INTRODUCTION
Pseudomonas aeruginosa is one of the leading pathogens responsible for healthcare-associated infections in hospitals worldwide [Reference Morrison and Wenzel1]. In France, it was ranked third of nosocomial pathogens according to the 2006 national nosocomial infections (NIs) prevalence survey [2]. Its intrinsic resistance to various antimicrobial agents [Reference Mesaros3] as well as its ability to acquire additional resistance mechanisms [Reference Strateva and Yordanov4], make treatment of infections caused by this microorganism difficult [Reference Vettoretti5]. P. aeruginosa is known to be a ubiquitous environmental opportunistic pathogen with on the one hand, an intermittent presence in normal human intestinal flora and on the other, causing intestinal colonization in up to 10% of hospitalized patients [Reference Murthy6, Reference Blanc, Francioli and Zanetti7]. Several studies, mainly in intensive care units (ICUs) have attempted to evaluate the respective contribution of endogenous versus exogenous sources of P. aeruginosa colonization or infection. This issue remains controversial. Indeed, some studies have argued for a major role (>80%) of endogenous flora of patients [Reference Berthelot8–Reference Speijer10]. Conversely, other authors have shown that exogenous sources (other patients and the inanimate environment such as taps and sinks) via cross-transmission played a substantial role (up to 50%) in the acquisition of P. aeruginosa [Reference Bergmans11–Reference Reuter14]. In the present study, we shed new light on this issue by means of a multilevel statistical approach. The aim of this work was to determine individual (patient)- and group (hospital)-level factors associated with prevalence of hospitalized patients infected with P. aeruginosa.
MATERIAL AND METHODS
Setting, period of study and data source
Data were from the 2006 national NIs point-prevalence survey for the eastern regions of France. These five regions (Alsace, Bourgogne, Champagne-Ardenne, Franche-Comté, Lorraine) account for 8·5 million inhabitants corresponding to 14% of the French population. This point-prevalence survey was performed in 2006, on a single day in June by trained investigators. Data were collected regarding hospitalized patients and healthcare facilities (HCFs) by means of standardized questionnaires. The resulting database, for the eastern regions of France included 52 720 in-patients and 343 HCFs (representing 83% of hospitals eligible for this survey). Our study was limited to HCFs of at least 300 beds.
Outcome variable
The outcome variable was binary and indicated whether a given hospitalized patient had, on the day of the survey, a P. aeruginosa NI acquired during his hospitalization [>48 h after admission, imported NIs (i.e. acquired in another hospital) were excluded]. Definitions of NIs were adapted from those of CDC [Reference Garner15], and McGeer et al. [Reference McGeer16] for patients from long-term care facilities. Asymptomatic bacteriurias were excluded. Regarding antibiotic resistance, only data regarding ceftazidime resistance were collected (resistant or not).
Data structure
Individual-level variables: patient characteristics
At the patient level (the lowest level of data), the following characteristics were considered: age (converted in binary data; <65 years or ⩾65 years), sex (male/female), immunocompromised status (patient with a malignant haemopathy/metastatic cancer, or having received immunosuppressive treatment, radiotherapy, or infected with human immunodeficiency virus with a CD4 count of <500 cells/l; yes/no), McCabe score (0, non-fatal disease; 1, ultimately fatal disease; 2, rapidly fatal disease) [Reference McCabe and Jackson17], surgery during the previous month (yes/no), and type of hospitalization ward (medical, surgical, ICU, other).
