Compared to singleton pregnancies, twin pregnancies are at increased risk for adverse perinatal outcomes, including preterm delivery, fetal growth restriction, congenital anomalies, neonatal intensive care unit admission and fetal loss (Chauhan et al., Reference Chauhan, Scardo, Hayes, Abuhamad and Berghella2010) It has also long been recognized that compared to first-born twins, second-born twins are at increased risk for morbidity and mortality independent of presentation, chorionicity or infant sex (Armson et al., Reference Armson, O’Connell, Persad, Joseph, Young and Baskett2006; Hartley & Hitti, Reference Hartley and Hitti2005). Such outcomes may be attributed to both antepartum and intrapartum events. As a result of a shared placental circulation, monochorionic (MC) gestations are at risk for additional complications compared to dichorionic (DC) gestations, including twin-to-twin transfusion syndrome (TTTS) and twin anemia polycythemia sequence (Hack et al., Reference Hack, Derks, Elias, Franx, Roos, Voerman and Visser2008). There is conflicting literature about whether chorionicity has intrapartum effects on acid-base physiology or neonatal outcomes of first- and second-born twins (Axelsdottir & Ajne, Reference Axelsdottir and Ajne2018; Ehrenberg et al., Reference Ehrenberg, Fischer, Westover and Mercer2004; Hjorto et al., Reference Hjorto, Nickelsen, Petersen and Secher2014).
Umbilical cord blood that obtained promptly after delivery allows for determination of newborn acid-base status at birth. Arterial pH and base excess, in particular, are important measures of the condition of the neonate; abnormal values may indicate the occurrence of an acute intrapartum hypoxic event. The risk of neonatal morbidity is inversely related to arterial pH (i.e., highest risk at lowest pH). In twin pregnancies, a prolonged twin-to-twin delivery interval (TTDI) is associated with pathologic fetal acidemia and adverse neonatal outcomes in the second twin (Axelsdottir & Ajne, Reference Axelsdottir and Ajne2018; Leung et al., Reference Leung, Lok, Tam, Leung and Lau2004; Stein et al., Reference Stein, Misselwitz and Schmidt2008) The effect of other intrapartum and clinical factors, such as chorionicity, on the acid-base physiology of second-born twins is less certain; a review of the literature reveals conflicting results. Recently, one large study by Axelsdottir and Ajne (Reference Axelsdottir and Ajne2018) concluded that chorionicity does not affect twin cord gas results. Others have speculated that the presence of placental vascular anastomoses in MC twin pregnancies would produce more concordant acid-base parameters than in DC twin pregnancies (Ehrenberg et al., Reference Ehrenberg, Fischer, Westover and Mercer2004). Leung et al. (Reference Leung, Lok, Tam, Leung and Lau2004) concluded that there may be impairment of uteroplacental exchange after delivery of the first twin. If true, this finding may be more pronounced in MC compared to DC pregnancies due to the shared placental circulation. The impact of this, if any, may only be relevant when vaginal delivery is attempted, given the risk of additional exposure to intrauterine hypoxic events in the second twin.
Prior studies investigating acid-base physiology in twin pregnancies have often been limited by small sample sizes (Ehrenberg et al., Reference Ehrenberg, Fischer, Westover and Mercer2004; Leung et al., Reference Leung, Lok, Tam, Leung and Lau2004) with comparatively few MC compared to DC gestations (Hjorto et al., Reference Hjorto, Nickelsen, Petersen and Secher2014), racially homogenous populations that may hinder generalizability (Axelsdottir & Ajne, Reference Axelsdottir and Ajne2018), and scant neonatal outcome data. The aim of our study was to ascertain whether chorionicity affects the umbilical cord blood acid-base parameters of the second twin in a large, diverse population at a single tertiary referral hospital with detailed neonatal records. Our primary objective was to determine whether chorionicity is associated with acidosis of the second twin. Secondary objectives were to determine whether chorionicity affects arterial pH discordance or neonatal outcomes among twin-pairs.
Materials and Methods
This retrospective cohort study evaluated all women with twin gestations who delivered at greater than 23 weeks of gestational age at a single, tertiary, university medical center from January 1, 2010 to December 31, 2016. Patients were included if umbilical cord gas results (both arterial and venous) were available for both newborns, and histological evaluation of the placenta was performed after delivery to determine chorionicity. Exclusion criteria included intrauterine fetal demise of either twin prior to labor, major fetal anomalies, monoamniotic twins, uncertain chronicity and TTTS. The Northwell Health Human Research Protection Program and Institutional Review Board approved the study protocol. This research adhered to the STROBE checklist for observational studies (https://www.strobe-statement.org).
