Dengue is the most common mosquito-borne viral infection worldwide, affecting as many as 100 million people annually( Reference L’Azou, Moureau and Sarti 1 , Reference Wilson and Chen 2 ). The typical presentation of dengue is as a febrile illness with a variety of accompanying symptoms. In a few cases, the disease can progress to a life-threatening syndrome characterised by plasma leakage. The factors that induce progression from dengue fever (DF) to these potentially fatal forms of the disease, including dengue haemorrhagic fever (DHF) and dengue shock syndrome (DSS), are uncertain. They may involve secondary infections by new viral serotypes, agent virulence and host characteristics( Reference Guzman, Alvarez and Halstead 3 ).
Among the host factors that could influence the risk of developing DHF/DSS, the nutritional status could play an important role because many nutrients serve key immunomodulatory functions. For example, vitamin E supplementation to patients with DF resulted in increased platelet counts in India( Reference Vaish, Verma and Agarwal 4 ); 25-hydroxy vitamin D levels were positively related to severe dengue in Colombia( Reference Villamor, Villar and Lozano 5 ) and India( Reference Alagarasu, Bachal and Bhagat 6 ) whereas 1,25-hydroxyvitamin D was inversely associated with DHF/DSS in Nicaragua( Reference Voge, Perera and Mahapatra 7 ), and Zn and Cu serostatus were related to dengue disease severity in cross-sectional studies in Indonesia( Reference Yuliana, Fadil and Chairulfatah 8 ) and India( Reference Soundravally, Sherin and Agieshkumar 9 ). Fatty acids (FA) are salient amidst the nutrients that could affect pathophysiological pathways leading to severe forms of dengue virus (DENV) infection. FA influence the organisation of cell membranes and the composition of lipid rafts through their incorporation as phospholipids and sphingolipids( Reference Turk and Chapkin 10 ). Lipid raft microdomains play an essential role in DENV protein synthesis and replication( Reference Garcia Cordero, Leon Juarez and Gonzalez 11 ). In addition, some n-3 PUFA, including EPA (20 : 5n-3) and DHA (22 : 6n-3) could reduce expression of pro-inflammatory cytokines through different mechanisms including down-regulated gene expression in mononuclear cells, decreased synthesis of pro-inflammatory eicosanoids derived from n-6 PUFA, reduced chemotaxis and lymphocyte proliferation, enhanced apoptosis of Th-1 cells, and decreased endothelial activation and dysfunction( Reference Calder 12 ). Some n-6 PUFA, including arachidonic acid (AA, 20 : 4n-6), are considered pro-inflammatory while others such as dihomo-γ-linolenic acid (DGLA, 20 : 3n-6) exhibit anti-inflammatory properties( Reference Wang, Lin and Gu 13 ). Despite the potential of FA to regulate pathways involved in the aetiology of severe dengue, they have not been systematically studied in the context of this infection. Some investigations indicate that FA concentrations are altered during the acute stages of dengue infection( Reference Cui, Lee and Kumar 14 , Reference Khedr, Hegazy and Kamal 15 ). Because therapies to prevent progression of DF to DHF/DSS are virtually inexistent, identifying modifiable factors associated with risk of these severe forms of the disease is a high research priority.
We conducted a prospective case–control study nested in a cohort of patients who were diagnosed with DF and followed during the acute episode. The primary aim was to investigate the associations of FA concentrations in serum collected <96 h from the onset of fever with progression to DHF/DSS. A secondary aim was to determine whether serum FA could be affected by acute inflammation at the early stages of DF. This was realised by assessing the correlations between inflammatory biomarkers and FA and by comparing FA concentrations during the acute episode and a disease-free period, after >1 year post-convalescence from the disease.