Group-level variables: HCF/hospital characteristics
Regarding the HCF level, this study included the following variables: region (Alsace, Bourgogne, Champagne-Ardenne, Franche-Comté, Lorraine), type (university, general, psychiatric, other), status (public/private) and number of beds (<900 or ⩾900 beds); this threshold was retained because it made a better fit of the data possible. Moreover, individual factors relating to an exposure, on the day of survey, to invasive devices [intravascular device, tracheal catheter and urinary catheter (within the 7 previous days)] and to antibiotic treatments were aggregated at the HCF level. In fact, we considered that the measure of exposure was biased at patient level for these extrinsic factors. Indeed, data about these type of exposures were collected only for the day of the survey (except for the urinary catheter) and nothing was recorded regarding chronology of exposure in relation to infection. Thus, exposures to invasive devices were taken into account as proportions (%) of patients exposed to at least one invasive device, intravascular device, tracheal catheter and urinary catheter. Similarly, exposures to antibiotic agents were taken into account as proportions of patients exposed to antibiotic treatments, antipseudomonal antibiotics, non-antipseudomonal antibiotics, penicillins, cephalosporins, aminoglycosides, quinolones, macrolides and other classes of antibiotics. In addition, HCFs were divided into two categories using the median as cut point for each of the proportions mentioned above. In this study, antipseudomonal antibiotics correspond to the following drugs: ticarcillin±clavulanic acid, piperacillin±tazobactam, some third-generation cephalosporins (ceftazidime, cefsulodine), all the fourth-generation cephalosporins (e.g. cefepime, cefpirome), aztreonam, carbapenems (imipenem, meropenem), aminoglycosides, ciprofloxacin and colistin.
Statistical analysis
To take into account the hierarchical structure of data, patients hospitalized in HCFs, and the possibility of intra-HCF correlation regarding the probability of being infected with P. aeruginosa, we used a two-level hierarchical logistic regression analysis. Initially, each independent variable was tested in a univariate analysis using Stata software, version 10 (Stata Corp., USA), and subsequently a final model was built by introducing variables with P<0·20 in the univariate analysis. The model building used a forward stepwise selection process. Five consecutive multilevel models were fitted to the data. Model 1 (unconditional, baseline), which included only the constant (i.e. no explanatory variables) was assessed in order to determine the initial distribution of the variance of the dependent variable between the two levels: the variance partition coefficient (VPC) was calculated using the Snijders & Bosker approximation [Reference Goldstein18, Reference Snijders and Bosker19]:
where σu02 is the variance of the HCF-level random intercept and π=3·14 159. VPC is the proportion of total variance of the outcome that is explained at the HCF level. Furthermore, σu02 (HCF-level variance) represents the heterogeneity between HCFs in terms of the outcome. This heterogeneity can be explained by compositional effects (patient factors) and contextual effects (HCF factors). In model 2 (individual model), we entered patient-level variables and assessed whether any of the coefficients of any of the explanatory variables had a significant variance component between HCFs (heterogeneity of the effect across HCFs). In models 3a, 3b and 4 (final models) HCF-level variables were added. These final models differed in the way in which variables relating to invasive devices and antibiotic treatments were included: model 3a (i.e. ⩾1 invasive device and ⩾1 antibiotic treatment), model 3b (⩾1 invasive device, ⩾1 antipseudomonal antibiotic, ⩾1 non-antipseudomonal antibiotic) and model 4 (each type of invasive devices and each class of antibiotics). Finally, we tested cross-level interactions (between patient- and HCF-level variables). The percentage of proportional change in variance (PCV) was calculated:
PCV represented the percentage of HCF-level variance (heterogeneity of HCFs) that was explained by variables retained in model 2 (i.e. patient-level variables). Significance of parameters was assessed with the Wald test. Modelling was performed using MLwiN software, version 2.02 [Reference Rasbash20]. A P value <0·05 was considered as significant.
RESULTS
Overall prevalence
From the database source, 51 HCFs (⩾300 beds) were included in the study corresponding to 26 249 in-patients. Because of missing values, only 25 533 in-patients (97·3%) were retained for analysis with a median number of 376 (range 224–1964) patients per HCF. The overall prevalence of patients infected with P. aeruginosa was 0·37% (95% confidence interval 0·30–0·45) with a median prevalence of 0·25% (range 0–1·65) in HCFs. Prevalence was nil in 35% of HCFs. Overall, 94 patients were infected during their hospitalization for a total of 98 infections (28% of isolates were ceftazidime-resistant). Main sites of infection were respiratory tract (32%), urinary tract (32%) and skin/soft tissue (15%).