Medical records were reviewed to obtain baseline characteristics of the study population, including maternal age, gravidity, parity, race, body mass index, medical and surgical history, and obstetrical and gynecological history. For each patient, the antepartum course and obstetrical outcomes were reviewed, and the following data were collected: chorionicity, gestational age at delivery, mode of delivery, indication for primary cesarean, newborn birth weights, Apgar scores, TTDI and pregnancy complications. Neonatal complications were identified, including neonatal intensive care unit (NICU) admission, neonatal death, respiratory distress syndrome, sepsis, intraventricular hemorrhage, blood transfusion, hyperbilirubinemia and others. Chorionicity was confirmed by placental histology evaluation after delivery. All data were stored in a secure REDCap database.
At our institution, umbilical cord blood (both arterial and venous) is routinely collected for the assessment of fetal oxygenation after all live births greater than 23 weeks of gestational age unless an insufficient quantity (<0.5 ml) is obtained. Cord clamping is typically performed within 90 s of birth, and sampling and laboratory analysis is performed within 60 min of birth. Arterial and venous pH, pCO2, PO2, HCO3 and base excess are reported.
The primary outcome was the umbilical artery (UA) pH of the second twin (evaluated as a continuous variable). Threshold values of arterial pH <7.00 and <7.20 were evaluated (as binary variables). Secondary outcomes included NICU admissions and neonatal outcomes and complications.
For patient demographics, mean and standard deviations were reported for continuous clinical variables and frequency for categorical variables. When clinical variables were compared between groups of patients (e.g., between DC and MC), t test or the Wilcoxon rank-sum test were used to compare continuous variables contingent on the normality of the variable distribution. Chi-square and Fisherʼs exact test were used to compare the distribution of categorical variables between groups. Statistical significance was defined as p < .05. A multivariable logistic regression was carried out to examine the association between UA pH < 7.20 in the second-born twin and the following potential explanatory variables: chorionicity, maternal age, multiparity, chronic hypertension, diabetes, hypertensive disorders or pregnancy, assisted reproductive technology (ART), gestational age at delivery, TTDI, cesarean delivery for NRFHT, cesarean delivery for baby B only, 1- or 5-min Apgar score < 7 for baby A and UA pH < 7.2 for baby A. Significant predictors were selected using the stepwise Akaike information criterion (AIC) method. Odds ratios and corresponding 95% confidence intervals are presented. Analyses were generated with RStudio Desktop 1.1.463 (RStudio Inc., Boston, MA, USA) using R 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) (R Core Team, 2013) and/or SAS Studio 3.8 Enterprise Edition build on SAS 9.04 (SAS Institute Inc, Cary, NC, USA).
Results
During the study period, a total of 1277 twin pregnancies delivered at ≥23 weeks of gestational age and were evaluated for inclusion. After applying exclusion criteria, 679 twin pregnancies (593 DC; 86 MC) remained for analysis. The study population had a mean maternal age of 33.8 ± 5.3 years and was approximately 70% Caucasian (Table 1). Patients in the DC group were older and more likely to have had a pregnancy resulting from ART compared to the MC group. There was no difference in race, prepregnancy BMI, gestational or pregestational diabetes, or hypertensive disorders of pregnancy between groups. As would be expected based on current recommendations from the American College of Obstetrics and Gynecology (ACOG), MC pregnancies delivered at earlier gestational ages than DC pregnancies (35.6 vs. 36.1 weeks) and were more likely to result in preterm birth (70.9% vs. 49.6%; ACOG, 2019). The rate of cesarean delivery was high in both groups, but even more so in the DC group.
Table 1. Maternal demographics and obstetrical outcomes of monochorionic and dichorionic twin pregnancies included in study population
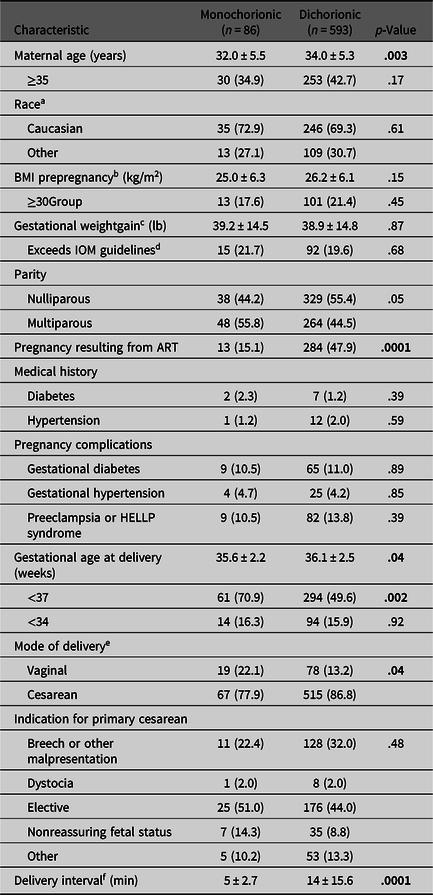
Note: BMI, body mass index; HELLP, hemolysis, elevated liver enzymes, low platelet count; IOM, Institute of Medicine; ART, assisted reproductive technology. Data are n (%) and mean ± standard deviation unless otherwise specified. Bold values denote statistical significance at p < .05.
