Introduction
Invasive mammals are major drivers of extinction and ecosystem change. Nowhere is this more apparent than on islands, on which more than half of all historical vertebrate extinctions have occurred (Aguirre-Muñoz et al., Reference Aguirre-Muñoz, Croll, Donlan, Henry, Hermosillo and Howald2008). Rats (Rattus sp.) have been introduced to > 80% of the world’s island groups and are associated with the extinction or decline of insular plants, vertebrates and invertebrates (Towns et al., Reference Towns, Atkinson and Daugherty2006). In response to these negative impacts techniques to eradicate rats using rodenticide baits were developed in New Zealand over 3 decades ago (Towns & Broome, Reference Towns and Broome2003) and since then there have been more than 332 successful rat eradications worldwide (Howald et al., Reference Howald, Donlan, Galván, Russell, Parkes and Samaniego2007). The biodiversity benefits of those rat eradications are increasingly well documented (Towns et al., Reference Towns, Atkinson and Daugherty2006; Donlan & Heneman, Reference Donlan and Heneman2007). Practitioners are now eradicating invasive rats from larger and more biologically diverse islands. As they do so they face the challenge of mitigating for potential non-target impacts on native insular biodiversity that may be susceptible to rodenticides.
Anticoagulant rodenticides are the most widely used toxin for rodent control (Eason et al., Reference Eason, Murphy, Wright and Spurr2002; Hoare & Hare, Reference Hoare and Hare2006). They act by inhibiting the synthesis of vitamin K-dependent clotting factors in the liver, which ultimately results in death by internal haemorrhaging, typically within 3–10 days (Hadler & Sahdbolt, Reference Hadler and Sahdbolt1975). The anticoagulant brodifacoum (3-[3-(4′-bromobiphenyl-4-yl)-1,2,3,4-tetrahydro-1-naphthy]-4 hydroxycoumarin) is the preferred rodenticide for invasive rodent eradications (Howald et al., Reference Howald, Donlan, Galván, Russell, Parkes and Samaniego2007). Brodifacoum is more potent than other anticoagulants: a single feeding of a few grams of bait can be lethal (Eason et al., Reference Eason, Murphy, Wright and Spurr2002). The high toxicity of brodifacoum is an advantage in eradicating target species; however, that same toxicity makes non-target species more vulnerable. In particular, native insular rodents, granivorous birds and raptors are susceptible to primary and secondary brodifacoum poisoning during eradication operations (Empson & Miskelly, Reference Empson and Miskelly1999; Howald et al., Reference Howald, Mineau, Elliott and Cheng1999; Eason et al., Reference Eason, Murphy, Wright and Spurr2002).
Here we report on the eradication of black rats Rattus rattus from Anacapa Island, US Channel Islands National Park, California, which was the first invasive rodent eradication from an entire island where an endemic rodent was present. The rodent eradication was consistent with the National Park’s general management plan to restore altered ecosystems. The eradication was further justified to improve nesting seabird habitat and aid in the recovery of Xantus’s murrelet Synthliboramphus hypoleucus scrippsi, a Californian and Federal Species of Special Concern (US National Park Service, 2000). The Anacapa deer mouse Peromyscus maniculatus anacapae presented the challenge of eradicating invasive rats without eradicating an endemic subspecies equally susceptible to rodenticide exposure. This was also the first aerial application of a rodenticide in North America. We describe the planning, environmental compliance, legal challenges, environmental monitoring and methods employed, including actions taken to mitigate for non-target impacts. Details of the captive holding, reintroduction and demography of deer mice will be reported elsewhere (Gellerman, Reference Gellerman2007; B. Tershy et al., unpubl. data). The potential impacts and benefits of the rat eradication on the reptiles and amphibians of Anacapa Island were documented and will also be reported elsewhere (T. Comendent et al., unpubl. data).
Study area
Anacapa Island consists of three islets: East (43 ha), Middle (71 ha) and West (182 ha; Fig. 1). Black rats were introduced to Anacapa Island sometime before 1939, probably in supplies transported onto the island or from a shipwreck (Collins, Reference Collins and Power1979). Rats were having negative impacts on the Xantus’s murrelet, as well as on terrestrial and intertidal marine invertebrates (Erickson & Halvorson, Reference Erickson and Halvorson1990; Jones et al., Reference Jones, Henry, Howald, Tershy and Croll2005; Whitworth et al., Reference Whitworth, Carter, Young, Koepke, Gress and Fangman2005). Rats compete with native rodents in the Galapagos Islands (Harris & Macdonald, Reference Harris and Macdonald2007) and have been observed depredating deer mice on Anacapa Island (Gellerman, Reference Gellerman2007).
