Introduction
Mild cognitive impairment (MCI) is a term that refers to a condition in which an essentially spared global cognition and normal or slightly impaired activities of daily living (ADL) coexist with a mild decline of cognitive functions greater than that expected for age and education.Reference Petersen, Smith, Waring, Ivnik, Tangalos and Kokmen1,Reference Petersen2 Four MCI phenotypes have been recognizedReference Petersen2 as follows: amnestic MCI single domain (a-MCI), amnestic MCI multiple domain (a-MCImd), nonamnestic MCI single domain (na-MCI), and nonamnestic MCI multiple domain (na-MCImd). Extracellular deposition of β-amyloid (Aβ) peptide, intracellular deposition of hyper-phosphorylated tau protein, and atrophy of frontal, parietal, and medial temporal cortices, i.e., neurodegeneration of Alzheimer disease (AD) signature cortical regions,Reference Jack3 are the key elements of the pathophysiology of ADReference McKhann, Knopman and Chertkow4 and MCI due to AD.Reference Albert, Dekosky and Dickson5 The prevalence of Aβ positivity among subjects with MCI increases from age 50 to 90 years from 27% to 71%; nonamnestic MCI types have lower prevalence estimates of Aβ positivity than amnestic MCI types, but higher than subjects cognitively normal (CN), and both amnestic and nonamnestic MCI are at increased risk for AD.Reference Jansen, Ossenkoppele and Knol6 Concerning tau, MCI and AD individuals have tau accumulation in the basal and mild-temporal, retrosplenial, posterior cingulate, and entorhinal regions greater than CN individuals Aβ positive.Reference Jack, Wiste and Schwarz7
The observation that 21.5% and 35% of individuals with amnestic and nonamnestic MCI types, respectively, are Aβ negative,Reference Petersen, Aisen and Boeve8 has suggested that these individuals are not on the AD pathway and that vascular pathology may be one of the possible non-AD causes of MCI. Among vascular pathology, cerebral small vessel disease (SVD) plays a pivotal role. SVD affects the smallest cerebral small vessels, increases throughout the lifespan,Reference Pantoni9 and contributes to the risk of MCIReference Luchsinger, Brickman and Reitz10 and dementia.Reference Gorelick, Scuteri and Black11 White matter hyperintensities (WMH), lacunes, small subcortical infarcts, microbleeds, enlarged perivascular spaces, and central atrophy are the imaging markers of SVD.Reference Wardlaw, Smith and Biessels12 LacunesReference Reijner, Freeze, Leemans and Biessels13 and WMHReference Lambert, Narean, Benjamin, Zeestraten, Barrick and Markus14 disrupting locally the structural integrity of white matter induce thinning of the connected cortical regions through Wallerian degeneration. Lacunes are associated with widespread cortical thinning, atrophy in multiple subcortical structures, and ventricular enlargement.Reference Thong, Hilal and Wang15 In subjects with MCI, expansion of the lateral ventricles is associated with atrophy of frontal, parietal, and temporal regions affected by AD.Reference Madsen, Gutman and Joshi16 Furthermore, age per se is associated with atrophy of the cerebellum, striatum, and prefrontal, parietal, and temporal association cortices.Reference Raz, Lindenberger and Rodrigue17 The pattern of cortical atrophy induced by severe WMH overlaps substantially with the patterns of age-related cortical atrophy and of AD-related cortical atrophy.Reference Habes, Erus and Toledo18 Since cortical atrophy induced by WMH drives cognitive decline,Reference Rizvi, Narkhede and Last19 age and SVD may contribute to the onset of cognitive decline through the overlapping atrophy of cortical regions vulnerable to AD pathology.
Cerebral SVD, Aβ, tau, and atrophic changes fragmenting over time brain networks into disconnected parts not only contribute to cognitive decline but also contribute to the presentation of a wide range of neurological signs. We have shown that neurologically and cognitively healthy (NCH) aging subjects frequently present at the routine neurological examination isolated, subtle neurological abnormalities (ISNAs) which do not have any immediate diagnostic relevance, cannot be attributed to any definite, overt neurological disease, are associated with atrophy of the caudate nuclei, and with parietal WMH and lacunes, and probably constitute a red flag for future cognitive decline given that they show poor performance in test evaluating global cognition, executive function, and language.Reference Camarda, Torelli, Camarda, Battaglini, Gagliardo and Monastero20 Past reports on neurological signs in MCI have primarily focused on extrapyramidal features,Reference Louis, Schupf, Manly, Marder, Tang and Mayeux21,Reference Boyle, Wilson and Aggarwal22 while reports on signs other than extrapyramidal are sparse. Therefore, the aims of our study are: (1) to investigate the prevalence of ISNAs in the four MCI types and (2) to verify whether in the individual MCI types the probability of having ISNAs is differently associated with the topographical location of WMH and lacunes, periventricular WMH (WMH-PV), apolipoprotein E (APOE) ϵ4 allele, and with two linear measures of central atrophy,Reference Wardlaw, Smith and Biessels12 i.e., the bicaudate ratio (BCr) as proxy of subcortical atrophy and the lateral ventricles to brain ratio (LVBr) as proxy of global brain atrophy. In the present paper, the terms “adult”, “elderly”, “old”, and “oldest-old” will be used to indicate people aged 45–64, 65–74, 75–84, and >85 years, respectively.
Methods
Participants
Data were used from the Cognitive Impairment through Aging (CogItA) study, a hospital-based prospective study focused on normal and pathological aging in middle-aged and older individuals launched in January 2000. CogItA’s participants were outpatients self-referred or referred by general practitioners for neurological and/or cognitive screenings to the clinics of the Department of Neurology and Cognitive Disorders of the teaching Hospital (AOUP “P.Giaccone”) of the School of Medicine of the University of Palermo, Italy. Details of the inclusion and exclusion criteria of the CogItA study have been reported elsewhere.Reference Camarda, Torelli, Camarda, Battaglini, Gagliardo and Monastero20 Informed consent was obtained from all participants and relatives. The study was approved by the University Hospital ethics committee and complies with the declaration of Helsinki.
According to the published criteria,Reference Petersen, Smith, Waring, Ivnik, Tangalos and Kokmen1,Reference Petersen2 CogItA participants with preserved global cognition at the Mini-Mental State examination (MMSE score ≥ 23.74),Reference Folstein, Folstein and McHugh23 subjective cognitive concerns, objective impairment in one or more cognitive domains, Clinical Dementia Rating = 0.5,Reference Morris24 no impaired or minimally impaired functional status on the ADLReference Katz, Ford, Moskowitz, Jackson and Jaffe25 and the instrumental ADL (IADL)Reference Lawton and Brody26 scales, and no dementia were classified as MCI and categorized as a-MCI, a-MCImd, na-MCI, and na-MCImd. MCI subjects included in the present paper were stroke-free first-ever diagnosed cases (n = 1250) aged 45–95 years (mean age = 70.52 ± 9.41 years), who remained in the MCI status for at least 3 years (mean follow-up = 64.98 ± 28.94 months). During this period, some of these subjects changed their MCI typology, but in the present paper, first-ever MCI diagnoses were considered. Subjects who during the follow-up reverted to normal cognition or converted to dementias different from AD and vascular dementia, such as dementia with Lewy bodiesReference McKeith, Galasko and Kosaka27 or frontotemporal dementia,Reference Brun, Englund and Gustafson28 were not considered.