a Race data available for 59.4% of population (n = 403).
b Prepregnancy BMI data available for 80.5% of population (n = 546).
c Gestational weight gain data available for 79.2% of population (n = 538).
d For twin pregnancy, the IOM recommends a gestational weight gain of 37–54 lb for women of normal weight, 31–50 lb for overweight women and 25–42 lb for obese women.
e Combined vaginal cesarean performed for four dichorionic and zero monochorionic pregnancies.
f After vaginal delivery of twin A (n = 19 monochorionic, n = 82 dichorionic).
No differences in umbilical cord blood gas parameters were observed between MC and DC twins for either the first or second twin (Table 2). UA pH < 7.20 was seen in 13% (n = 88) of second twins compared to 11% (n = 76) of first twins (p = .32). UA pH < 7.00 was seen in only four first twins and four second twins (0.6%). Among vaginally delivered twins (n = 97), the UA pH of the first twin was higher than the second twin (7.26 vs. 7.24; p = .01). In addition, among deliveries in which the first twin delivered vaginally and the TTDI was ≥20 min, the first twin had a significantly higher UA pH than the second twin (7.25 vs. 7.16 respectively; p = .006). MC first twins were more likely than DC first twins to be admitted to the NICU (55.8% vs. 43.8%, p = .03); no difference was found between MC and DC second twins.
Table 2. Neonatal outcomes and umbilical cord blood gas results of monochorionic and dichorionic twin pregnancies
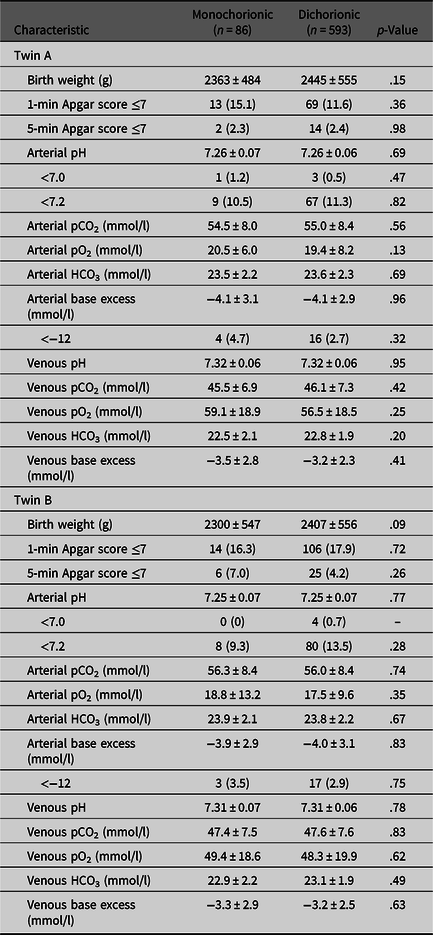
Note: Data are n (%) and mean ± standard deviation unless otherwise specified.
Overall, MC twins were more likely to be admitted to the NICU than DC twins (55.8% vs. 44.5% respectively; p = .005), a finding likely attributable to the increased incidence of preterm delivery in MC compared to DC twins. However, regardless of chorionicity, second twins did not have an increased rate of neonatal complications or NICU admissions compared to first twins (Table 3).