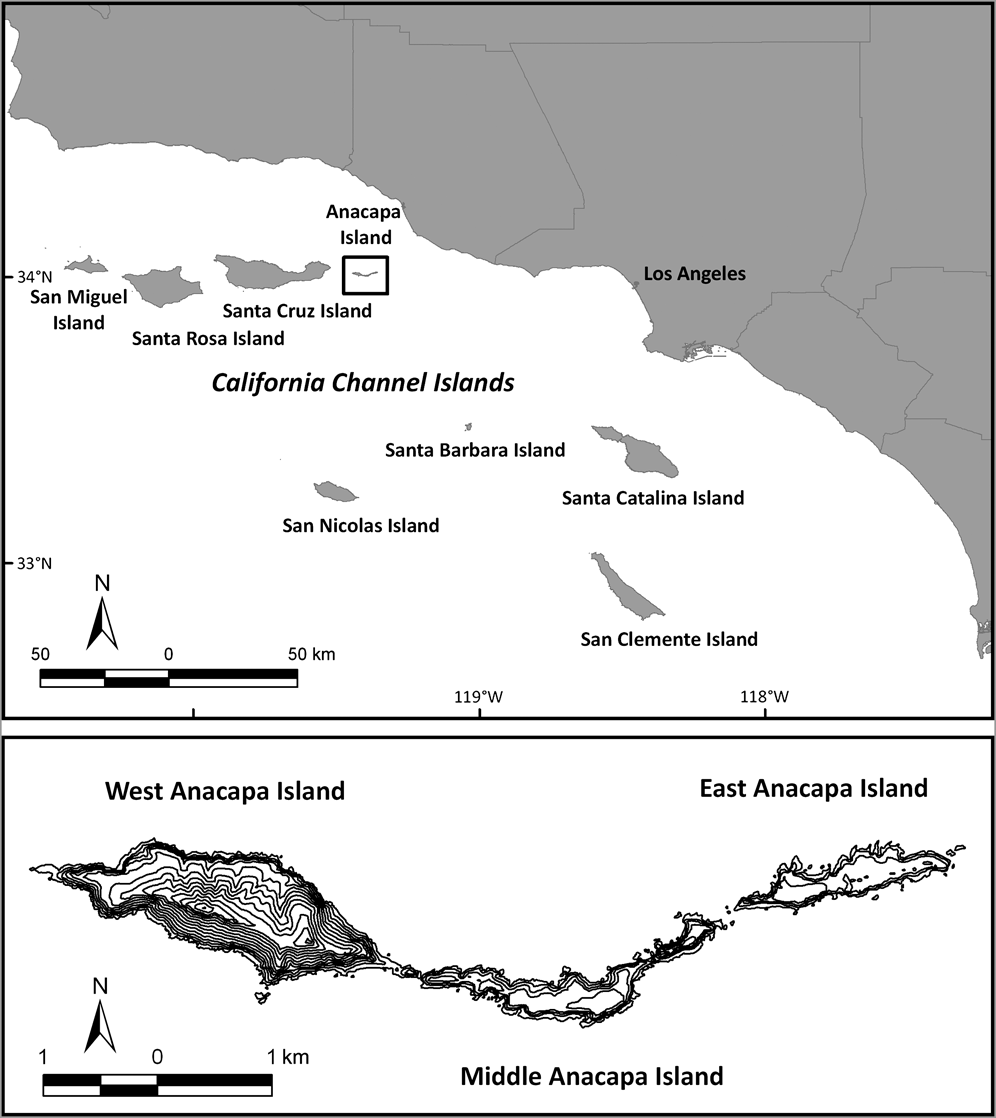
Fig. 1 Located off southern California, Anacapa Island is part of the US Channel Islands National Park. The rodenticide was broadcast over the three islets in two stages: East Anacapa in 2001 and West and Middle Anacapa in 2002.
Despite the island’s small size, a number of factors presented challenges to rat eradication on Anacapa Island, including (1) steep and rugged topography, (2) presence of legally protected breeding seabirds (California brown pelicans Pelecanus occidentalis californicus), which limited physical access to portions of the island, and (3) presence of native species susceptible to rodenticide, particularly granivorous land-birds and the Anacapa deer mouse. Pre-eradication trials revealed that the latter was equally susceptible to and competed with rats for bait, making it difficult to deliver bait to all the rats without making it available to the endemic mice. Because of these challenges, we developed an overall strategy and specific techniques to limit potential environmental impacts while ensuring that enough bait would be delivered to eradicate rats.
Background and methods
Planning, bait delivery and eradication efficacy
Aerial spread of a rodenticide on Anacapa Island required compliance with a host of environmental laws, a process that began > 2 years prior to the first rodenticide application (Fig. 2). Under the US National Environmental Policy Act an Environmental Impact Statement was undertaken, and a 3-year quarantine exemption registration for an aerial broadcast of bait containing brodifacoum was granted by the Environmental Protection Agency (US National Park Service, 2000; Howald et al., Reference Howald, Faulkner, Tershy, Keitt, Gellerman, Creel, Garcelon and Schwemm2005). The project was not subject to any specific animal welfare legislation; however, best practices were followed for all project activities, including captive breeding, bait applications and monitoring (US National Park Service, 2000; Gellerman, Reference Gellerman2007).