Methods
Study design
This was a case–control study nested within a cohort of patients diagnosed with DF who were followed during the acute episode. Ambulatory patients with suspected dengue were recruited during non-epidemic (May 2003–September 2009) and epidemic (October 2009–December 2010) periods at healthcare centres in five areas of Bucaramanga, a city in northeast Colombia. Eligible participants had an acute febrile syndrome caused by DENV infection, had onset of symptoms <96 h before consultation, and were ≥5 years of age. Exclusion criteria were as follows: history of diabetes, acquired immunodeficiency syndrome, haematological disorders, cancer or CVD; or, at baseline, DHF or DSS (case definition below), major bleeding, hypoalbuminaemia, effusions or shock. At the time of recruitment, we elicited information on sociodemographic characteristics, medical history and symptoms through a questionnaire. Height and weight were measured on calibrated instruments with the use of standardised techniques and a complete physical examination was carried out. Blood samples were obtained to determine haematocrit, platelet counts and albumin concentration. To identify the occurrence of DHF/DSS, we followed participants during the acute episode daily at their homes until the 7th day of disease or the day of hospital discharge if they were admitted. Data collected during this period included signs and symptoms of DHF/DSS, as well as daily haematocrit measures. To detect thrombocytopenia, a DHF/DSS diagnostic criterion, we started daily platelet count measurements when patients had spontaneous haemorrhage, signs of effusion, oedema, a haematocrit change >10 % or a platelet count <120 000/mm3. We saw the patients again in the convalescent period, 7–15 d after the onset of symptoms, and obtained a new blood sample. DENV infection was confirmed according to a diagnostic algorithm that included IgM seroconversion from the acute to the convalescent samples (shift from negative to positive or a titre increase ≥4) plus either a positive result for NS1 antigen or viral genome amplification per RT-PCR in acute serum (<96 h from the onset of fever). IgM antibodies were determined with use of an enzyme-linked immunosorbent assay (Panbio Dengue IgM Capture ELISA; Alere), NS1 antigen was quantified with the ELISA NS1 Dengue kit (Panbio; Alere), and RT-PCR amplification of viral RNA was conducted with the QIAamp Viral RNA kit (Qiagen). We determined whether DENV infection was secondary with use of the Panbio Dengue IgG Capture ELISA test (Alere).
Case definition
Cases were patients who developed DHF or DSS during follow-up, according to the 1997 WHO criteria, prevalent at the time( 16 ). DHF cases met all of the following criteria: a platelet count ≤100 000/mm3, any spontaneous haemorrhage or ≥1 positive tourniquet test, and evidence of plasma leakage (i.e. pleural effusion, ascites, hypoalbuminaemia <30 g/l or an increase in haematocrit >10 %). A haemoconcentration of 10 %, instead of 20 %, was chosen because this criterion has greater sensitivity in identifying dengue-related complications( Reference Rigau-Perez and Bonilla 17 ) and has been associated with severe morbidity in patients from areas endemic for dengue( Reference Diaz-Quijano, Martinez-Vega and Villar-Centeno 18 ). DSS cases had all criteria above plus any reading of mean arterial pressure <70 mmHg or pulse pressure <20 mmHg during follow-up.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki. All participants gave written informed consent before recruitment. Among children, written consent was sought from the primary care provider and assent from the children was confirmed before recruitment. The study protocol was approved by the Medical Ethics Committee of the Industrial University of Santander. The University of Michigan Health Sciences and Behavioral Sciences Institutional Review Board approved the use of data and samples from the study.
Study population
We recruited 820 participants into the cohort. Of them, 173 (21·1 %) developed DHF/DSS during follow-up. For the case–control study, we selected all 109 cases (63·0 % of all cases in the cohort) whose first available serum sample (at the time of the initial consultation) had been collected within 96 h from the onset of fever (‘acute’ serum sample) and had sufficient volume for FA quantification. Next, we randomly selected a group of controls among the patients who did not reach DHF/DSS criteria during follow-up, using a 2:1 ratio of controls per case plus an additional 10 % in anticipation of losses due to lack of an acute serum sample. The final number of controls was 235. Selected controls were comparable to those not selected with respect to sex, age, socio-economic status, hours with fever before consultation and signs of severity. Nevertheless, selected controls were more likely to have become infected during the epidemic period and were taller and heavier compared with non-selected controls. Selected cases were compared with non-selected cases in the same manner as controls.