Baseline Clinical Assessment
Participants to the present study underwent an extensive assessment of variables such as demographics, medical history, laboratory tests, neurological and functional examinations, cognitive testing, carotid ultrasonography, and brain magnetic resonance imaging (MRI). Many vascular risk factors (VRFs) and vascular diseases (VDs) were considered and assessed as reported elsewhere.Reference Camarda, Torelli, Camarda, Battaglini, Gagliardo and Monastero20,Reference Camarda, Torelli, Pipia, Azzarello, Battaglini, Sottile, Cilluffo and Camarda29 Since multiple VRFs and VDs often coexist, we created the VRF and VD summary scores indicating for each participant the sum of the individual VRFs and VDs that were concurrently present. APOE genotypes were determined by using standard methods.Reference Hixson and Vernier30 Participants with at least 1 APOE ϵ4 allele were classified as APOE ϵ4 carriers.
Assessment of ISNAs
All participants underwent a standardized neurological examination reflecting that routinely performed in the clinical practice. Subjects presenting at baseline or during follow-up meaningful neurological signs such as visual field defects, language deficits, cranial nerves deficits, hemimotor and hemisensory dysfunction, brachial or crural weakness, brachial or crural sensory dysfunction, Babinski sign, spastic rigidity, and hemiplegic gait were excluded. The ISNAs evaluated were: (1) mild dysphagia, (2) slurred speech, (3) central facial weakness, (4) mixed rigidity, i.e., a condition in which spastic and plastic rigidity coexist; (5) hyperreflexia (bilateral increased deep tendon reflexes), (6) reflex asymmetry, (7) tremor (resting tremor and postural/kinetic tremor), (8) plastic rigidity, (9) bradykinesia, (10) gait/balance/axial dysfunction, (11) dysmetria, (12) atactic type gait defined as a gait pattern broadly indicative of cerebellar involvement, and (13) primitive reflexes (PRs), i.e., glabellar tap, snout, palmomental, grasping, and sucking reflexes.Reference Rossor31,Reference Schott and Rossor32 To evaluate tremor, rigidity, bradykinesia, and gait/balance/axial dysfunction, collectively called mild parkinsonian signs (MPSs),Reference Louis and Bennett33 the items of motor section of the Unified Parkinson’s Disease Rating ScaleReference Fahn, Elton, Fahn, Marsden, Goldstein and Calne34 were used and were considered present when any one of the following conditions was met: (1) two or more items with a score of 1; or (2) one item with a score ≥ 2.Reference Louis, Schupf, Manly, Marder, Tang and Mayeux21 The ISNA were dichotomised as absent (score = 0) or present (score = 1) and were clustered into four categories as following: central-based signs (Cs) (signs 1–6), MPSs (signs 7–10), cerebellar-based signs (CLs) (signs 11–12), and PR (signs 13). We defined the presence of Cs, MPS, CLs, and PR as the presence of at least one sign within those included in each of these clusters. Accordingly, subjects were divided into subjects without ISNAs (ISNA−) and with ISNAs (ISNA+) if at least one sign within the above clusters was present. The neurological examination of each participant was always performed by two neurologists blinded to the patient’s history and neuroimaging. The interrater reliability assessed over time in random samples always showed excellent agreement with weighted Cohen’s kappa ranging between 0.88 and 0.91 (p < 0.001).
Functional and Neuropsychological Assessments
The functional status of participants was assessed through the ADL and the IADL scales. Cognitive functions were assessed using an extensive neuropsychological battery as reported elsewhere,Reference Camarda, Torelli, Camarda, Battaglini, Gagliardo and Monastero20,Reference Camarda, Pipia and Azzarello35 including the MMSE as test of global cognition and 12 tests to evaluate memory, attention, executive function, language, constructional ability, and visuospatial skill. Impaired cognitive domains were identified using a cut-off of 1.5 standard deviation (SD)Reference Petersen2 below the Italian normative data adjusted for age, sex, and education.Reference Carlesimo, Caltagirone and Gainotti36
Carotid Ultrasonography and Imaging Assessments
Intimal-medial thickness (IMT) and stenosis of internal carotid arteries (SICAs) were assessed as reported elsewhere.Reference Camarda, Torelli, Camarda, Battaglini, Gagliardo and Monastero20 Participants had brain MRI on a 1.5T scanner (GE Signa HDxt, Milwaukee, WI, USA). Details of the image acquisition protocol have been published previously.Reference Camarda, Torelli, Camarda, Battaglini, Gagliardo and Monastero20 The BCr and the LVBr were calculated as reported elsewhere.Reference Camarda, Torelli, Camarda, Battaglini, Gagliardo and Monastero20,Reference Camarda, Pipia and Azzarello35 WMH and lacunes were assessed according to the published criteria.Reference Wardlaw, Smith and Biessels12 The Wahlund scale (range 0–3) Reference Wahlund, Barkhof and Fazekas37 was used to obtain the scores of WMH of the frontal, parieto-occipital, and temporal areas (WMH-SC), infratentorial (WMH-INF), basal ganglia (WMH-BG), and the WMH total score (WMH-T). To define the WMH status, a cut-off score ≥2 in at least one of the above regions was used. WMH-PV were evaluated with the Fazekas scale (range 0–3),Reference Fazekas, Chawluk, Alavi, Hurtig and Zimmerman38 and a cut-off score ≥2 was used. Lacunes were assessed topographically according to the Wahlund regions used to score WMH and categorized as lacunes-SC, lacunes-BG, lacunes-INF, and lacunes-T. A cut-off score ≥2 in at least one of the Wahlund regions was used to define the status of lacunes. Subjects having WMH and/or lacunes with a score ≥2 in at least one of the Wahlund scale topographical locations were categorized as SVD positive (SVD+), and those having WMH and/or lacunes with a score ≤1 were categorized as SVD negative (SVD−).