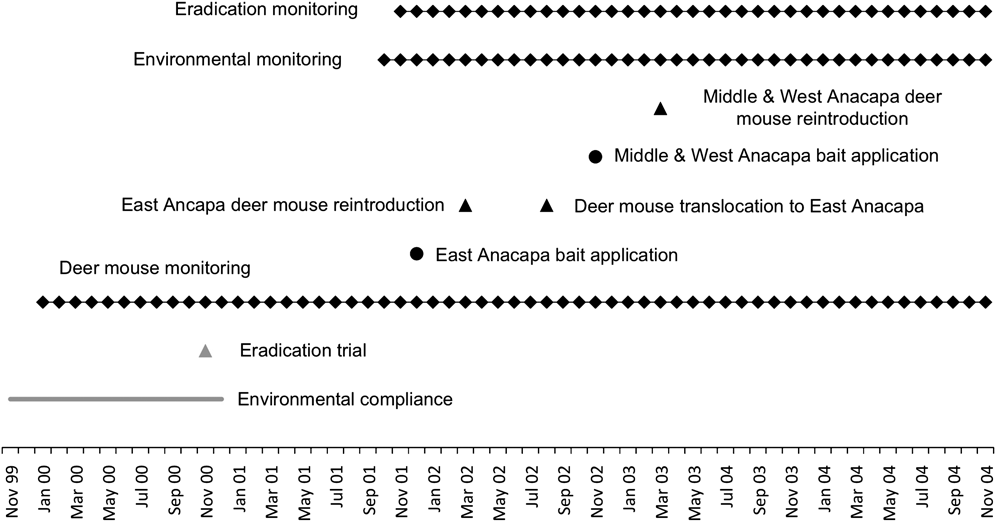
Fig. 2 Timeline of planning, implementation and mitigation during the rat eradication on Anacapa Island (Fig. 1).
Local animal rights groups voiced their opposition to the rat eradication. Primary issues raised were the killing of animals and the aerial application of the rodenticide. Shortly before the rodenticide application an animal rights organization notified the National Park Service that it intended to request a temporary restraining order. The Park Service agreed to delay the rodenticide application until after the court hearing. The plaintiffs alleged that the rat eradication was in violation of the National Park Service Organic Act, the National Environmental Policy Act and Migratory Bird Treaty Act; their legal case focused particularly on the latter. The Migratory Bird Treaty Act prohibits the taking of migratory birds without a permit issued by the US Fish and Wildlife Service. While the latter did not consider the unintentional mortality of migratory birds to fall within the permitting requirements of the Migratory Bird Treaty Act, the Park Service subsequently applied for and was granted a Migratory Bird Treaty Act permit based on the expected benefits from the rat eradication to migratory birds. The US District Court issued a memorandum opinion denying the plaintiffs’ request for a preliminary injunction (US District Court for the District of Columbia, Judge Ellen Segal Huvelle, 29 November 2001).
Eradication of rats is only possible if sufficient bait containing rodenticide is delivered to every rat territory on an island. Because of steep cliffs on Anacapa, the rodenticide was broadcast aerially by helicopter (Plate 1). Bait consisted of green, non-waxed compressed grain pellets (c. 1–2 g, 9 mm diameter) containing 25 ppm brodifacoum (see US National Park Service, 2000, for a discussion on bait selection). Both laboratory and field trials demonstrated that the bait was palatable and lethal to rats (Howald et al., Reference Howald, Faulkner, Tershy, Keitt, Gellerman, Creel, Garcelon and Schwemm2005). Bait was broadcast at 15 kg ha-1 from an agricultural hopper suspended under a Bell 206 helicopter fitted with an on-board differential global positioning system (GPS). The shoreline and steep cliffs near the ocean were treated with a specially designed deflector fitted to the hopper that delivered bait to one side only, preventing deliberate bait spread into the marine environment. Bait stations were used in a designated ‘no-drop’ zone to mitigate for non-target impacts (see below). They were also employed in and around the few human structures on the island to ensure that bait was always available in areas with potential alternative food resources.

Plate 1 (a) Delivery of rodenticide bait by helicopter on Anacapa Island. (b) On-island captive-holding facility that held native deer mice Peromyscus maniculatus anacapae during rodenticide applications; deer mice were later reintroduced to the island following the rat eradication. (c) Peregrine falcons Falco peregrinus were live captured and held during rodenticide applications to prevent non-target impacts from secondary poisoning.
We applied bait during the dry season (November–December) when the rat population was probably food stressed and thus more likely to consume bait (Erickson & Halvorson, Reference Erickson and Halvorson1990). We staggered bait delivery (Figs 1 & 2), beginning with a 2.5-ha trial rodenticide application on Middle Anacapa in November 2000 (Howald et al., Reference Howald, Faulkner, Tershy, Keitt, Gellerman, Creel, Garcelon and Schwemm2005). Next, bait was broadcast on East Anacapa in December 2001 and the eastern section of Middle Anacapa, the latter to reduce the possibility of rat movement between the islets. Finally, bait was broadcast on Middle and West Anacapa in November 2002. To prevent rats moving from Middle to East Anacapa between December 2001 and November 2002, we maintained rodenticide bait stations along opposite coastlines of East and Middle Anacapa.
We used live trapping, wax chew blocks and radio telemetry to assess eradication efficacy. We conducted live trapping in targeted areas before and after rodenticide applications. Tomahawk live traps baited with peanut butter were placed every 15–25 m along transect lines located across the islets in relatively accessible areas. Deer mice were able to move freely through the traps because of the large mesh size and were not captured. Flavoured wax chew blocks were placed along transect lines in areas with relative ease of access across the islets or in conjunction with live traps. Incisor width was used to distinguish between black rats and deer mice. Trapping and chew block data are reported as percentage trap success or detection [number of rats trapped (number rats or deer mice detected)/number of trap nights (number of chew block nights)]. Effort was corrected for sprung traps with no animal caught and for melted chew blocks.