Univariate analyses, patients and HCF characteristics
In Table 1, we report characteristics of patients depending on whether they were infected or not with P. aeruginosa. Regarding HCFs, these were mainly general hospitals, which admitted almost half of patients, with public status and <900 beds (Table 2). Other HCF characteristics including patients' data aggregated in relation to invasive devices and antibiotic treatments are shown in Table 2. Univariate analyses revealed on the one hand, that patient's age, region where the HCF was located, as well as its status, and high proportion (⩾median) of patients exposed to quinolones were not significantly associated with prevalence of cases. On the other hand, infected patients were significantly (Table 3) more often male with an immunocompromised status, a McCabe score ⩾1, a previous surgery, hospitalized in an ICU in a university hospital of at least 900 beds with a high proportion of patients exposed to invasive devices (intravascular device, tracheal catheter, urinary catheter) and antibiotic agents (all classes except quinolones).
Table 1. CharacteristicsFootnote * of patients with and without Pseudomonas aeruginosa (Pa) infection
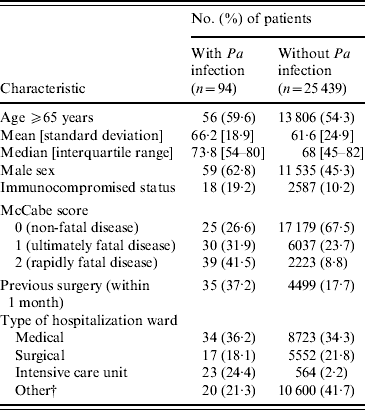
* P<0·05 for all patient characteristics except age.
† Other: rehabilitation, long term-care and psychiatry.
Table 2. Characteristics of healthcare facilities (HCFs⩾300 beds): HCF-level factors
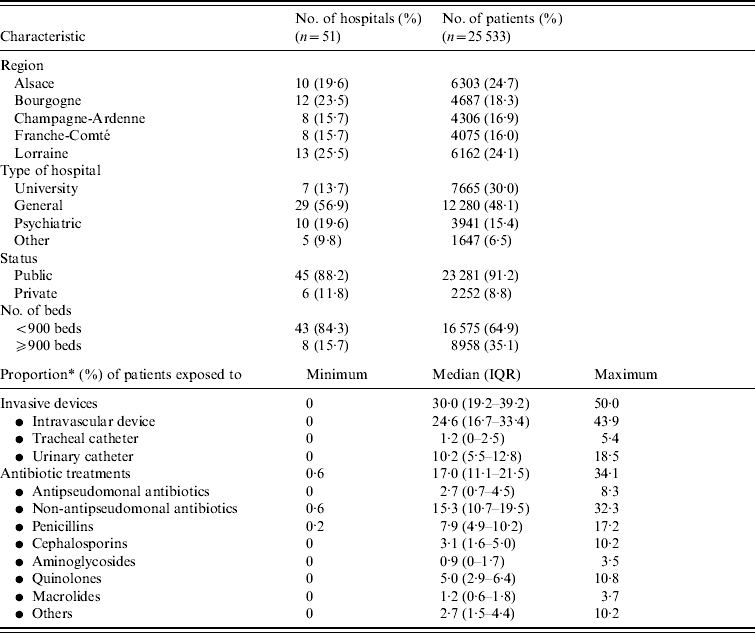
IQR, Interquartile range.
* Proportion (%) of patients exposed on the day of the survey to at least one invasive device [intravascular device, tracheal catheter or urinary catheter (within 7 previous days)], at least one antibiotic treatment.