Table 3. Neonatal complications of newborn twins admitted to the neonatal intensive care unit (NICU) by chorionicity
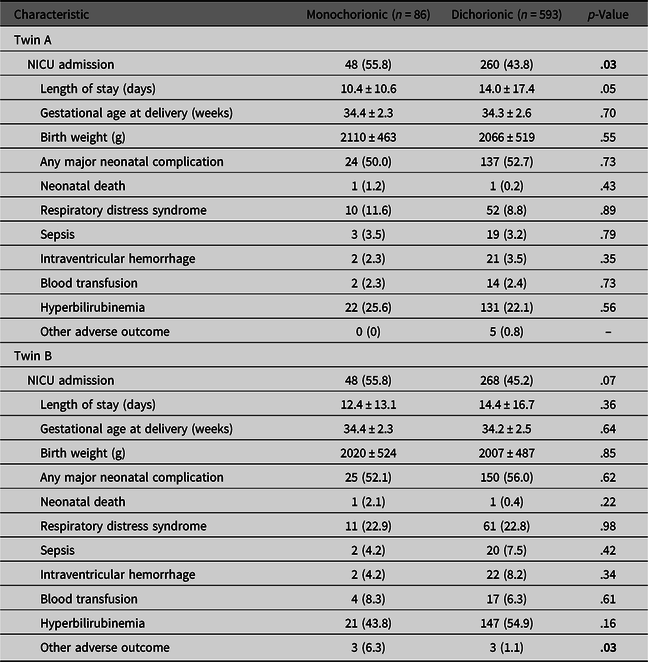
Note: Data are n (%) and mean ± standard deviation unless otherwise specified. Bold values denote statistical significance at p < .05.
In the final multivariable logistic regression model to predict arterial pH < 7.20 for the second twin, multiple statistically significant factors were identified (Table 4). However, the low odds ratios (i.e., <2) for most variables indicate that their effects are likely small. The stepwise AIC method was used to select significant predictors from the full model with all potential explanatory variables (described above); the selected parameters in the final model included chronic hypertension, ART, TTDI, first twin birth weight, second twin birth weight and first twin arterial pH < 7.20. Chorionicity was not selected by the algorithm but it was added to the model because of its clinical importance rather than its statistical significance. In cases where arterial pH was less than 7.20 for the first twin, the second twin was 14 times more likely to have an arterial pH less than 7.20, provided that all other variables in the model remain constant. In addition, when the mother had a diagnosis of chronic hypertension, the second twin was nearly six times more likely to have an arterial pH < 7.20, again, when all other predictors are held constant. The effect of TTDI was muted in this model by the high prevalence of cesarean section. Although the birth weight of each twin was statistically significant, this effect may be statistical artifact given the odds ratio of approximately 1 and extremely narrow 95% confidence intervals for both.
Table 4. Results of multivariable logistic regression model to predict umbilical artery pH < 7.20 in the second-born twin
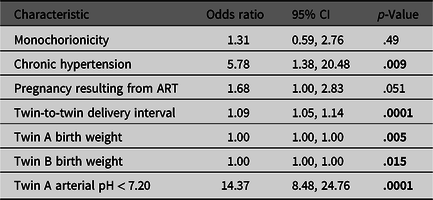
Note: ART, assisted reproductive technology. Bold values denote statistical significance at p < .05.
Discussion
In our study population, chorionicity was not associated with any acid-base parameter of umbilical cord blood in either the first or second twin. Furthermore, no differences in neonatal outcomes were observed based on chorionicity or birth order. Neither ACOG nor the Society for Maternal–Fetal Medicine currently recommends any altered intrapartum management based on chorionicity. Our findings are reassuring and provide more support for a single intrapartum management protocol for twin pregnancies, despite different recommendations regarding delivery timing based on chorionicity.
Previous studies have not adequately evaluated the contribution of maternal comorbidities to the arterial pH of the second twin. In singleton pregnancies, it is well established that maternal perfusion of the placenta is reduced with hypertensive disorders (Kovo et al., Reference Kovo, Bar, Schreiber and Shargorodsky2017). We observed that chronic hypertension was strongly associated with twin B arterial pH less than 7.20. In addition, when twin A arterial pH was less than 7.20, twin B was much more likely to have an arterial pH of less than 7.20 as well. Thus, even in DC twin pregnancies, in which there are two separate placentas, fetal hypoxia is not independent from one twin to the other. We also confirmed previous reports that among vaginally delivered twins, UA pH is on average lower in second twins compared to first-born twins (McGrail & Bryant, Reference McGrail and Bryant2005; Suh et al., Reference Suh, Park, Hong, Yoon, Shim, Park and Syn2007). Prolonged TTDI, herein defined as greater than 20 min, was associated with a significantly decreased UA pH in the second twin, consistent with prior studies (Hartley & Hitti, Reference Hartley and Hitti2005; Stein et al., Reference Stein, Misselwitz and Schmidt2008). Of note, most of our study population was delivered by cesarean section (>80%), which functionally eliminates prolonged TTDI. Other studies of umbilical cord pH in twin gestations have typically focused exclusively on vaginal deliveries (Axelsdottir & Ajne, Reference Axelsdottir and Ajne2018; Hjorto et al., Reference Hjorto, Nickelsen, Petersen and Secher2014; Leung et al., Reference Leung, Lok, Tam, Leung and Lau2004) or populations in which vaginal deliveries comprised approximately half of cases (Ehrenberg et al., Reference Ehrenberg, Fischer, Westover and Mercer2004; Schneuber et al., Reference Schneuber, Magnet, Haas, Giuliani, Freidl, Lang and Bjelic-Radisic2011; Usta et al., Reference Usta, Nassar, Awwad, Nakad, Khalil and Karam2002; Young et al., Reference Young, Suidan, Antoine, Silverman, Lustig and Wasserman1985). MC pregnancies had a significantly shorter TTDI than DC pregnancies (after vaginal delivery of twin A); this finding has been demonstrated in other studies but not adequately explained (Axelsdottir & Ajne, Reference Axelsdottir and Ajne2018; Hoffmann et al., Reference Hoffmann, Oldenburg, Rode, Tabor, Rasmussen and Skibsted2012).