Thirty-four radio-collared rats were followed before and after rodenticide applications. Rats were live captured, anaesthetized with isoflurane (IsoFlo), fitted with a radio collar (AVM Instruments, Livermore, USA) programmed to a unique frequency, and released. We attempted to locate each rat nightly to determine its approximate position and whether it was active. Prior to rodenticide applications, burrow locations were mapped by tracking signals during daylight hours when rats were not active. This facilitated locating rats that died below ground.
Environmental monitoring
To evaluate the movement of brodifacoum on Anacapa Island and the toxicological risks and impacts on non-target species we studied multiple aspects of the marine and terrestrial ecosystems. We placed groups of 10 rodenticide bait pellets in rodent-proof cages, which were placed haphazardly in different habitats on the islets, and then sampled them at 1 week, 6 weeks and 6 months. The 10 bait pellets were homogenized into one sample and tested for brodifacoum.
Brodifacoum levels in the soil, sampled over time, indicated its potential movement in the ecosystem. We collected soil samples at 1, 3, 6 and 12 months following rodenticide applications. We haphazardly sampled and analysed the top 5 cm of soil collected from directly under a bait pellet (or from an area marked where a pellet had resided, for the later time periods). In addition, we haphazardly collected samples of grasses (Poaceae) on Anacapa 6 weeks after the rodenticide applications for brodifacoum testing.
Because brodifacoum does not persist in invertebrate tissues (Booth et al., Reference Booth, Eason and Spurr2001) its presence is indicative of recent exposure and thus serves as a proxy for the biological availability of the compound. We haphazardly collected samples of the terrestrial arthropod community found under rocks and shrubs. We collected samples (5 g each) at c. 5, 30, 90 and 180 days post-rodenticide application; these were homogenized, chemically cleaned, and frozen.
We assessed unintentional bait drift into the coastal environment using divers and with land- and boat-based observers. Two divers each observed a c. 500-m area at seven separate locations during the rodenticide applications; sites were selected under steep cliffs where it was more likely that bait would accidentally enter the marine environment. If bait was detected, behavioural observations of the fish and invertebrates in the surrounding area were made along with the estimates of density and condition of the bait pellets. We collected ocean water samples (500 ml) for brodifacoum analysis at 24 and 48 hours following rodenticide applications. Concurrently, we haphazardly sampled intertidal marine organisms from five locations for the presence of brodifacoum at 15, 30 and 90 days following rodenticide applications.
Terrestrial native vertebrates are at risk of inadvertent exposure to rodenticide by consuming bait directly or from contaminated prey items. To assess these primary and secondary impacts we sampled rats, deer mice, passerines, raptors and seabirds for brodifacoum. Rats were haphazardly collected below and above ground; liver, gastrointestinal tract and whole carcass samples were tested. Deer mice were also opportunistically collected and whole carcass samples were tested. Following rodenticide applications we conducted systematic searches across East and Middle Anacapa to collect carcasses of terrestrial passerines, raptors and seabirds. Bird livers were tested. In addition, we systematically conducted carcass searches in 46 10-m-diameter circular plots on all three islets during the 45 days following rodenticide applications. We also collected carcasses along established random transects on Middle and West Anacapa for up to 82 days following rodenticide applications.
Water samples were refrigerated and shipped to the US California Department of Fish and Game Pesticide Investigation Unit (Rancho Cordova, California) for brodifacoum analysis. All other samples were frozen and shipped to the Illinois Department of Agriculture (Centralia, Illinois) for brodifacoum analysis. The samples were processed following Hunter (Reference Hunter1983). The limit of brodifacoum detection was 0.001–0.07 ppm (or the equivalent mg kg-1). Results are reported on a wet weight basis.
Mitigating non-target impacts
Our mitigation plan for the Anacapa deer mouse was based on the established genetic connectivity of the mouse populations between the three Anacapa islets, and a population viability analysis specifically tailored to develop a management plan (Pergams et al., Reference Pergams, Lacy and Ashley2000). To ensure the presence of two viable deer mouse populations throughout the eradication, we staggered the rodenticide application over 2 years so that a wild population was always present on one or more islets (Fig. 1), and held a representative sample of the mouse population in captivity (Plate 1). An on-island captive-holding facility was constructed to hold captive deer mouse populations during and following rodenticide applications. Captive populations were reintroduced to the wild the following spring, and supplemental feed (Purina Mouse Breeder Chow #5015) was provided on East Anacapa in an effort to enhance survival (Bright & Morris, Reference Bright and Morris1994). With the exception of a single broadcast of feed at the release sites just prior to the reintroduction, no supplemental feeding took place on Middle and West Anacapa (Gellerman, Reference Gellerman2007).