A post-convalescence blood sample was obtained in a subgroup of fifteen cases and twenty-nine controls who were visited at home after a median 1·8 years (range 1·3–2·5) from the acute episode.
Laboratory methods
Serum fatty acids
Samples were shipped frozen to the University of Michigan where total serum FA were quantified at the Regional Comprehensive Metabolomics Resource Core. Total lipids were extracted from 200 μl serum per the methods described by Bligh & Dyer( Reference Bligh and Dyer 19 ). About 10 μl of 4 mm non-adecanoic acid (C19 : 0) was added as an internal standard. Boron trifluoride–methanol was used to derivatise the FA portion of the total lipids into their methyl esters as previously described( Reference Morrison and Smith 20 ). To extract the methyl esters, a 2:1 hexane–water mixture was added and the sample was centrifuged. The hexane layer containing the methyl esters was removed from the aqueous layer and dried, and the methyl esters were resuspended in 100–200 μl of hexane, according to the volume of the original sample. About 1–2 μl was injected via an autosampler and analysed on a gas chromatographer (Model 6890N; Agilent) equipped with a flame ionisation detector and a 100 m×0·25 mm×0·2 μm SP-2560 column (Sigma-Aldrich). To quantify FA, known amounts of C19 : 0 and other authentic methyl esters were used to create a calibration curve. The authentic methyl esters were also used to identify FA in samples based on their retention times. Eluted peaks were analysed with Chemstation software (Agilent). FA concentrations were expressed as the percentage of each FA relative to the total FA concentration (FA %). This FA % was estimated by dividing the area of each FA by the total area. The limit of detection for pentadecanoic acid (C15 : 0), a type of FA present at low concentrations, was 50 pmol.
The activity of key enzymes in FA metabolism was estimated from desaturation and elongation indices calculated as the product/substrate ratios: stearoyl-coA-desaturase (SCD) as 18 : 1n-9/18 : 0, elongase as 18 : 1n-7/16 : 1n-7, Δ6-desaturase as 18 : 3n-6/18 : 2n-6 and Δ5-desaturase (D5D) as 20 : 4n-6/20 : 3n-6.
Cytokines
We determined concentrations of pro- and anti-inflammatory cytokines that may be independent predictors of progression to DHF/DSS. Interferon (IFN)-γ, IL-10, IL-6 and TNF-α were measured at the University of Michigan Cancer Center Immunology Core with the use of Luminex assays (Thermo Fisher Scientific Inc.) in all acute sera and in the samples collected post-convalescence.
Dengue virus serotype
DENV serotypes were identified in a subsample of forty-nine controls and twenty-eight cases using conventional and real time RT-PCR assays. Viral RNA was isolated from serum using the commercial kit QIAamp® Viral RNA. Conventional RT-PCR tests were performed following the Lanciotti protocol( Reference Lanciotti, Calisher and Gubler 21 ). Real-time RT-PCR were conducted with the CDC Kit DENV-1-4 Real-Time RT-PCR (Centers for Disease Control and Prevention)( Reference Santiago, Vergne and Quiles 22 ).
Data analysis
Variables
Case (DHF/DSS) status was the outcome of interest. FA % were the primary exposures and enzyme activity indices were secondary exposures. We considered as covariates baseline characteristics that could confound the associations between FA status and progression to DHF/DSS. These included sociodemographic (age, sex, socio-economic status), anthropometric (height and BMI as nutritional status indicators), clinical (time since the beginning of fever, early signs of severity), virological (secondary infection, serotype) and immunological (cytokine concentrations) characteristics.
