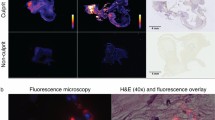
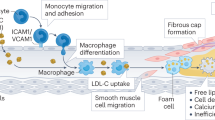
Abstract
Angiogenesis within human atherosclerotic plaques has an important role in plaque progression as immature blood vessels leak red blood cells and inflammatory mediators into the plaque center. Accumulation of free cholesterol from red blood cell membranes potentially increases the size of the necrotic core and triggers a chain of events that promote plaque destabilization. Antiangiogenic agents have been shown to prune some tumor vessels and 'normalize' the structure and function of the remaining vasculature, thereby improving the access of chemotherapeutic agents to tumors. We propose that antiangiogenic therapy can similarly stabilize vulnerable 'rupture-prone' plaques by pruning and normalizing immature intraplaque vessels, preventing further intraplaque hemorrhage. This normalization would limit necrotic core enlargement, further luminal narrowing and the degree of inflammation. Such normalization has been realized using vascular endothelial growth factor antagonists for the treatment of cancer and age-related macular degeneration. The development of this novel approach to prevent plaque progression might add to the armamentarium of preventive measures for acute myocardial infarction, stroke and sudden cardiac death.
Key Points
-
Although the causes of atherosclerotic lesion progression from an asymptomatic fibroatheromatous plaque to a lesion at high risk of rupture (thin-cap fibroatheroma or 'vulnerable plaque') are not fully understood, data support the concept that intraplaque hemorrhage is critical for the progression of plaques into high-risk unstable lesions
-
On the basis of similarities with the structure of tumor vessels, we propose that judicious application of antiangiogenic agents could prune and normalize immature intraplaque blood vessels, thereby preventing intraplaque hemorrhage
-
A similar paradigm has been described in the wet form of age-related macular degeneration; pegaptanib, a aptamer that inhibits vascular endothelial growth factor, and ranibizumab, an antibody fragment that inhibits vascular endothelial growth factor, maintain or improve vision in these patients, suggesting that these agents normalize the vasculature in the eye
-
At present, there are serious obstacles to the testing of this concept, such as the lack of an appropriate animal model, uncertainty regarding the optimum agents and targets and appropriate dosing and delivery method, and difficulties in selecting patients suitable for this therapy
-
Further research in this nascent field of vascular normalization could result in the prevention of plaque progression and its clinical consequences
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Burke AP et al. (1997) Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med 336: 1276–1282
Milei J et al. (1998) Carotid rupture and intraplaque hemorrhage: immunophenotype and role of cells involved. Am Heart J 136: 1096–1105
Kolodgie FD et al. (2003) Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 349: 2316–2325
Takaya N et al. (2005) Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: a high-resolution magnetic resonance imaging study. Circulation 111: 2768–2775
Virmani R et al. (2005) Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol 25: 2054–2061
Jeziorska M and Woolley DE (1999) Local neovascularization and cellular composition within vulnerable regions of atherosclerotic plaques of human carotid arteries. J Pathol 188: 189–196
Kockx MM et al. (2003) Phagocytosis and macrophage activation associated with hemorrhagic microvessels in human atherosclerosis. Arterioscler Thromb Vasc Biol 23: 440–446
Virmani R et al. (1998) When neoangiogenesis ricochets. Am Heart J 136: 937–939
Jain RK (2005) Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307: 58–62
Jain RK et al. (2006) Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol 3: 24–40
Batchelor TT et al. (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11: 83–95
Jain RK (2001) Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7: 987–989
Virmani R et al. (2000) Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 20: 1262–1275
Gerrity RG (1981) The role of the monocyte in atherogenesis: II. Migration of foam cells from atherosclerotic lesions. Am J Pathol 103: 191–200
Tabas I et al. (1996) Evidence that the initial up-regulation of phosphatidylcholine biosynthesis in free cholesterol-loaded macrophages is an adaptive response that prevents cholesterol-induced cellular necrosis. Proposed role of an eventual failure of this response in foam cell necrosis in advanced atherosclerosis. J Biol Chem 271: 22773–22781
Tabas I (2000) Cholesterol and phospholipid metabolism in macrophages. Biochim Biophys Acta 1529: 164–174