We closely monitored deer mouse populations throughout the eradication campaign. We established two live-trapping grids each on East and Middle Anacapa and sampled them on a monthly basis during 2000–2004, with sampling gaps during winter months. We sampled two additional grids each on East Anacapa (2002) and Middle Anacapa (2003) for 1 year following reintroduction. Restricted access because of breeding seabirds limited sampling efforts on West Anacapa to the autumn only, and only a single grid was sampled. Trapping grids consisted of 100 Sherman traps (23 × 8 × 10 cm) with 7-m spacing. Traps were baited with rolled oats and polyester fill was added to provide insulating nesting material. Mice were trapped for 3 consecutive nights each month.
While all bird species known from Anacapa Island are also found on other Channel Islands or on the adjacent mainland, we took three explicit measures to minimize potential non-target impacts: (1) colouring and sizing the rodenticide bait to deter gulls and granivorous birds from consuming it (Day & Matthews, Reference Day and Matthews1999), (2) live trapping and captive holding (or translocating) resident raptors prior to rodenticide applications, and (3) the establishment of a 15-ha no-drop zone on West Anacapa to create a refuge for granivorous birds, particularly the Santa Cruz Island rufous-crowned sparrow Aimophila ruficeps obscura, which was suspected of being particularly vulnerable to non-target poisoning because of its sedentary nature and granivorous diet. This subspecies is endemic to the Channel Islands and breeds on Santa Cruz Island and West and Middle Anacapa (Collins, Reference Collins and Poole1999). Within the no-drop zone rats were eradicated using bait stations that were inaccessible to granivorous birds, thereby reducing the probability of them ingesting bait (Thomas & Taylor, Reference Thomas, Taylor, Veitch and Clout2002).
We monitored rufous-crowned sparrows on West Anacapa using wandering surveys and playback recordings in areas of the islet known for high sparrow densities. The no-drop zone and parts of West Anacapa were monitored pre- and post-rodenticide application in 2002 and in the autumn of 2003–2005 and 2008. The surveys and their replication were constrained because of logistics and weather. In addition, restricted access to the islet because of breeding seabirds prohibited surveys in some locations and during certain time periods, including the spring breeding season. In addition to wandering surveys, we conducted playback surveys using standardized transects during the rodenticide application (November–December 2002). Every 100 m along transects (0.5–1.5 km) researchers would wait 1 minute, play songs, wait another minute, play an alarm call and wait an additional minute. All individual birds that responded were recorded.
Results
Planning, bait delivery and eradication efficacy
Bait was broadcast by helicopter in December 2001 and November 2002 on East and Middle/West Anacapa, respectively (Fig. 2). The on-board differential GPS ensured that bait was applied to all areas of the islets and identified areas that needed supplemental baiting by hand. Areas around shorelines, including caves, were baited by hand using small boats for access.
Of the radio-collared rats known active just prior to the rodenticide applications, all stopped moving between 0 and 14 days post-application (East Anacapa, mean = 6.3 days, n = 18; Middle/West Anacapa, mean = 3.9 days, n = 10). Of the rats whose fate was followed (n = 28), 71% died underground. Observations, however, suggest that an even higher percentage of the rat population died underground. Two rats showed direct evidence that they had been scavenged or preyed on. Monthly rat trapping success fluctuated between 0 and 55% prior to rodenticide applications (Fig. 3). Following the rodenticide applications, there was no sign of rats in 21,382 trap nights and 17,218 wax chew block nights (Fig. 3).

Fig. 3 The efficacy of the Anacapa Island (Fig. 1) rat eradication campaign. (a) Rat trapping success on East, Middle and West Anacapa before and after rodenticide applications. (b) Percentage detection of rats using chew blocks concurrently with the trapping efforts. (c) Percentage detection of native deer mice before and after the bait applications. The grey line in each panel shows the cumulative effort in trap or block nights.
Environmental monitoring
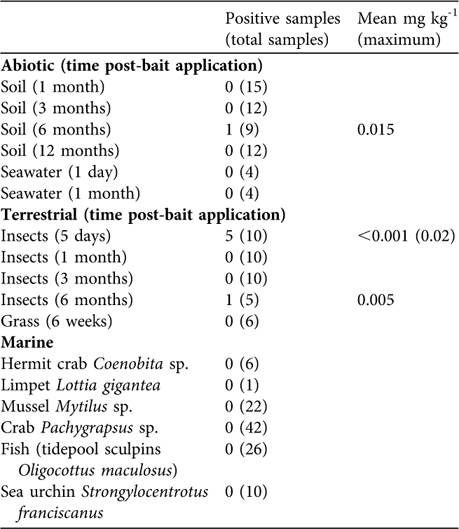
Brodifacoum concentration in the bait pellets declined by > 90% in a 6-month period (Table 1). Of the 48 soil samples only one taken at 6 months post-rodenticide application tested positive for brodifacoum (Table 2). Seventeen percent of terrestrial insect samples tested positive for brodifacoum, whereas all vegetation samples were negative (Table 2). No bait was observed being spread directly into the ocean. Small amounts of bait were observed entering the ocean indirectly by bouncing off cliffs. Divers detected bait entering the marine environment at three locations; densities were estimated at 0.15 pellets m-2. Neither fish nor marine invertebrates were observed consuming the bait. Seawater and marine invertebrates tested negative for brodifacoum residues (Table 2). Bait in the ocean had completely dissolved within 5 hours.