Statistical Analysis
Descriptive statistics (percentages, mean and SD, median and interquartile range) were used to summarize data. Continuous variables were compared between subjects ISNA− and ISNA+ by using one-way analysis of variance, and differences were tested with a post hoc F-test. Categorical variables were evaluated by contingency tables, and the hypotheses of independence were tested with χ2 test. Logistic ridge regression modelsReference Hoerl and Kennard39 were used to evaluate for each MCI type the risk of having ISNA co-varying for age, sex, years of education, and the variables found significant in the univariate analysis. In general, ridge regression method is the most applied solution for addressing problems of multicollinearity.Reference El-Dereny and Rashwan40 It implies adding a small positive constant (λ), i.e., the ridge parameter, to the main diagonal elements of the information matrix. The ridge parameter was selected using likelihood cross-validation.Reference Goeman41 IMT, BCr, and LVBr values were scaled to work on percentage of increments. All tests were two-tailed, and statistical significance was set at p ≤ 0.05. Results are presented as odds ratios with 95% confidence interval. All analyses were performed using R statistical software (version 3.5.1; The R Foundation for Statistical Computing).
Results
The demographic characteristics of the MCI types are shown in Table 1. In almost all MCI types, the majority of subjects were elderly followed by old, adult, and oldest-old individuals. The MCI types single domain were more common among the adults, while those multiple domain were more common within the elderly, old, and oldest-old participants. In each age class, the mean age did not vary significantly among the various MCI types. Since the oldest-old subjects were few, subsequent analysis was conducted pooling the old and oldest-old classes in the class of old-oldest old.
Table 1: Demographic characteristics of MCI types
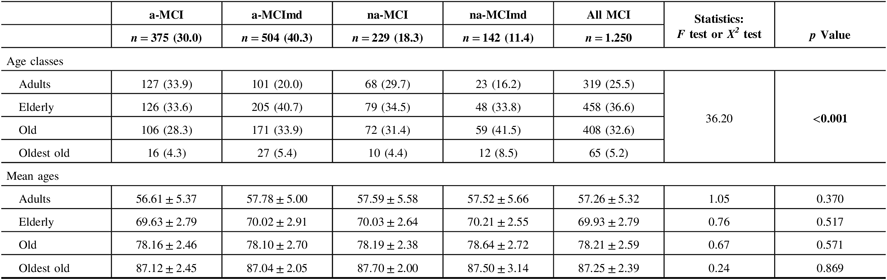
MCI = mild cognitive impairment; a-MCI = amnestic mild cognitive impairment single domain; a-MCImd = amnestic mild cognitive impairment multiple domain; na-MCI = nonamnestic mild cognitive impairment single domain; na-MCImd = nonamnestic mild cognitive impairment multiple domain.
Data presented are number (%) for categorical and mean ± SD for continuous data.
Tests F and X2 were performed across four MCI types.
Bold values indicate significance at p ≤ 0.05.
Within the sample, 175 (14.0%) subjects did not show any of the selected ISNA, while 1075 (86.0%) presented at least one ISNA (Table 2). Overall, subjects ISNA− were more common among the MCI types single domain, and subjects ISNA+ were more common among those multiple domain. PRs were the most frequent ISNA, followed by ISNA central-based and MPSs, while ISNA cerebellar-based were the rarest. PRs were exhibited by 864 subjects (69.1%) of the sample and were more common among the MCI types multiple domain than those single domain. In all MCI types, snout reflex was the most common PR followed by glabellar tap and palmomental reflex, while grasping and sucking reflexes were the rarest. Snout and palmomental reflexes were more common among the MCI types multiple domain than those single domain, while glabellar tap was more common among the amnestic MCI types than the nonamnestic types. The mean number of PRs was greater among the amnestic MCI types than the nonamnestic types. Central-based signs were exhibited by 645 subjects (51.6%) of the sample. Subjects with Cs were more common among the nonamnestic MCI types than the amnestic types. In all MCI types, reflex asymmetry was the most common Cs, followed by bilateral hyperreflexia, central facial weakness, mixed rigidity, slurred speech, and dysphagia. Reflex asymmetry, bilateral hyperreflexia, and central facial weakness were more common among the nonamnestic MCI types than the amnestic types, while the frequency of the other Cs did not vary among the four MCI types. The mean number of Cs was greater among the nonamnestic MCI types than the amnestic types. MPSs were found in 46.2% (n = 578) of the sample. In all MCI types, bradykinesia was the most common MPS, followed by gait/balance/axial dysfunction, and tremor, while rigidity was the rarest. Individual MPSs were more common among the MCI types multiple domain than the MCI single domain. The mean number of MPS was greater in the former than in the latter. CLs were the rarest ISNA encountered. Dysmetria was the most common CLs, and the atactic type gait was the rarest. The frequency of dysmetria did not vary among the four MCI types, while the atactic type gait was more common among the a-MCImd than the other MCI types. The mean number of CLs did not vary among the MCI types.
Table 2: Frequency of ISNA selected in MCI types
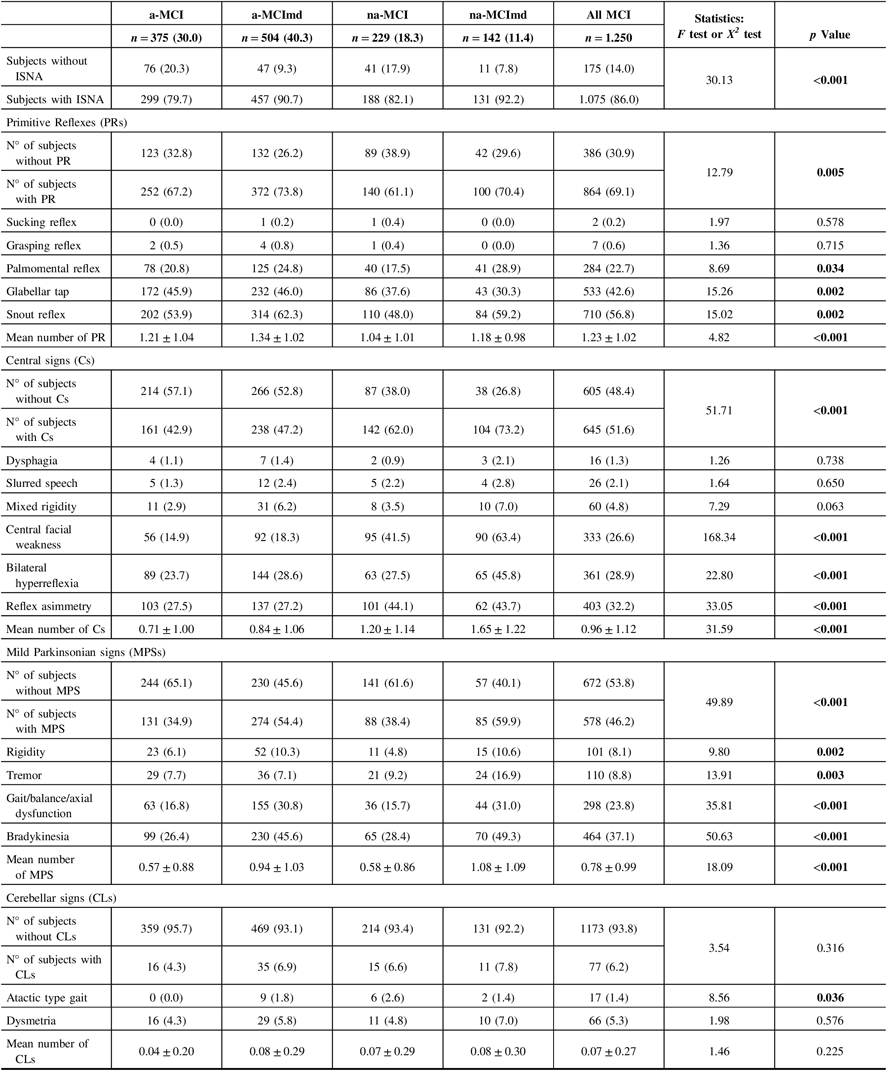
ISNA = isolated, subtle neurological abnormalities; MCI = mild cognitive impairment; a-MCI = amnestic mild cognitive impairment single domain; a-MCImd = amnestic mild cognitive impairment multiple domain; na-MCI = nonamnestic mild cognitive impairment single domain; na-MCImd = nonamnestic mild cognitive impairment multiple domain.