Davis GE (1992) The Mac-1 and p150,95 beta 2 integrins bind denatured proteins to mediate leukocyte cell-substrate adhesion. Exp Cell Res 200: 242–252
Hynes RO (1992) Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69: 11–25
Darbonne WC et al. (1991) Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J Clin Invest 88: 1362–1369
Vlodavsky I and Friedmann Y (2001) Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. J Clin Invest 108: 341–347
Kim-Shapiro DB et al. (2006) Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arterioscler Thromb Vasc Biol 26: 697–705
Graversen JH et al. (2002) CD163: a signal receptor scavenging haptoglobin-hemoglobin complexes from plasma. Int J Biochem Cell Biol 34: 309–314
Moreno PR et al. (2006) Neovascularization in human atherosclerosis. Circulation 113: 2245–2252
Van den Heuvel MM et al. (1999) Regulation of CD 163 on human macrophages: cross-linking of CD163 induces signaling and activation. J Leukoc Biol 66: 858–866
Philippidis P et al. (2004) Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ Res 94: 119–126
Levy AP et al. (2007) Haptoglobin genotype is a determinant of iron, lipid peroxidation, and macrophage accumulation in the atherosclerotic plaque. Arterioscler Thromb Vasc Biol 27: 134–140
O'Brien KD et al. (1996) Neovascular expression of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in human atherosclerosis and their relation to intimal leukocyte content. Circulation 93: 672–682
Melder RJ et al. (1996) During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat Med 2: 992–997
Burke AP et al. (1999) Plaque rupture and sudden death related to exertion in men with coronary artery disease. JAMA 281: 921–926
Fleiner M et al. (2004) Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation 110: 2843–2850
Moreno PR et al. (2004) Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation 110: 2032–2038
Folkman J (1971) Tumor angiogenesis: therapeutic implications. N Engl J Med 285: 1182–1186
Carmeliet P and Jain RK (2000) Angiogenesis in cancer and other diseases. Nature 407: 249–257
Zhang Y et al. (1993) Immunohistochemical study of intimal microvessels in coronary atherosclerosis. Am J Pathol 143: 164–172
Dunmore BJ et al. (2007) Carotid plaque instability and ischemic symptoms are linked to immaturity of microvessels within plaques. J Vasc Surg 45: 155–159
Jain RK (2003) Molecular regulation of vessel maturation. Nat Med 9: 685–693
Van den Brenk HA et al. (1977) The significance of free blood in liquid and solid tumours. Br J Exp Pathol 58: 147–159
Kohn S et al. (1992) Pathways of macromolecular tracer transport across venules and small veins. Structural basis for the hyperpermeability of tumor blood vessels. Lab Invest 67: 596–607
Heistad DD and Armstrong ML (1986) Blood flow through vasa vasorum of coronary arteries in atherosclerotic monkeys. Arteriosclerosis 6: 326–331
Moulton KS et al. (1999) Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolipoprotein E-deficient mice. Circulation 99: 1726–1732
Chen YX et al. (1999) Immunohistochemical expression of vascular endothelial growth factor/vascular permeability factor in atherosclerotic intimas of human coronary arteries. Arterioscler Thromb Vasc Biol 19: 131–139
Hansson GK (2001) Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol 21: 1876–1890
Laine P et al. (2000) Adventitial mast cells connect with sensory nerve fibers in atherosclerotic coronary arteries. Circulation 101: 1665–1669
Shah PK et al. (1995) Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation 92: 1565–1569
Lappalainen H et al. (2004) Mast cells in neovascularized human coronary plaques store and secrete basic fibroblast growth factor, a potent angiogenic mediator. Arterioscler Thromb Vasc Biol 24: 1880–1885
Toth-Jakatics R et al. (2000) Cutaneous malignant melanoma: correlation between neovascularization and peritumor accumulation of mast cells overexpressing vascular endothelial growth factor. Hum Pathol 31: 955–960
Des Guetz G et al. (2006) Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer 94: 1823–1832
Winkler F et al. (2004) Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell 6: 553–563
Manegold C et al.; the BO17704 study group (2007) Randomised, double-blind multicentre phase III study of bevacizumab in combination with cisplatin and gemcitabine in chemotherapy-naïve patients with advanced or recurrent non-squamous non-small cell lung cancer (NSCLC): BO17704. J Clin Oncol 25 (Suppl): 18S
Willett CG et al. (2004) Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med 10: 145–147
Willett CG et al. (2005) Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J Clin Oncol 23: 8136–8139
Aiello LP et al. (1995) Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc Natl Acad Sci USA 92: 10457–10461
Gragoudas ES et al. (2004) Pegaptanib for neovascular age-related macular degeneration. N Engl J Med 351: 2805–2816