Table 1 Brodifacoum residue decline in degrading bait pellets. The initial concentration of toxin in bait pellets was 25 ppm. Mean ppm (± SD) is shown for samples in the natural environment. Each replicate was 10 bait pellets homogenized into one sample.

Table 2 Movement of brodifacoum into the abiotic, terrestrial and marine environments following rodenticide applications, with number of positive samples and mean mg kg-1 (maximum) concentrations.
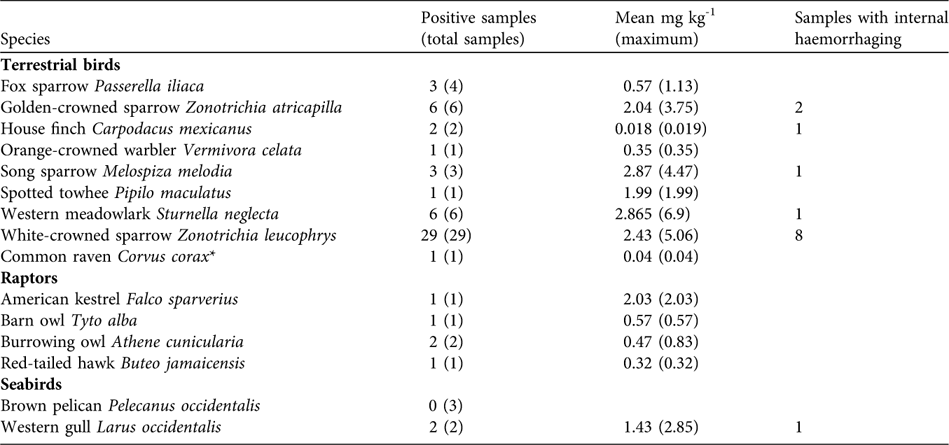
As expected, rats and mice collected following rodenticide applications showed high levels of brodifacoum (mean mg kg-1: rat liver, 9.7 ± SD 2.4, n = 8; rat gastrointestinal tract, 1.5 ± SD 0.88, n = 5; rat carcass, 0.90 ± 0.18, n = 5; mouse carcass, 2.7 ± SD 1.1, n = 10). A total of 94 birds (16 species) were identified from carcass searches following rodenticide applications (49 in 2001 and 45 in 2002). Of the 63 birds tested for brodifacoum, 59 (94%) tested positive. All raptor carcasses collected tested positive for brodifacoum, as did two western gull Larus occidentalis carcasses and many passerines (Table 3).
Table 3 Brodifacoum concentrations in terrestrial birds, raptors and seabirds collected during carcass searches following rodenticide applications, with number of positive samples, mean mg brodifacoum kg-1 (maximum), and number of samples with internal haemorrhaging.
* Serum tested
Minimizing non-target impacts
As expected, the majority, if not the entirety, of the deer mouse population was killed along with black rats (Figs 3 & 4). Prior to the East Anacapa rodenticide application 185 deer mice were live captured and held in captivity. Five months following the rodenticide application (April 2002) the captive population of 174 mice was reintroduced to East Anacapa (Fig. 2). The release date coincided with the beginning of the breeding season along with lunar dark phases to minimize risk from nocturnal raptor predation. In addition, 60 kg of supplemental feed was hand broadcast throughout the release areas. Prior to the second rodenticide application 373 and 365 deer mice were captured from Middle and West Anacapa, respectively, and held in captivity. Concurrently, 715 and 308 mice from Middle and West Anacapa, respectively, were captured and translocated to East Anacapa (Fig. 2). In March 2003, 358 and 360 captive mice were reintroduced to Middle and West Anacapa, respectively.
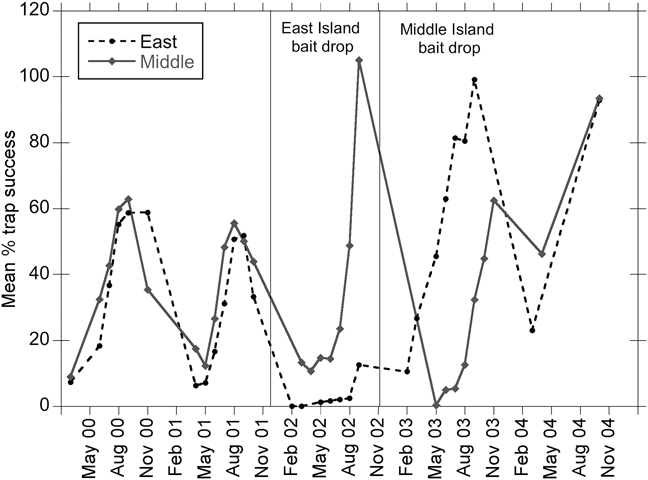
Fig. 4 Mean percentage trap success for deer mouse on monitoring grids on East and Middle Anacapa before and after bait applications. Trap success is > 100% because of multiple animals caught in a single trap.
On the monitoring grids deer mouse populations declined to zero following rodenticide applications and then recovered to pre-eradication levels or higher following the reintroduction of captive populations (Figs 3 & 4). East and Middle Anacapa showed similar dynamics: cyclical trap success with peaks in the autumn and higher trap success post-rodenticide application compared to the previous 2 years (Fig. 4). The West Anacapa grid was sampled twice prior and once 2 years after the rodenticide application and provided additional evidence of recovery (trap success: pre-eradication, November 2000 = 37% and November 2001 = 41%; post-eradication, November 2004 = 60%).