Data presented are number (%) for categorical and mean ± SD for continuous data.
Tests F and X2 were performed across four MCI types.
Bold values indicate significance at p ≤ 0.05.
ISNA increased with age reaching a peak in the old-oldest old individuals (Table 3). Among the adults, 210 subjects (65.8%) were ISNA+ and 109 subjects (34.2%) were ISNA−. PRs were the most frequent ISNA followed by Cs, MPS, and CLs categories. PR, Cs, and MPS were more common among the MCI types multiple domain than those single domain, while CLs did not vary in the four MCI types. The mean number of ISNA was greater in the former than in the latter. Within the elderly, 405 subjects (88.4%) were ISNA+ and 53 subjects (11.6%) were ISNA−. The frequency of subjects ISNA+ did not vary among the four MCI types. PRs were the most frequent ISNA followed by Cs, MPS, and CLs categories. PRs were almost equally distributed in the four MCI types, MPS and CLs were more common among the MCI types multiple domain, and Cs were more common among the nonamnestic MCI types. The mean number of ISNA was greater in the nonamnestic MCI types than in the amnestic types. Among the old-oldest old subjects, 461 individuals (97.5%) presented at least one ISNA and 12 individuals only (2.5%) were ISNA−. CLs and MPS did not vary among the four MCI types. Cs were significantly more common among the nonamnestic MCI types, while PRs were significantly more common among the amnestic MCI type. The mean number of ISNA was significantly greater in the na-MCImd type than in the other MCI types. The co-occurrence of multiple ISNAs increased with age in all MCI types.
Table 3: Frequency of ISNA categories in MCI types according to age classes
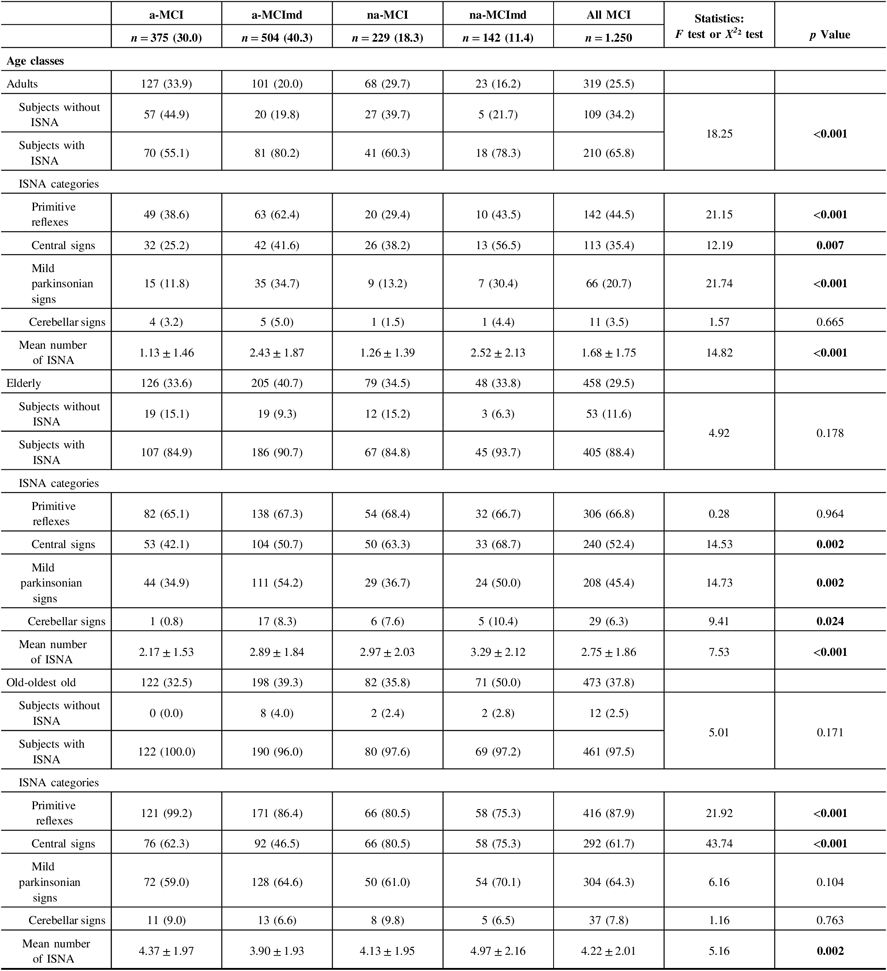
ISNA = isolated, subtle neurological abnormalities; MCI = mild cognitive impairment; a-MCI = amnestic mild cognitive impairment single domain; a-MCImd = amnestic mild cognitive impairment multiple domain; na-MCI = nonamnestic mild cognitive impairment single domain; na-MCImd = nonamnestic mild cognitive impairment multiple domain; MPSs = mild parkinsonian signs; Cs = central-based signs; CLs = cerebellar-based signs.
Data presented are number (%) for categorical and mean ± SD for continuous data.
Tests F and X2 were performed across four MCI types.
Bold values indicate significance at p ≤ 0.05.