Comparison of controls and cases
We first compared the distribution of baseline covariates between controls and DHF/DSS cases, which were categorised as presented in Table 1. Case–control differences were tested with the use of χ 2 and Wilcoxon rank-sum tests for categorical and continuous characteristics, respectively.
Table 1 Characteristics of dengue fever controls and dengue haemorrhagic fever/dengue shock syndrome cases at the time of uncomplicated dengue fever diagnosis (baseline) (Mean values and standard deviations; medians and ranges)
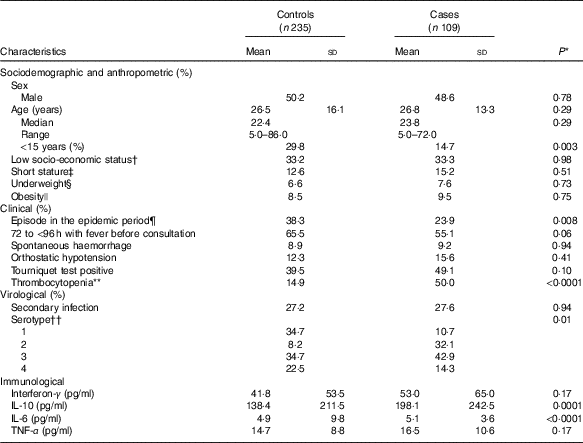
DENV, dengue virus.
* χ 2 and Wilcoxon rank-sum tests for dichotomous and continuous characteristics, respectively.
† Strata 1 and 2 (out of five) of the local government’s socio-economic status classification of households for tax and planning purposes.
‡ For participants <18 years of age, height-for-age <–1 Z according to the WHO sex-specific growth reference for school-aged children and adolescents. For participants ≥18 years of age, height <–1 Z of the sex-specific distributions of controls (<151 cm for women and <167 cm for men).
§ For participants <18 years of age, BMI-for-age <–2 Z according to the WHO sex-specific growth reference for school-aged children and adolescents.( Reference de Onis, Onyango and Borghi 51 ) For participants ≥18 years of age, BMI <18·5 kg/m2.
|| For participants <18 years of age, BMI-for-age ≥2 Z according to the WHO sex-specific growth reference for school-aged children and adolescents. For participants ≥18 years of age, BMI ≥30 kg/m2.
¶ The epidemic period occurred between October 2009 and December 2010.
** Platelet count <100 000/mm3.
†† DENV serotyping was conducted in a subsample of forty-nine controls (20·9 %) and twenty-eight cases (25·7 %).
Fatty acids and progression to dengue haemorrhagic fever/dengue shock syndrome
We compared the distribution of FA between controls and cases using means and standard deviations and the Wilcoxon rank-sum test. Next, we categorised each FA into quintiles according to the distribution among controls and estimated OR with 95% CI with the use of unconditional logistic regression. Tests for linear trend were conducted by introducing into the models a variable representing the median values of each quintile as a continuous predictor. Multivariable-adjusted OR and 95 % CI were estimated for each FA in models that included as covariates sex, age, hours with fever before consultation, and IL-10 and TNF-α concentrations at the time of consultation. Finally, we fitted a model with simultaneous adjustment for the FA that were statistically significant predictors of DHF/DSS progression in multivariable analyses and that remained significant in the final model at P<0·05.
Acute inflammation and fatty acids
To ascertain whether acute inflammation in the course of DENV infection was related to FA concentrations, we estimated sex- and age-adjusted partial Spearman’s correlation coefficients between the cytokines (IFN-γ, IL-10, IL-6 and TNF-α) and each FA separately for controls and cases. Next, we estimated the correlations between the analytes measured in the samples collected after >1 year post-convalescence. We also examined the prevalence of thrombocytopenia at baseline by FA quintiles since platelet count is a strong predictor of progression to severe dengue.