Approximately 68% of the known raptors were live captured prior to rodenticide applications (37 birds in total, including eight peregrine falcons Falco peregrinus, nine red-tailed hawks Buteo jamaicensis, four barn owls Tyto alba and six burrowing owls Athene cunicularia; Howald et al., Reference Howald, Faulkner, Tershy, Keitt, Gellerman, Creel, Garcelon and Schwemm2005). Most were released on the mainland in suitable habitat; peregrine falcons were held and released back onto Anacapa 3 weeks after rodenticide applications. Some raptors not captured, including a burrowing owl, survived the rodenticide applications. Three barn owls, six burrowing owls and an American kestrel Falco sparverius either died in captivity or were found dead during carcass searches; all tested positive for brodifacoum (Table 3).
Evidence suggests that resident rufous-crowned sparrows experienced significant mortality from the rodenticide application. Transect surveys the month before and after the rodenticide application on West Anacapa indicated a decline (mean number of birds detected per transect (total number of birds detected, total number of transects) October/November 2002 = 1.15 (103, 90); November/December 2002 = 0.47 (77, 190)). Although quantitative abundance estimates prior to the bait application were not available, wandering surveys, in areas known anecdotally for high densities of sparrows, over the month following the bait application also indicated a decline: five surveys revealed only 11 birds. Monitoring trips to West Anacapa in 2003, 2004 and 2005 (using wandering surveys) did not locate any rufous-crowned sparrows but are difficult to interpret because of rain, high winds and limited access. A survey in March 2008 revealed two pairs of rufous-crowned sparrows.
Discussion
Black rats were successfully eradicated from Anacapa Island. Live trapping and chew block data confirm the absence of rats along with successful mitigation for the native deer mouse. The successful Anacapa deer mouse mitigation plan was based on a priori research that recommended a captive population consists of a minimum of 1,000 individuals across all three islets to maintain population persistence with minimal loss of genetic diversity (Pergams et al., Reference Pergams, Lacy and Ashley2000). By taking captive populations and staggering the rodenticide applications over 2 years, two viable deer mouse populations were present at all times during the eradication. While deer mouse detection rates from both chew blocks and trapping grids fell to zero following rodenticide applications, it is equivocal whether deer mice were eradicated along with rats. Regardless, deer mice populations were severely depressed during the rodenticide applications and then recovered to pre-eradication levels following reintroduction. The recovery of the Anacapa deer mouse following the eradication demonstrates the feasibility of eradicating invasive rodents from islands with native rodents and, by extension, other susceptible native animals that can be held in captivity.
Like the Anacapa deer mouse, raptors and granivorous birds suffered impacts from the eradication; however, as observed with invasive rodent eradications elsewhere, those impacts are probably ephemeral (Howald et al., Reference Howald, Donlan, Galván, Russell, Parkes and Samaniego2007). Captive holding and translocation significantly reduced raptor mortality. Approximately 68% of all raptors present on Anacapa were taken into captivity; two of those 37 birds died, probably from brodifacoum poisoning (attempts to live-capture birds continued up to and during the rodenticide applications). All raptor carcasses collected post-eradication tested positive for brodifacoum (n = 5) and probably died of secondary poisoning. Peregrine falcons, which were reintroduced to the islets, were breeding 6 months after the rodenticide applications. Because of the close proximity to the mainland (20 km), other raptors were released at mainland sites. Multiple raptors were observed on Anacapa during a 1-day survey in March 2008, including sharp-shinned hawks Accipiter striatus, northern harriers Circus cyaneus, peregrine falcons and red-tailed hawks (R. Hamilton, pers. comm.).
Nearly all the passerines collected showed evidence of brodifacoum poisoning. While pre-eradication abundance estimates for passerines are lacking for Anacapa, evidence to date suggests the population-level impacts were ephemeral. Multiple passerine species that suffered from non-target impacts were observed during a brief survey in 2008 (R. Hamilton, pers. comm.). Non-target impacts on land-birds from invasive rodent eradication campaigns elsewhere have been short term (Howald et al., Reference Howald, Donlan, Galván, Russell, Parkes and Samaniego2007). Future surveys will reveal potential impacts or benefits from the rat eradication campaign.
Evidence suggests that rufous-crowned sparrows declined significantly following, and because of, rodenticide applications and were probably more susceptible than other passerines because of their resident year-round territories and granivorous diet. Unfortunately, our surveys and experimental design lacked the rigour to capture the dynamics and details of the decline. Captive holding or other mitigation measures may be necessary for sedentary granivorous passerines during future invasive rodent eradications. A similar no-drop zone approach was used as a mitigation strategy for fernbirds Bowdleria punctata wilsoni during the eradication of rats from Codfish Island, New Zealand. The majority of the fernbird population is thought to have died because of brodifacoum poisoning; few birds were recorded 2 years following the eradication but the population later recovered to pre-eradication abundance (McClelland, Reference McClelland, Veitch and Clout2002). The observed decline of the rufous-crowned sparrow suggests that more rigorous monitoring may be needed for some granivorous birds during eradication campaigns and also demonstrates the need for well-designed data-driven mitigations.