Table 3. Univariate analyses of patient- and healthcare facility (HCF)-level factors associated with Pseudomonas aeruginosa infection
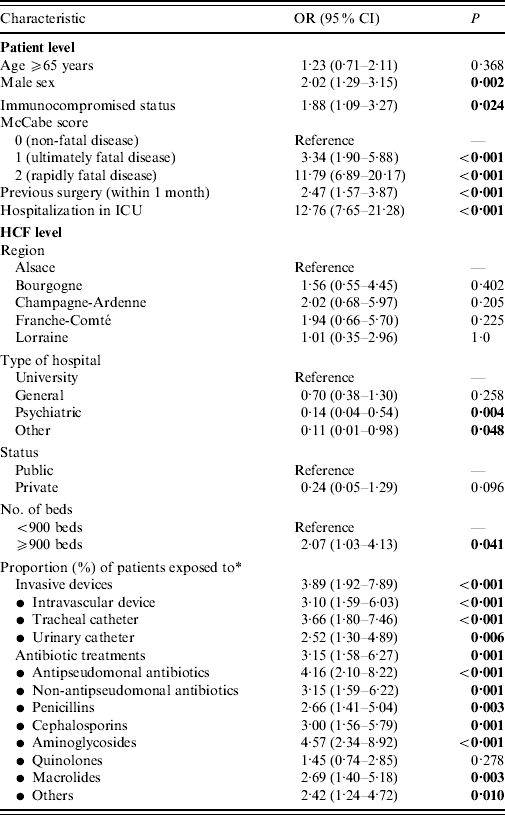
OR, odds ratio; CI, confidence interval; ICU, intensive care unit.
Bold values are significant.
* Odds ratios are reported for proportions higher than median.
Multilevel modelling
Results of multilevel modelling are presented in Table 4. First, model 1 (no explanatory variables, i.e. with only random intercept) revealed a significant heterogeneity of HCFs in term of prevalence of cases (σu02, HCF-level variance was significantly different from zero). VPC was equal to 14% and represented the initial share of total variance (heterogeneity) of outcome variable attributable to HCF level. Second, after adjusting for patient-level variables (model 2, individual model), HCF-level variance decreased and became non-significantly different from zero, i.e. there was no more significant heterogeneity between HCFs. PCV was equal to 52% [(0·533–0·258)×100/0·533]. In other terms, patient-level variables retained in model 2 explained 52% of HCF-level variance. Moreover, VPC was equal to 7·3% in the adjusted model for patient-level variables. No significant random effect was detected regarding patient-level variables, i.e. effects (coefficients) of these variables did not significantly vary across HCFs. Third, the three final models showed that on the one hand the following patient-level variables were independently and positively associated with prevalence of cases: male sex (P<0·05), McCabe score ⩾1 (P<0·001), a previous surgery (P<0·01) and a hospitalization in ICU (P<0·001). On the other hand, HCFs with a high proportion of patients exposed to invasive devices (model 3a, P<0·05), antipseudomonal antibiotics (model 3b, P<0·01) and aminoglycosides (model 4, P<0·01) were positively associated with outcome variable. Finally, the model-building process led to random-intercept and fixed-coefficient models (final models). No significant cross-level interaction was identified.