Baseline characteristics of MCI types ISNA+ and ISNA− are shown in Table 4. In all MCI types, no difference was found in the distribution of female and male among the two ISNA groups. In all MCI types, the mean age of female was greater in subjects ISNA+ than in subjects ISNA−, while the mean age of male was greater in subjects ISNA+ than in subjects ISNA− in the a-MCI only. Level of education did not vary among the ISNA groups in MCI types single domain and in a-MCImd type, while in the na-MCImd type, subjects ISNA+ were less educated than subjects ISNA−. The ADL scores were worse in the MCI types single domain ISNA+ only, and the IADL scores were worse in the MCI types single domain and a-MCImd ISNA+. In the na-MCImd type, ADL and IADL scores did not vary among the two ISNA groups. Out of the VRFs evaluated, arterial hypertension only was significantly more common among subjects ISNA+ than subjects ISNA− with the na-MCImd type, while no difference was found in the distribution of VRFs among the two ISNA groups of the other MCI types. However, in a-MCI and na-MCImd, the VRF summary score was greater in subjects ISNA+ than those ISNA−. No difference was found in the frequency of the VD evaluated among subjects ISNA+ and ISNA− of almost all MCI types, with the exception of history of TIA that was significantly more common among the subjects a-MCI ISNA+. The VD summary score was greater in subjects ISNA+ than in those ISNA− in the a-MCI only. In the a-MCI type, APOE ϵ4 carriers were significantly more common in subjects ISNA+ than in subjects ISNA− and APOE ϵ4 noncarriers were significantly more common in subjects ISNA− than in subjects ISNA+. The distribution of APOE ϵ4 carriers and noncarriers did not differ in the two ISNA groups of the other MCI types.
Table 4: Baseline characteristics of MCI types with at least one ISNA (ISNA+) and without ISNA (ISNA−)
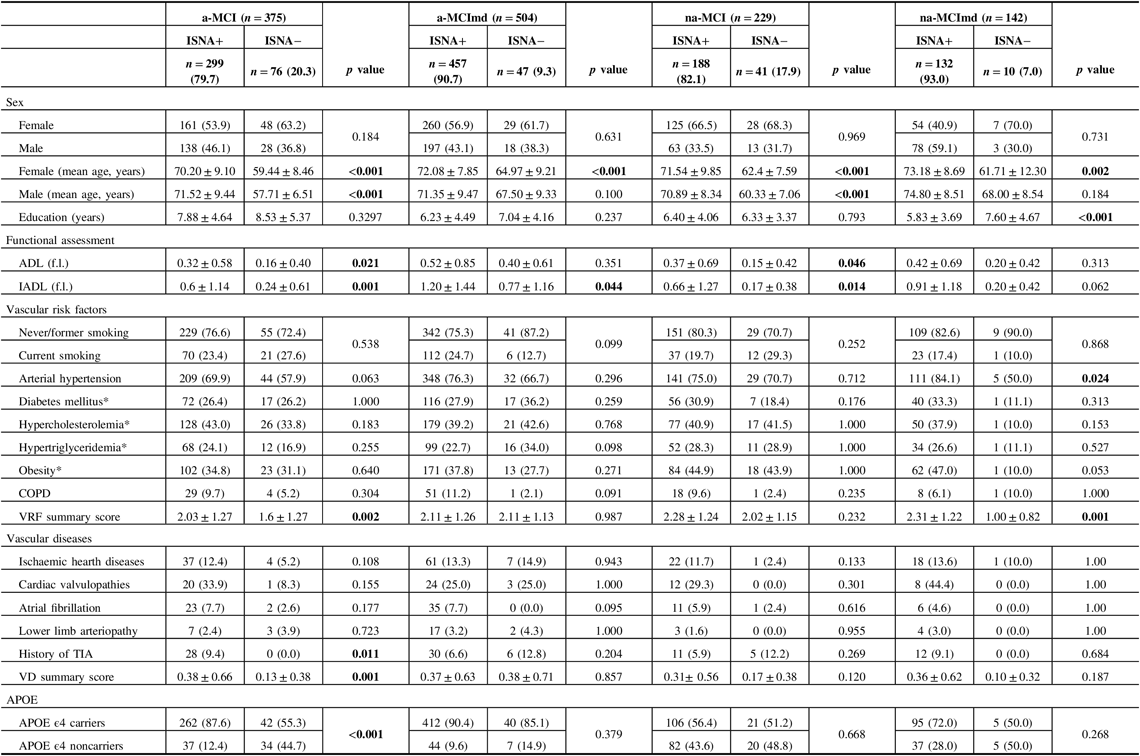
MCI = mild cognitive impairment; ISNA = isolated, subtle neurological abnormalities; a-MCI = amnestic mild cognitive impairment single domain; a-MCImd = amnestic mild cognitive impairment multiple domain; na-MCI = nonamnestic mild cognitive impairment single domain; na-MCImd = nonamnestic mild cognitive impairment multiple domain; ADL (f.l.) = activities of daily living (functions lost); IADL (f.l.) = instrumental activities of daily living (functions lost); COPD = chronic obstructive pulmonary disease; TIA = transient ischemic attacks; APOE = apolipoprotein E.
Data presented are number (%) for categorical and mean ± SD for continuous data.
Tests F and X 2 were performed across the subjects ISNA+ and ISNA− of each MCI type.
Bold values indicate significance at p ≤ 0.05.
* Number and percentage of missing data:
Diabetes mellitus: a-MCI ISNA+ = 25 (8.0%); a-MCI ISNA− = 12 (16.0%); a-MCImd ISNA+ = 40 (9.0%); a-MCImd ISNA− = 1 (2.0); na-MCI ISNA+ = 7 (4.0%); na-MCI ISNA− = 3 (8.0%); na-MCImd ISNA+ = 12 (9.0%); na-MCImd ISNA− = 1 (10.0%).
Hypertriglyceridemia: a-MCI ISNA+ = 16 (5.0%); a-MCI ISNA− = 6 (8.0%); a-MCImd ISNA+ = 20 (4.0%); a-MCImd ISNA− = 1 (2.0); na-MCI ISNA+ = 4 (2.0%); na-MCI ISNA− = 3 (8.0%); na-MCImd ISNA+ = 4 (3.0%); na-MCImd ISNA− = 1 (10.0%).
Obesity (Body Mass Index): a-MCI ISNA+ = 5 (2.0%); a-MCI ISNA− = 3 (4.0%); a-MCImd ISNA+ = 3 (1.0%); a-MCImd ISNA− = 1 (2.0); na-MCI ISNA+ = 1 (1.0%); na-MCI ISNA− = 0 (0.0%).
Neuropsychological performances of MCI types ISNA+ and ISNA− are summarized in Table 5. The MMSE score was above the cut-off level in all MCI types, but subjects a-MCI ISNA+ performed significantly less than subjects ISNA−. As expected, a worse performance on memory task was exhibited by the amnestic MCI types ISNA+ and ISNA−. In subjects with a-MCI, the nonmemory domains were not impaired, but subjects ISNA+ performed significantly less than subjects ISNA− in attention, executive function, and language. In subjects a-MCImd ISNA+ and ISNA−, the nonmemory domains were impaired and subjects ISNA+ performed significantly worse than subjects ISNA− in tests evaluating language. In the nonamnestic MCI types, no significant difference was found in the performance of nonmemory domains among subjects ISNA+ and ISNA−, although a trend of worse performance of subjects ISNA+ in almost all cognitive domains was evident.