Change in fatty acid concentrations from the acute episode to post-convalescence
In the subgroup with a postconvalescence blood sample, we estimated the difference in FA concentrations between after >1 year of recovery and the acute episode with the use of a repeated-measures linear regression model with each FA as the continuous outcome and order of measurement as the predictor. An exchangeable covariance matrix was specified to account for within-subject correlations. Participants with a post-convalescence sample were more likely to have been recruited during the epidemic period, were younger and of lower socio-economic status, and consulted later after the onset of fever than those without these samples. They also differed with respect to some of the FA at baseline.
Associations of dengue virus serotype with fatty acids
Because DENV serotype is a strong independent predictor of progression to severe dengue( Reference Vaughn, Green and Kalayanarooj 23 ) and we lacked this information on the majority of participants, we examined the associations of serotype with serum FA in the subset with available data to explore whether serotype could have confounded the key associations between FA and case status. We compared the distribution of FA by serotype with the use of Wilcoxon rank-sum tests.
All analyses were carried out with Statistical Analysis Software version 9.4 (SAS Institute Inc.).
Results
Characteristics of controls and cases
In all, 50 % (n 173) of participants were female. Age was 26·6 (sd 15·2) years (range 5–86); 25 % of participants (n 86) were <15 years old. Compared with controls, cases were older, consulted earlier after the onset of fever, had lower platelet counts and had higher cytokine concentrations at baseline (Table 1).
Fatty acids and progression to dengue haemorrhagic fever/dengue shock syndrome
Mean pentadecanoic (15 : 0) and stearic (18 : 0) acid concentrations were significantly lower in cases compared with controls (Table 2, online Supplementary Fig. S1), whereas behenic acid (22 : 0) levels were higher. There were no significant differences in MUFA concentrations by case status. Mean concentrations of the long-chain n-3 PUFA docosapentaenoic acid (DPA) and DHA and of the n-6 PUFA DGLA, AA and adrenic acid were significantly higher in cases compared with controls (Table 2, online Supplementary Fig. S1). SCD and D5D activities were higher in cases than controls. When the FA exposures were considered in quintiles of their distributions among controls (online Supplementary Table S1), pentadecanoic acid and DGLA were inversely associated with case status whereas DHA, AA, SCD and D5D were positively related to progression to DHF/DSS, after adjustment for sex, age, hours with fever before consultation and IL-10 and TNF-α concentrations. In a multivariable model adjusted for these FA, simultaneously pentadecanoic acid, DHA and DGLA remained significantly associated with progression to DHF/DSS (Table 3) whereas the associations with AA and SCD were attenuated and became non-statistically significant. For pentadecanoic acid, compared with patients in the lowest quintile those in the highest had a 76 % lower odds of progression (P=0·002). DHA was positively related to case status in a non-linear manner; compared with patients in the lowest quintile, odds of progression were five times higher (OR: 4·96; 95 % CI 2·05, 11·99; P=0·0004) for patients with concentrations in the second quintile or above. DGLA was inversely related to DHF/DSS progression; the OR (95 % CI) between quintiles 5 and 1 was 0·30 (95 % CI 0·13, 0·69; P=0·005). When D5D was substituted for DGLA in the model, D5D was positively associated with DHF/DSS progression (Q5 v. Q1 OR: 3·28; 95 % CI 1·42, 7·58; P=0·006).
