 8.3.1. GM/CA@APS is supported by the National Cancer Institute (ACB-12002) and the National Institute of General Medical Sciences (AGM-12006 and P30GM138396), and the Eiger 16M detector by the National Institutes of Health (NIH; S10 OD012289). We also acknowledge the Advanced Photon Source, supported by the US Department of Energy (DE; contract DE-AC02-06CH11357), and beamline 8.3.1 at the Advanced Light Source operated by the University of California at San Francisco with support from the NIH (R01 GM124149 and P30 GM124169), Plexxikon and the Integrated Diffraction Analysis Technologies programme (US Department of Energy Office of Biological and Environmental Research). The Advanced Light Source at Lawrence Berkeley National Laboratory is supported by DE (DE-AC02-05CH11231). H.T.K. was supported by the NIH (K99GM138753). W.F.D. was supported by the NIH (R35 GM122603), NSF (CHE 1709506) and the Air Force Office of Scientific Research (FA9550-19-1-0331). L.C.W. and G.A.V. were supported by the NIH (R01 GM053148). J.M.N. was supported by the NIH (5T32HL007731 and F32GM133085). J.L.T. was supported by the NIH (R35GM122603).
Author information
Authors and Affiliations
Contributions
H.T.K. designed the channels, ran flux measurements, crystallized and collected the X-ray diffraction data, and ran and analysed the classical MD simulations. L.C.W. ran the MS-RMD simulations. L.C.W. and G.A.V. analysed the data. M.M. ran classical MD simulations. J.L.T, J.M.N. and L.L. processed and refined the crystal structures. H.T.K. and W.F.D. analysed the experimental data. All authors contributed to the data analysis and writing of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Mihail Barboiu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Composite omit maps (2mFo-DFc) of designed proton channels.
Composite omit maps of the asymmetric unit for a, LQLL, b, LLQL, and one pentamer from the asymmetric unit for c, QQLL, and d, QLQL (shown only for the waters for clarity). All contours at σ = 1.0. Omit maps with simulated cartesian annealing were generated using Phenix, using methodology described in Hodel, et al.104.
Extended Data Fig. 2 Pulse diagram and water-edited 13 C spectra, water buildup curves of membrane-bound LQLL and LLLL peptides.
a, Pulse diagram of water-edited 13 C CP experiment. b, Representative water-edited 13 C spectra of I13 in LQLL and LLLL, measured with 225 ms and 49 ms 1H mixing. The relative intensities of the 49 ms spectrum to the 225 ms spectrum are higher for LQLL Ile13 than LLLL Ile13, especially for the sidechain Cγ2 and Cδ carbons. c, Site-resolved water buildup curves for Ile13 in LQLL and LLLL. For all 13C sites, LQLL shows a faster water buildup than LLLL, consistent with water molecules in the pore lumen due to the Gln10 PLS.
Extended Data Fig. 3 Overview of proton flux measurements.
a, Full schematic for proton flux measurement including CCCP step, which is included to check vesicle leakiness and confirm proton selectivity. b, Chemical structures of key components of vesicle assay. c, Calibration curves for HPTS at ~5 µM in 12 solutions of 50 mM K2SO4, 30 mM K2HPO4 at different pH values for two plate readers used in data collection process. Unless stated, all data collected with instrument that generated the blue calibration curve. Fits used for downstream data processing shown for each of the two instruments with adjusted R-squared values of 0.9866 and 0.9998 for the left and right curves, respectively. Data for n = 3 independent samples shown as mean values +/− SD.
Extended Data Fig. 4 All proton flux assay data for long kinetics runs.
Long kinetics runs of about 5 hours total for a, empty, b, LLLL, and c, LQLL vesicles. Dotted lines denote times in experiment when valinomycin and CCCP were added to the samples. Three samples of the different conditions were measured in triplicate. These long-time measurements reveal that the vesicles are not leaky to Na+, K+, or H+ and maintain their cargo and assembly over the entire course of the measurement. d, From the linear regression fits of the first 220 s following addition of valinomycin, all slopes (which give the initial rates (in M/s)) were used to calculate the mean and standard error. The one-way ANOVA analysis (with Dunn’s test) reveals that LLLL rates are not significantly different (p > 0.05, adjusted p = 0.4021) when compared to the control empty vesicles. LQLL rates, however, are statistically significant (p < 0.0001, p = 3.23E-5) when compared to the control empty vesicles using one-way ANOVA analysis with Dunn’s test. All data from n = 3 independent samples are shown as mean values +/− SD.