Table 5: Neuropsychological performances of MCI types with at least one ISNA (ISNA+) and without ISNA (ISNA−)
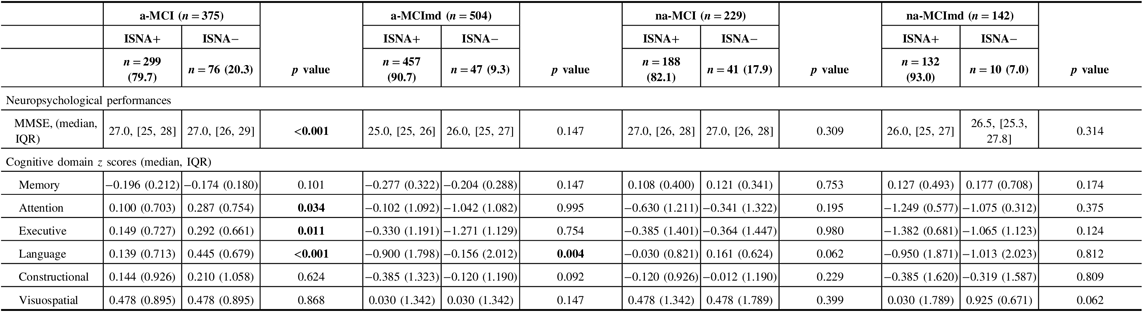
MCI = mild cognitive impairment; ISNA = isolated, subtle neurological abnormalities; a-MCI = amnestic mild cognitive impairment single domain; a-MCImd = amnestic mild cognitive impairment multiple domain; na-MCI = nonamnestic mild cognitive impairment single domain; na-MCImd = nonamnestic mild cognitive impairment multiple domain; MMSE = Mini-Mental State Examination.
Data presented are median, interquartile range (IQR), for continuous data, and number (%) for categorical data.
Tests F and X 2 were performed across the ISNA+ and ISNA− of each MCI type.
Bold values indicate significance at p ≤ 0.05.
Carotid ultrasonography and imaging findings in the MCI types ISNA+ and ISNA− are reported in Table 6. In all MCI types, the frequency of IMT was significantly higher in subjects ISNA+ than subjects ISNA−, while the frequency of SICA did not differ between the two ISNA groups. In a-MCI, na-MCI, and na-MCImd, WMH-SC, WMH-T, and WMH-PV were significantly higher in subjects ISNA+ than subjects ISNA−, while in a-MCImd, WMH-BG only were significantly higher in subjects ISNA+. Lacunes-BG and lacunes-T were significantly higher in MCI types single domain ISNA+, and lacunes-T were significantly higher in na-MCImd ISNA+. In a-MCI, na-MCI, and na-MCImd, SVD+ was more common in subjects ISNA+ than ISNA−, while SVD− was more common among the subjects ISNA− than ISNA+. In a-MCImd, the distribution of subjects SVD+ and SVD− did not differ among the two ISNA groups. BCr was significantly higher in all MCI types ISNA+ with the exception of a-MCImd type. LVBr was significantly higher in the MCI types single domain ISNA+, while no difference among the ISNA groups was found in the MCI types multiple domains.
Table 6: Carotid ultrasonography and imaging findings of MCI types with at least one ISNA (ISNA+) and without ISNA (ISNA−)
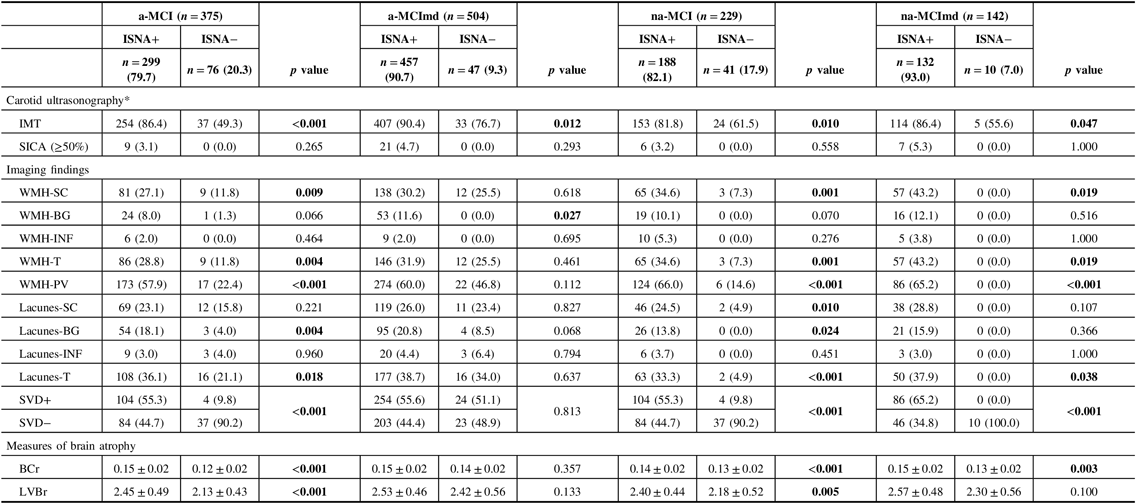
MCI = mild cognitive impairment; ISNA = isolated, subtle neurological abnormalities; a-MCI = amnestic mild cognitive impairment single domain; a-MCImd = amnestic mild cognitive impairment multiple domain; na-MCI = nonamnestic mild cognitive impairment single domain; na-MCImd = nonamnestic mild cognitive impairment multiple domain; IMT = intimal-medial thickness; SICA = stenosis of the internal carotid artery; WMHs = white matter hyperintensities; SC = deep/subcortical; BG basal ganglia; INF = infratentorial; T = total; PV = periventricular; SVD+ = small vessel disease (presence of WMH and lacunes with a score ≥ 2); SVD− = small vessel disease (presence of WMH and lacunes with a score ≤ 1); BCr = bicaudate ratio, LVBr = lateral ventricles to brain ratio.
* Missing data: a-MCI ISNA+ =5 (2.0%); a-MCI ISNA− = 1 (1.0%); a-MCImd ISNA+ =7 (2.0%); a-MCImd ISNA− = 4 (8.0%); na-MCI ISNA+ = 1 (1.0%); na-MCI ISNA− = 2 (5.0%); na-MCImd ISNA− = 1 (10.0%).
Data presented are number (%) for categorical and mean ± SD for continuous data. Tests F and X 2 were performed across the ISNA+ and ISNA− of each MCI type.
Bold values indicate significance at p ≤ 0.05.