Table 2 Distribution of serum fatty acids (FA) percentage in dengue fever controls and dengue haemorrhagic fever/dengue shock syndrome cases (Mean values and standard deviations)
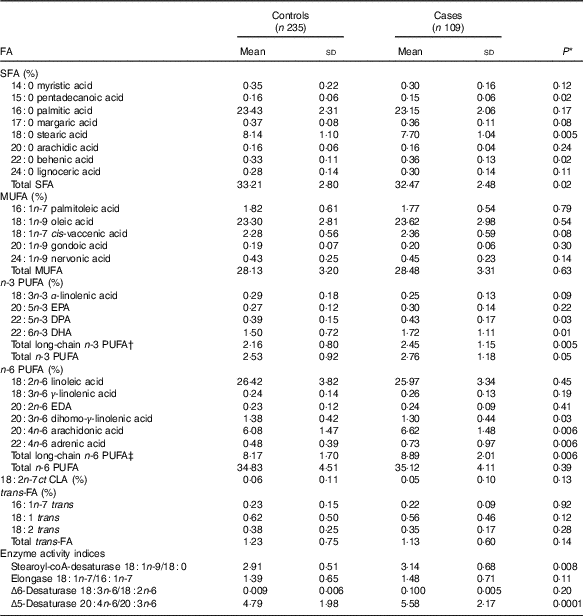
DPA, docosapentaenoic acid; EDA, eicosadienoic acid; CLA, conjugated linoleic acid.
* Wilcoxon rank-sum test.
† Sum of 20 : 5n-3 EPA, 22 : 5n-3 DPA and 22 : 6n-3 DHA.
‡ Sum of 20 : 2n-6 EDA, 20 : 3n-6 dihomo-γ-linolenic acid, 20 : 4n-6 arachidonic acid and 22 : 4n-6 adrenic acid.
Table 3 Adjusted OR for progression to dengue haemorrhagic fever/dengue shock syndrome according to baseline characteristics and serum fatty acids (FA) percentage (Odds ratios and 95 % confidence intervals)
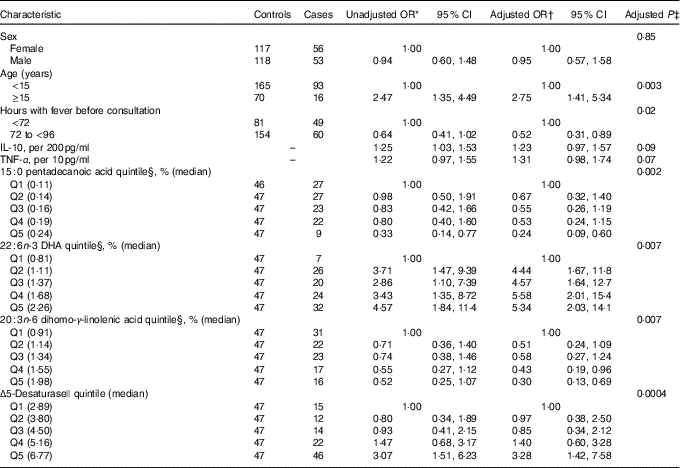
* From unconditional logistic regression models with case status (dengue haemorrhagic fever/dengue shock syndrome) as the outcome and each covariate presented as predictor.
† From an unconditional logistic regression model with case status as the outcome and predictors that included sex (one indicator for male), age (one indicator for <15 years), hours with fever before consultation (one indicator for ≥72 h), IL-10 (continuous), TNF-α (continuous), 15 : 0 pentadecanoic acid quintile (four indicators), 22 : 6n-3 DHA quintile (four indicators) and 20 : 3n-6 dihomo-γ-linolenic acid quintile (four indicators). Estimates for Δ5-desaturase were obtained from a model that excluded 20 : 3n-6 dihomo-γ-linolenic acid due to collinearity.
‡ For sex, age, hours with fever before consultation and cytokines, Wald test. For FA, test for linear trend when a variable representing the median of each quartile was introduced into the logistic regression model as a continuous predictor.
§ Percentage of total serum FA.
|| Ratio of 20 : 4n-6/20 : 3n-6.
Acute inflammation and fatty acids
Correlations between FA and cytokine concentrations were generally low (online Supplementary Table S2). DGLA was weakly, inversely correlated with IL-10 at baseline but not post-convalescence. DPA, DHA and D5D were related to low platelet counts, whereas the n-6 PUFA γ-linolenic acid (GLA, 18 : 3n-6) and DGLA were associated with less thrombocytopenia (online Supplementary Table S3). Pentadecanoic acid was not related to platelet counts at baseline.