Table 4. Multilevel logistic regression models of patient and healthcare facility (HCF) characteristics associated with Pseudomonas aeruginosa infection [adjusted odds ratio (OR) and 95% confidence interval (CI)]
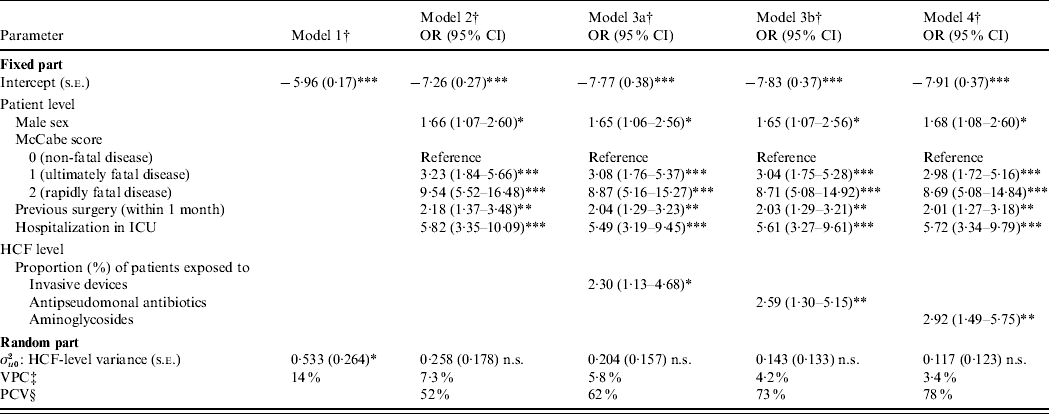
ICU, Intensive care unit; n.s., non-significant; s.e.: standard error.
† The model-building strategy used a forward stepwise selection process of patient- and HCF-level factors. Model 1 (unconditional model): without covariates, baseline. Model 2 (individual model): includes only patient-level variables. Three final models: model 3a (includes invasive devices and antibiotic treatments on the whole), model 3b (includes invasive devices on the whole and antipseudomonal/non-antipseudomonal antibiotics) and model 4 [includes separately each type of invasive devices (intravascular device, tracheal catheter, urinary catheter) and each class of antibiotics (penicillins, cephalosporins, aminoglycosides, quinolones, macrolides and others)].
‡ VPC: variance partition coefficient (%), [calculated using Snijders & Bosker approximation, σu02/(σu02+π2/3)].
§ PCV: proportional change in variance (%), [(σu02 (model 2)−σu02 (model 1)×100)/σu02 (model 1)], for model 2 in this case.
* P<0·05, ** P<0·01, *** P<0·001 (P values are from Wald χ2 test).
DISCUSSION
In this study, we tried by means of a complex statistical approach to shed new light on the respective contribution of patient- and ecological-level factors associated with P. aeruginosa infections. Thus, we used multilevel modelling in order to quantify higher-level variations (at HCF level), some of which could be explained by the heterogeneity of the effect of patient-level factors (across HCFs) and HCF-level factors. This statistical approach makes possible a simultaneous and appropriate analysis of patient- and HCF-level characteristics [Reference Diez Roux and Aiello21]. Indeed, the implementation of multilevel logistic regression models has the following advantages: the correction of underestimation of standard errors (due to clustered data), the examination of cross-level interactions, the estimation of the variability of coefficients at the HCF level, the determination of distribution of total variance of outcome variable between patient and HCF levels, and the analysis of contextual effects after adjusting for individual variables. The unconditional model (model 1) showed that 14% (VPC) of variability of the outcome variable was explained by differences between HCFs. After adjusting for patient-level variables (model 2), VPC was equal to 7·3% and residual variance (HCF-level variance) decreased and became non-significantly different from zero. This is an indication of a substantial compositional effect explaining the heterogeneity of the prevalence of cases in the HCFs in eastern of France. Moreover, at the patient level, factors significantly associated with prevalence of cases were consistent with those reported in previous studies [Reference Morrison and Wenzel1, Reference Cross22]. Furthermore, in the full model 3a, the proportion of patients exposed to ‘invasive devices’ was positively associated with the outcome variable after adjusting for patient factors (control for ecological bias). This can be interpreted as a significant association between the level of invasive procedures of the hospital and prevalence of cases. Similarly, proportions of patients exposed to ‘antipseudomonal antibiotics’ (model 3b) especially ‘aminoglycosides’ (model 4) were positively associated with outcome variable. These findings can be explained by the fact that HCFs with higher prevalence of P. aeruginosa more often use antipseudomonal antibiotics. Another possible interpretation, which seems predominant, is that these HCFs are probably those with higher prevalence of multidrug-resistant strains. This hypothesis is sustained first by the significant and positive association (P=0·021) between a high prevalence of patients infected with ceftazidime-resistant P. aeruginosa and a high proportion of patients exposed to ceftazidime in HCFs. Second, when we included a binary variable at the HCF level ‘high vs. low prevalence of patients infected with P. aeruginosa’ in final models (not shown), a high proportions of patients exposed to antipseudomonal antibiotics, aminoglycosides and invasive devices remained significantly and positively