To assess the effects of the variables evaluated on the estimated probability of having at least one ISNA, logistic ridge regression analysis was carried out in each MCI type (Table 7). Age resulted associated with a-MCI type only. For each year of age increase, the odds of having at least one ISNA went up about 3%. Being female increased by 7.7% the probability of having at least one ISNA in the a-MCI type only. Education and VRF summary score did not influence the probability of having at least one ISNA in any of the MCI types. VD summary score increased the probability of having at least one ISNA in a-MCI (18.1%), na-MCI (11.1%), and na-MCImd (4.5%). Being carrier of the APOE ϵ4 allele increased by 52.7%, 16.5%, and 5.1% the probability of having at least one ISNA in a-MCI, a-MCImd, and na-MCImd, respectively. At the increase of a single percentage point of the IMT, the probability of having at least one ISNA in a-MCI, a-MCImd, na-MCI, and na-MCImd went up by 52.7%, 36.9%, 14.8%, and 16.3%, respectively. The presence of WMH-PV increased by 36.7%, 58.4%, and 14.3% the probability of having at least one ISNA in a-MCI, na-MCI, and na-MCImd, respectively. The presence of SVD in the frontal region increased the risk of having at least one ISNA in a-MCI (16.4%), na-MCI (54.5%), and na-MCImd (16%) types. The presence of SVD in the parieto-occipital region increased the risk of having at least one ISNA in a-MCI, na-MCI, and na-MCImd by 22.3%, 30.3%, and 10.5%, respectively. The presence of SVD in the lateral temporal region increased the risk of having at least one ISNA in a-MCI, a-MCImd, na-MCI, and na-MCImd MCI by 14.0%, 15.9%, 19.8%, and 8.4%, respectively. The presence of SVD in the basal ganglia increased the risk of having at least one ISNA in a-MCI, a-MCImd, na-MCI, and na-MCImd by 29.9%, 31.1%, 51.8%, and 9.7%, respectively. BCr and LVBr resulted associated with all the MCI types. At the increment of a single percentage point of BCr, the probability of having at least one ISNA increased by 15.6% in a-MCI, 31.9% in a-MCImd, 65.8% in na-MCI, and 32.4% in na-MCImd. At the increment of a single percentage point of LVBr, the odds of having at least one ISNA increased by 13.1% in a-MCI, 6.2% in a-MCImd, 9.6% in na-MCI, and 5.2% in na-MCImd.
Table 7: Association between VRF and VD summary scores, APOE ϵ4 carriers, IMT, topographical location of SVD, WMH-PV, BCr, LVBr, and the risk of having at least one ISNA in individual MCI types
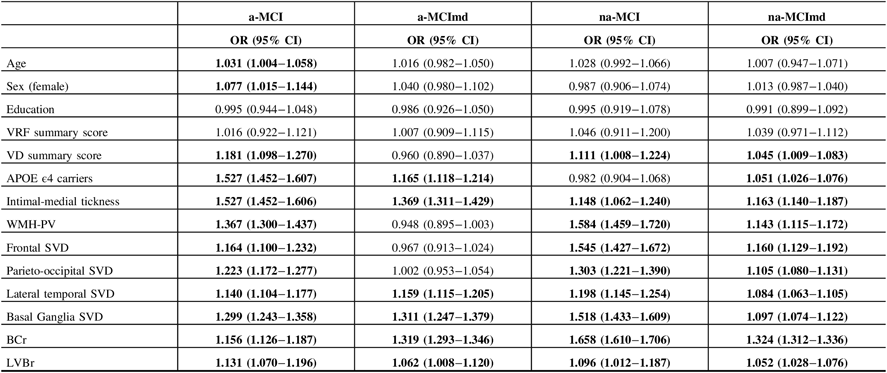
VRFs = vascular risk factors; VDs = vascular diseases; APOE = apolipoprotein E; IMT = intimal-medial thickness ; WMHs = white matter hyperintensities; PV = periventrivular; SVD = small vessel disease (presence of WMH and lacunes with scores ≥ 2); BCr = bicaudate ratio; LVBr = lateral ventricle to brain ratio; ISNAs = isolated, subtle neurological abnormalities; OR = odd ratio; CI = confidence interval; a-MCI = amnestic mild cognitive impairment single domain; a-MCImd = amnestic mild cognitive impairment multiple domain; na-MCI = nonamnestic mild cognitive impairment single domain; na-MCImd = nonamnestic mild cognitive impairment multiple domain.
For each MCI types, association of the variables with ISNA categories was estimated using logistic regression analysis with adjustments for age, sex, and education (years).
Bold values indicate significance at p ≤ 0.05.
Discussion
The frequency of ISNA detected in subjects with MCI was greater (86%) than that found in NCH aging individuals (57.2%).Reference Camarda, Torelli, Camarda, Battaglini, Gagliardo and Monastero20 In all MCI types, and in all age classes, PRs were the ISNA most frequently encountered, followed by Cs, MPS, and CLs. All ISNA categories increased with age and were presented by both the amnestic and nonamnestic MCI types, particularly by those multiple domain, with the exception of Cs and PR that in the old-oldest old subjects were more common among the MCI types single domain.
Copious data of the literature underline the importance of midlife vascular risks and Aβ burden to neurodegenerative processes and to the development of cognitive decline in older adults. In CN individuals, midlife VRFs accelerate structural brain agingReference Debette, Seshadri and Beiser42 and are associated with greater prospective cognitive decline,Reference Gottesman, Schneider and Albert43 current and later-life smaller brain volumes,Reference Pase, Davis-Plourde and Himali44 and risk of dementia.Reference Whitmer, Gunderson, Quesenberry, Zhou and Yaffe45 Further, midlife but not later-life exposure to VRF is important also for Aβ deposition, as shown by the fact that having two or more midlife VRFs compared with none is significantly associated with elevated Aβ deposition (61.2% vs 30.8%).Reference Gottesman, Schneider and Zhou46 In addition, in CN and MCI individuals, VRFs interact with Aβ to reduce cortical thickness in frontotemporal and parietal regions vulnerable to ADReference Villeneuve, Reed and Madison47 and are associated with prospective cognitive decline, both alone and synergistically with Aβ burden.Reference Rabin, Schultz and Hedden48 However, VRF summary score was not associated with ISNAs in any of the four MCI types, and VD summary score increased the probability of having at least one ISNA in the MCI types single domain and poorly in the na-MCImd. These data need attention. A high vascular risk burden at younger ages is indeed indicative of early vascular aging,Reference Nilsson49 but in the later years of life, it is less relevant regarding the MCI typology since vascular and neurodegenerative processes have already occurred at younger ages when the MCI typology is likely to be of single domain type. The fact that subjects with MCI types multiple domain were older than subjects with MCI types single domain supports this hypothesis. IMT increased the probability of having at least one ISNA in all MCI types, and the risk was greater for the amnestic MCI types than for the nonamnestic types. These results are in agreement with a previous study showing that IMT is associated with WMH, infarcts, brain atrophy, and with poor cognitive performance particularly in the executive function.Reference Romero, Beiser and Seshadri50 Further, IMT significantly increases the risk of conversion of a-MCI to dementia.Reference Viticchi, Falsetti and Vernieri51 WMH-PV increased greatly the probability of having at least one ISNA in MCI types single domain and in the na-MCImd. It has been shown that WMH-PV are associated with elevated Aβ deposition independent from age and presence of APOE ϵ4 alleleReference Marnane, Al-Jawadi and Mortazavi52 and induce thinning of prefrontal, parietal, temporal cortices, anterior insula, and atrophy of the caudate nuclei,Reference Lambert, Narean, Benjamin, Zeestraten, Barrick and Markus14 and cognitive decline through the cortical atrophy of the above disconnected regions.Reference Rizvi, Narkhede and Last19 Therefore, it is reasonable to suspect that even in our MCI types, WMH-PV may also have induced atrophy of the caudate nuclei, and thinning of the frontal, parietal, and temporal cortices through likely disruption of periventricular long associating tracts.