We observed no apparent impact on the marine environment during the eradication. During pre-eradication trials on Kapiti Island, New Zealand, three fish species consumed rodenticide bait pellets; however, concurrent laboratory rodenticide feeding trials and monitoring during the actual eradication found no impacts on the fish community (Empson & Miskelly, Reference Empson and Miskelly1999). An unintentional experiment occurred in New Zealand when a transport truck crash dumped 20 t of brodifacoum into the marine environment. Brodifacoum concentrations in water and sediment declined to < 0.02 ppb within 3–9 days, and concentrations in mussels Mytilus edulis peaked at 0.41 ppm 1 day after the spill and declined to < 0.02 ppb in 1 month (Primus et al., Reference Primus, Wright and Fisher2005). To date, there is no evidence that marine fish or invertebrates are at risk from invasive rodent eradications.
The detection of brodifacoum in arthropods was expected as they play an important role as detritivores. Arthropods and microbes remove rodenticide from the environment by digesting residual rodenticide pellets and rodent carcasses, ultimately breaking down brodifacoum to its non-toxic base components of water and CO2 (Shirer, Reference Shirer1992). While toxicity has been demonstrated with high levels of intake in the laboratory, impacts on terrestrial invertebrates have not been observed in natural settings and population-level impacts are unlikely (Howald et al., Reference Howald, Donlan, Galván, Russell, Parkes and Samaniego2007). Arthropods that tested positive for brodifacoum probably did so because of the presence of the compound in the gut; brodifacoum is not known to persist in terrestrial invertebrate tissue for any significant time (Booth et al., Reference Booth, Eason and Spurr2001). Thus, arthropods do represent a pathway of exposure for insectivores. The recovery of the deer mouse population, following reintroduction, supports the hypothesis that the persistence of brodifacoum levels in the invertebrate community was negligible on Anacapa Island. A concurrent study on the impacts of rodenticide applications on Anacapa reptile and amphibian populations also supports this hypothesis (T. Comendant, pers. comm.).
The conservation benefits of the eradication to seabirds were quickly realized. Exploiting the eradication campaign as an experimental manipulation, Jones et al. (Reference Jones, Henry, Howald, Tershy and Croll2005) documented elevated predation on Xantus’s murrelet by rats using artificial nests. A concurrent study documented the recovery of nesting Xantus’s murrelets on Anacapa following rat eradication: average hatching success increased from 42 to 80%, average nest predation decreased from 52 to 7%, and average nesting attempts more than doubled (Whitworth et al., Reference Whitworth, Carter, Young, Koepke, Gress and Fangman2005). Four months following the rodenticide applications Cassin’s auklet Ptychoramphus aleuticus, a seabird highly susceptible to rat predation and previously not documented as nesting on Anacapa Island, began nesting there (Whitworth et al., Reference Whitworth, Carter, Young, Koepke, Gress and Fangman2005).
The successful removal of black rats from Anacapa Island demonstrates that it is possible to mitigate safely for native small mammals that are susceptible to rodenticides. However, like raptors, some granivorous birds may require captive-holding efforts to minimize risk for non-target impacts during rodent eradication in some scenarios. The total cost of the eradication campaign was c. USD 1.8 million, which included eradication, monitoring, administration and legal fees. While non-target impacts were documented on a suite of native vertebrates during the eradication campaign, some evidence on Anacapa, along with observations from other rodent eradications, suggests that those impacts were ephemeral. Future monitoring, however, should reveal more details on the dynamics of those impacts. Likewise, the biodiversity benefits from removing rats from Anacapa Island will probably continue to reveal themselves for decades to come.
Acknowledgements
We thank the American Trader Trustees, including J. Boyce, C. Gorbics and P. Kelly, the entire Channel Islands National Park, Island Conservation and Aspen Helicopters staff, especially F. Rodriguez, D. Burgess, R. Brooks, R. Bidwell, S. Buckelew, E. Creel, M. Grinnell and K. Miskel, B. Latta and the Predatory Bird Group, R. Lacy and G. Alaks for providing expertise in the captive holding of mice, K. Hulvey, R. Hamilton, S. Morrison and O. Pergams, and C. McKinley and the legal departments of the Department of Interior and Department of Justice.
Biographical sketches
Gregg Howald is interested in island restoration programme development and he provides technical oversight of Island Conservation’s rodent eradication projects. C. Josh Donlan’s research interests include restoration, removal of invasive species, the economics of biodiversity conservation, and market-based conservation strategies. Kate R. Faulkner is responsible for stewardship of the five northern Channel Islands and the surrounding marine ecosystem. Her work includes the removal of non-native animals and plants, re-establishment of extirpated species and long-term ecological monitoring. Steve Ortega’s interests include planning, designing and implementing natural resource restoration projects for the National Park Service. Holly Gellerman’s research interests include the implementation of invasive species prevention, rapid response and early detection and monitoring programmes in California. Donald A. Croll’s research focuses on the foraging ecology of seabirds and marine mammals and the direct and indirect impacts of predator introductions and removals on island ecosystems. Bernie R. Tershy’s research focuses on conservation effectiveness and the ecology of seabirds and island ecosystems.