Ferrara N et al. (2006) Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina 26: 859–870
Fung AE et al. (2006) The International Intravitreal Bevacizumab Safety Survey: using the internet to assess drug safety worldwide. Br J Ophthalmol 90: 1344–1349
Weiss MRD and Prenner JL (2007) The incidence of systemic adverse events in patients treated with intravitreal bevacizumab. Presented at the Association for Research in Vision and Ophthalmology, May 10 2007, Fort Lauderdale, FL
Skillings JR et al. (2005) Arterial thromboembolic events (ATEs) in a pooled analysis of 5 randomized, controlled trials (RCTs) of bevacizumab (BV) with chemotherapy [abstract]. J Clin Oncol 23 (Suppl): S3019
LiewM G and Mitchell P (2007) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 356: 747–748
Yang JC et al. (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349: 427–434
Ratner M (2004) Genentech discloses safety concerns over Avastin. Nat Biotechnol 22: 1198
Drimal J et al. (2006) Cardiovascular toxicity of the first line cancer chemotherapeutic agents: doxorubicin, cyclophosphamide, streptozotocin and bevacizumab. Neuroendocrinol Lett 27 (Suppl 2): S176–S179
Inai T et al. (2004) Inhibition of vascular endothelial growth factor (VEGF) signaling in cancer causes loss of endothelial fenestrations, regression of tumor vessels, and appearance of basement membrane ghosts. Am J Pathol 165: 35–52
Munoz-Najar UM et al. (2006) Hypoxia stimulates breast carcinoma cell invasion through MT1-MMP and MMP-2 activation. Oncogene 25: 2379–2392
Kamba T et al. (2006) VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am J Physiol Heart Circ Physiol 290: H560–H576
Autiero M et al. (2003) Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost 1: 1356–1370
Khurana R et al. (2005) Role of angiogenesis in cardiovascular disease: a critical appraisal. Circulation 112: 1813–1824
Johnson J et al. (2005) Plaque rupture after short periods of fat feeding in the apolipoprotein E-knockout mouse: model characterization and effects of pravastatin treatment. Circulation 111: 1422–1430
Schwartz SM et al. (2007) Plaque rupture in humans and mice. Arterioscler Thromb Vasc Biol 27: 705–713
Rosenfeld ME et al. (2000) Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler Thromb Vasc Biol 20: 2587–2592
Robertson AK (2003) Disruption of TGF-beta signaling in T cells accelerates atherosclerosis. J Clin Invest 112: 1342–1350
Sasaki T et al. (2006) A simple method of plaque rupture induction in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol 26: 1304–1309
Gordon MS and Cunningham D (2005) Managing patients treated with bevacizumab combination therapy. Oncology 69 (Suppl 3): S25–S33
Burke AP et al. (2001) Healed plaque ruptures and sudden coronary death: evidence that subclinical rupture has a role in plaque progression. Circulation 103: 934–940
Luttun A et al. (2002) Revascularization of ischemic tissues by PlGF treatment, and inhibition of tumor angiogenesis, arthritis and atherosclerosis by anti-Flt1. Nat Med 8: 831–840
Verheul HM and Pinedo HM (2007) Possible molecular mechanisms involved in the toxicity of angiogenesis inhibition. Nat Rev Cancer 7: 475–485
Weis M et al. (2002) Statins have biphasic effects on angiogenesis. Circulation 105: 739–745
Tong RT et al. (2004) Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res 64: 3731–3736
Acknowledgements
The authors thank Y Boucher, P Carmeliet, D Duda, D Fukumura, K Kozak, L Munn and T Padera for their input. RK Jain is grateful to the National Cancer Institute of USA for continuous support of his research on vascular biology since 1980.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
RK Jain is a consultant for AstraZeneca, Novartis, Pfizer and Thrombogenics. He has received honoraria from Pfizer and Roche, and a grant from AstraZeneca. The other authors declared no competing interests.
Rights and permissions
About this article
Cite this article
Jain, R., Finn, A., Kolodgie, F. et al. Antiangiogenic therapy for normalization of atherosclerotic plaque vasculature: a potential strategy for plaque stabilization. Nat Rev Cardiol 4, 491–502 (2007). https://doi.org/10.1038/ncpcardio0979
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio0979
This article is cited by
-
The potential role of nicotine in breast cancer initiation, development, angiogenesis, invasion, metastasis, and resistance to therapy
Breast Cancer (2022)
-
Diversity of macrophage phenotypes and responses in atherosclerosis
Cellular and Molecular Life Sciences (2020)
-
Coupled Modeling of Lipid Deposition, Inflammatory Response and Intraplaque Angiogenesis in Atherosclerotic Plaque
Annals of Biomedical Engineering (2019)
-
Biology and Novel Targets in Metaplastic Breast Cancer
Current Breast Cancer Reports (2012)
-
RANTES/CCL5-induced pro-angiogenic effects depend on CCR1, CCR5 and glycosaminoglycans
Angiogenesis (2012)