Overall, SVD at different topographical locations and BCr greatly increase the risk of having ISNA in all the MCI types. Given that cortex, cerebellum, and BG are strictly interconnected,Reference Caligiore, Strick and Jörntell53 it is likely that ISNAs are the by-product of the disconnection of the cortical-cerebellar-basal ganglia-thalamo-cortical circuits induced by vascular and degenerative processes. It is also likely that the caudate atrophy and the disruption of the internal circuits of BG may have induced an excessive inhibition to its output nuclei. As a consequence, the inhibitory drive to thalamus may have led to bradykinesia and that to brainstem structures controlling postural muscle tone and locomotion may have led to rigidity and gait/balance/axial dysfunction, respectively,Reference Takakusaki54 while the inhibition of the physiological inhibitor control of lower brainstem centers on stereotyped motor responsesReference Delwaide and Dijeux55 may have led to the reappearance of PRs.
Nevertheless, the above hypotheses underpinning the vascular contribution to ISNA do not fully explain their presentation in the various MCI types. Among the amnestic MCI types, 39% (n = 342) of subjects ISNA+ were SVD− and 5% (n = 45) of subjects ISNA− were SVD+. Similarly, among the nonamnestic MCI types, 51% (n = 190) of subjects ISNA+ were SVD− and 35% (n = 130) of subjects ISNA− were SVD+. These conflicting data suggest that co-occurring factors other than vascular contribute to the presentation of ISNA. The APOE ϵ4 allele can be one of these factors. In the present study, the APOE ϵ4 carriers were indeed over-represented relative to other studies, probably because the CogItA study assessed essentially subjects referred to the memory clinic, making the sample vulnerable to selection. Overall, APOE ϵ4 carriers were more common among subjects ISNA+ than subjects ISNA− in all MCI types, and being ϵ4 carrier increased the probability of having at least one ISNA greatly in the amnestic MCI types and scarcely in the nonamnestic MCI types, a finding in agreement with the notion that compared to non-ϵ4 carriers, MCI ϵ4 carriers have frequently the amnestic phenotype.Reference Snowden, Stopford and Julien56 The presence of APOE ϵ4 allele in nonamnestic MCI types is not surprising. Along with age, APOE ϵ4 allele is a significant predictor of amyloidosis. More than half of all MCI types is Aβ positive,Reference Wolk, Price and Saxton57 and APOE ϵ4 carriers are 2–3 times more likely to be amyloid positive than APOE ϵ4 non-carriers.Reference Jansen, Ossenkoppele and Knol6 Subjects with MCI have elevated Aβ deposition in frontal, parietal, temporal, and posterior cingulate cortices suggestive of early AD process,Reference Kemppainen, Aalto and Wilson58 and those converting to AD have a greater Aβ deposition in these regions, as well as in the putamen and in the caudate nuclei as compared to nonconverters.Reference Koivunen, Scheinin and Virta59 Furthermore, in subjects with MCI, the presence of the APOE ϵ4 allele is more frequent in the converters than in the nonconverters.Reference Okello, Koivunen and Edison60 Therefore, it is reasonable to suspect that subjects of all four MCI types carrying the APOE ϵ4 allele were also Aβ positive and that subjects ISNA+ being more APOE ϵ4 carriers than subjects ISNA− were candidates to convert to dementia faster than subjects ISNA−.
Limitations of the Study
Some limitations of our study are worth noting. First, CSF biomarkers of Aβ and tau and advanced imaging techniques up to now are not fully available in our country, particularly in a clinical setting. So, we do not know the distribution of Aβ and tau in subjects ISNA+ and ISNA− of the various MCI types. Second, we assessed cortical and subcortical atrophy using linear measurements well aware that they are rather crude estimates of brain atrophy. However, it has been shown that the BCr is a reliable marker of caudate atrophyReference Doraiswamy, Patterson and Na61 and that ventricular enlargement is a feasible, even if nonspecific, surrogate marker of neurodegeneration in MCI and AD.Reference Madsen, Gutman and Joshi16 Third, we did not estimate WMH volumetrically, but visually. However, it has been shown that WMH evaluated with visual scales correlates well with WMH volumetry.Reference Gao, Swartz and Scheltens62 Fourth, perhaps, we underestimated the magnitude of cerebral SVD in our cohort because we evaluated WMHs and lacunes only. Fifth, the generalizability of our results is limited because the patients have been selected in a hospital setting. Sixth, in the present paper, there is no mention of the ISNAs in neurologically and CN subjects because data on this topic have been already published.Reference Camarda, Torelli, Camarda, Battaglini, Gagliardo and Monastero20 Lastly, the cross-sectional design of our study does not allow causal inferences.
Conclusion
ISNAs are likely to have a multifactorial origin, increase with age, and are presented by both the amnestic and nonamnestic MCI types, particularly by those multiple domain, and carrying the APOE ϵ4 allele. Further, subjects ISNA+ perform less than subjects ISNA− in almost all nonmemory domains. Given that cortical and subcortical vascular and atrophic processes contribute to ISNAs, their presence in individuals with MCI must alert the practitioners to target timely interventions to slowing cognitive decline and delay progression of MCI to dementia. Longer prospective population-based studies are needed to clarify to what extent the presentation of ISNAs in middle-aged and older MCI individuals represents an additive risk for the conversion to dementia.
Acknowledgements
We gratefully thank all participants, as well as the neurologists and the neuropsychologists who over time collected patients data.
Disclosures
All the authors hereby declare that they have nothing to disclose.
Statement of Authorship
CC and RC were responsible for the study’s concept and design, data managements, and record linkage. GS and GC did the statistical analysis. CC, PT, CP, DA, and RC contributed to the analysis and interpretation of the data.
CC wrote the paper. All co-authors edited the paper and approved its final version.