Extended Data Fig. 5 All proton flux assay data for QLLL vesicle samples.
Nine samples (each run in triplicate with shaded error bars shown) containing 1:500 peptide:lipid ratio; samples were run independently in the assay. a, pHin as a function of time throughout the measurement for each independent sample. b, Mean and standard deviation for data collection. c, Data prior to CCCP addition shows little change in pHin after addition of valinomycin. d, Fits for the initial 50 seconds following addition of valinomycin. From the linear regression fits, all slopes (which give the initial rates (in M/s)) were used to calculate mean and standard error presented in Fig. 6g and Supplementary Table 3.
Extended Data Fig. 6 All proton flux assay data for LLQL vesicle samples.
Eight samples (each run in triplicate with shaded error bars shown) containing 1:500 peptide:lipid ratio; samples were run independently in the assay. a, pHin as a function of time throughout the measurement for each independent sample. b, Mean and standard deviation for data collection. c, Data prior to CCCP addition shows notable change in pHin after addition of valinomycin. d, Fits for the initial 50 seconds following addition of valinomycin. From the linear regression fits, all slopes (which give the initial rates (in M/s)) were used to calculate mean and standard error presented in Fig. 6g and Supplementary Table 3.
Extended Data Fig. 7 All proton flux assay data for QLQL vesicle samples.
Seven samples (each run in triplicate with shaded error bars shown) containing 1:500 peptide:lipid ratio; samples were run independently in the assay. a, pHin as a function of time throughout the measurement for each independent sample. b, Mean and standard deviation for data collection. c, Data prior to CCCP addition shows notable change in pHin after addition of valinomycin. d, Fits for the initial 50 seconds following addition of valinomycin. From the linear regression fits, all slopes (which give the initial rates (in M/s)) were used to calculate mean and standard error presented in Fig. 6g and Supplementary Table 3.
Extended Data Fig. 8 Determining orientation of pentamers in vesicles.
a, HPLC trace of unreacted and reacted peptides following reaction with the highly polar, amine-reactive methyltetrazine 3-sulfo-N-hydroxysuccinimide ester (methyltetrazine sulfo-NHS, see Methods). The only amine-reactive groups are the N-terminus or the N-terminal lysine sidechain. Thus, only peptides in which N-terminus is exposed on the outside of the vesicle should react to the dye. b, HPLC traces of mixtures of different ratios of non-reacted and reacted peptides. c, Calibration curves for area under the curve for nonreacted and reacted peaks in the HPLC traces corresponding to the different mixtures in b. Data shown are for n = 2 independent experiments and shown as mean values +/− SD. d, Traces of three independent samples of LQLL pentamers from vesicles after reaction with methyltetrazine sulfo-NHS. e, Using the calibration curves in c, the area under the curve was determined for each sample. The data indicate that half the amines react, as expected from a random orientation of pentamers in the lipid vesicle.
Supplementary information
Supplementary Information
Materials and Methods, Supplementary Figs. 1–25, Tables 1–6, a description of Video 1, and complete references and notes.
Supplementary Video 1
Snapshots from MS-RMD simulations along the MFEP (Fig. 5) depict the proton (represented as a yellow hydronium in the video) inducing the formation of water wires in hydrophobic regions of the channel lumen. The proton moves along these transiently forming water wires to make its way down to a region just below the PLS, formed by the green Gln residues. Once near this site, the presence of the proton enables the formation of a secondary water wire through the longer hydrophobic region. This allows the proton to traverse the rest of the way across the channel.
Source data
Source Data Fig. 2
Unclipped gel.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 6
All data for every panel in Fig. 6, with each panel in unique tabs.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 4
H+ concentration (M) for first 220 s after the addition of valinomycin for long kinetics runs.
Source Data Extended Data Fig. 5
All data for QLLL.
Source Data Extended Data Fig. 6
All data for LLQL.
Source Data Extended Data Fig. 7
All data for QLQL.
Source Data Extended Data Fig. 8
Data for calibration curves.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kratochvil, H.T., Watkins, L.C., Mravic, M. et al. Transient water wires mediate selective proton transport in designed channel proteins. Nat. Chem. 15, 1012–1021 (2023). https://doi.org/10.1038/s41557-023-01210-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-023-01210-4