It must be acknowledged that fetal blood gas analysis has inherent limitations. A UA pH less than 7.00 is often used as a threshold to define pathologic fetal acidemia but this value alone does not indicate whether the primary cause is a fetal, placental or maternal disorder. Moreover, in a normal fetus with normal placental function, acute acidosis in labor is only rarely associated with adverse outcomes. Thus, the consequences of acidosis depend on the condition of the fetus before the insult and the severity and duration of acidosis (acute vs. chronic). In twin gestations, as noted above, the most consistently identified factor contributing to acidosis of the second twin is the TTDI.
Our study has several strengths. We evaluated a large sample of twin pregnancies at a single institution with detailed documentation of maternal medical history, peripartum management and neonatal outcomes. Although the population was approximately two-thirds of Caucasian, this represents considerably greater racial diversity than similar previous studies (Axelsdottir & Ajne, Reference Axelsdottir and Ajne2018; Hjorto et al., Reference Hjorto, Nickelsen, Petersen and Secher2014). In order to eliminate the possibility of arterial–venous mismatch that may erroneously suggest a higher pH, deliveries were only included if paired arterial and venous cord gases were obtained for both twins (Westgate et al., Reference Westgate, Garibaldi and Greene1994); previous large studies did not employ such rigorous inclusion criteria (Axelsdottir & Ajne, Reference Axelsdottir and Ajne2018; Hjorto et al., Reference Hjorto, Nickelsen, Petersen and Secher2014). There are also several limitations associated with our study. The design is retrospective. A relatively high cesarean delivery rate hinders generalizability of our findings to other clinical settings in which twin vaginal delivery is more common. We were unable to sufficiently evaluate predictive factors associated with UA pH < 7.00 in the second twin due to the small number of cases (n = 4). Finally, this study only evaluated short-term clinical outcomes from the neonatal hospitalization and did not evaluate long-term neurodevelopment.
In summary, no significant differences in umbilical cord blood gas analysis and neonatal outcomes were observed in this large contemporary cohort of MC and DC diamniotic twin pregnancies. Therefore, intrapartum clinical management should not be affected by chorionicity. It is possible that populations with a lower cesarean delivery rate and concomitant prolongation of the TTDI may yield different findings. It should be emphasized that reduction in TTDI is not of sufficient benefit to justify planned cesarean section. ACOG recommends that women with diamniotic twin gestations whose presenting fetus is in a vertex position should be considered as candidates for a vaginal birth because no reduction in fetal or neonatal morbidity or mortality has been demonstrated with planned cesarean delivery (Barrett et al., Reference Barrett, Hannah, Hutton, Willan, Allen and Armson2013; Committee on Practice Bulletins — Obstetrics, Society for Maternal–Fetal Medicine, 2016). Further investigation is necessary to determine whether chorionicity independently affects UA pH in certain subgroups of twin pregnancies, including those affected by fetal growth restriction, abnormal UA Doppler studies and maternal comorbidities.
Acknowledgments
Results of this study were presented at the Society for Reproductive Investigation 65th Annual Scientific Meeting in San Diego, California in March 2018. We thank Michael Qiu and Shreya Sanghani of the Business Intelligence Competency Center of Northwell Health and Shonah Sandel of the Division of Neonatal-Perinatal Medicine at Cohen Childrenʼs Medical Center of New York for their assistance with data retrieval. We also thank Stephanie Augustine, RNC for administrative support.
Financial Support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of Interest
None.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.