associated with outcome. These variables regarding antibiotic exposures can be considered as estimations of antibiotic selective pressure which provides P. aeruginosa with a selective growth advantage. Several individual-level studies demonstrated a significant and positive association between exposure to some antipseudomonal agents especially aminoglycosides and acquisition of P. aeruginosa [Reference Bonten9, Reference Martinez23–Reference Fortaleza25]. In our study, this association involving aggregated data at the HCF level could simply be the reflection of the association identified at the patient level. The present study has several limitations. First, data are from a cross-sectional study (point prevalence survey). Thus, some findings, particularly the major role of compositional effect on the heterogeneity of HCFs for prevalence of infected patients with P. aeruginosa, should be confirmed using multilevel longitudinal studies. In addition, the outcome variable concerned infected patients, and not the acquisition (colonized and/or infected patients) of P. aeruginosa. Second, available data did not allow us to determine the intermediate level between the patient level and the HCF level, i.e. a middle level such as the unit of hospitalization. The latter would make it possible to better understand and model the patients' close environment (i.e. other patients, healthcare workers and inanimate hospital environment). Only the type of hospitalization ward was known and the variable ‘hospitalization in ICU’ which characterizes a diagnostic as well as this middle level was collapsed to patient level. However, no significant random effect at HCF level was identified with this factor. In other terms, the coefficient of the variable ‘hospitalization in ICU’ did not significantly vary across HCFs (33/51 HCFs had at least one ICU). Moreover, this variable was potentially biased because in some cases, patients can be admitted to the ICU as a consequence of a severe P. aeruginosa infection. Third, no data were available to assess some contextual effects that could modify the risk of cross-transmission such as colonization pressure (other patients colonized by P. aeruginosa or contamination of hospital environment) and nurse-to-patient ratio. These unobserved variables are part of the unexplained heterogeneity between HCFs. Fourth, some individual variables were not taken into account or were included as aggregated data at HCF level, for previously stated reasons. Indeed, some invasive devices (endotracheal tube, central venous catheter) are risk factors for P. aeruginosa infections [Reference Morrison and Wenzel1, Reference Cross22] and can illustrate the severity of underlying illness. However, these exposures were collected only for the day of the survey (except for the urinary catheter) and nothing was recorded regarding the chronology of exposure in relation to infection. This means, that it was impossible to unequivocally characterize the association of these exposures with the outcome; ‘possible cause or consequence’. This work was limited to HCFs with at least 300 beds. This restriction was informed by findings from Moineddin et al. [Reference Moineddin, Matheson and Glazier26] who recommended for low prevalence events (<10%) at least 50 groups with group size (i.e. no. of individuals per group) adjusted such that the expected number of events in each group should be >1. In our study, we tried to comply with this recommendation. To our knowledge, this is the first study which investigates, at two levels (patient and HCF), factors associated with P. aeruginosa infection using a multilevel statistical approach.
In conclusion, multilevel analysis results suggest that a compositional effect (patient factors) rather than a contextual effect (ecological factors) explains the heterogeneity of the prevalence of patients infected with P. aeruginosa in HCFs in the eastern regions of France. This finding is in line with studies that advocate a major role of endogenous flora in P. aeruginosa infections.
ACKNOWLEDGEMENTS
The authors thank the French hospitals that contributed to data collection and the nosocomial infections surveillance network of eastern regions of France [CCLIN-Est (Centre de Coordination de la Lutte contre les Infections Nosocomiales de l'Est de la France)].
DECLARATION OF INTEREST
None.