Change in fatty acid concentrations from the acute episode to post-convalescence
Among the forty-four patients with post-convalescent samples, concentrations of most SFA, MUFA and n-3 and n-6 PUFA changed from the time of the acute episode (online Supplementary Table S4). Concentrations of SFA, except for palmitic acid, tended to increase from the acute episode to postconvalescence, whereas MUFA concentrations, except for palmitoleic acid, decreased. α-Linolenic acid (ALA), EPA, DPA and all n-6 PUFA concentrations increased. DHA was higher during the acute episode than post-convalescence, but the change was not statistically significant. There were no changes in trans FA. D5D activity decreased. Changes did not differ by case status during the acute episode (online Supplementary Table S5).
Dengue virus serotype and fatty acids
The distributions of some FA varied significantly by DENV serotype in the subset with available data (online Supplementary Table S6). Of the FA significantly associated with progression to DHF/DSS, only DHA was related to DENV serotype. DHA of participants infected with serotypes 2 or 3 was lower than that of patients infected with DENV 1 or 4.
Discussion
In this prospective investigation of patients diagnosed with DF, serum DHA at the time of diagnosis was related to increased odds of progression to DHF/DSS whereas the SFA pentadecanoic acid and the n-6 PUFA DGLA were associated with decreased odds. In addition, increased D5D activity was related to higher odds of progression.
The positive association of DHA levels with progression to DHF/DSS is consistent with results from a small untargeted metabolomics study( Reference Voge, Perera and Mahapatra 7 ). Serum DHA within 4 d from the onset of fever was higher in sixteen Nicaraguan children diagnosed with DF who progressed to DHF/DSS compared with fifteen children who did not progress. This finding is unexpected because DHA contributes to the resolution of inflammation through different pathways( Reference Calder 12 ) and effective resolution of inflammation should be related to less risk of progression. Nevertheless, the association should also be interpreted in light of the comparison of DHA levels during the acute episode and post-convalescence. In our study, DHA was higher during the acute episode than in a presumably disease-free period. Although this difference was not statistically significant, it is in line with findings from a metabolomics study of adults with DF in which DHA was higher during the febrile stage of infection than it was 3–4 weeks after resolution of the episode( Reference Cui, Lee and Kumar 14 ). An increase in DHA levels during acute DF has been interpreted as an early attempt to initiate the resolution of inflammation, which could start concomitantly with the initial pro-inflammatory response( Reference Voge, Perera and Mahapatra 7 , Reference Cui, Lee and Kumar 14 ). A more severe inflammatory process during the early stages of DF predicts progression to DHF/DSS; thus, a correspondingly more aggressive anti-inflammatory response involving increased DHA levels could explain the association of DHA with progression even in the absence of an adverse causal effect of DHA. Of note, however, in our data, DHA was generally not correlated with cytokine concentrations and the association of DHA with progression was observed even after controlling for these inflammation biomarkers. An adverse effect of DHA cannot be discarded. Rodent models indicate that n-3 long-chain PUFA supplementation can be detrimental in infections by intracellular pathogens including influenza A and herpes simplex viruses by suppressing immune cell responses needed to eradicate infected cells( Reference Husson, Ley and Portal 24 ), although a study of mice showed no effect on n-3 PUFA on responses to vaccinia virus infection( Reference Jones and Roper 25 ). Evidence on the effects of PUFA on acute febrile illnesses in humans is scant. Some studies suggest that n-3 PUFA may reduce the incidence of pneumococcal infection in the elderly and n-3 PUFA supplementation to infants or school-age children has resulted in decreased respiratory morbidity( Reference Birch, Hoffman and Castaneda 26 – Reference Imhoff-Kunsch, Stein and Martorell 31 ), possibly of viral aetiology. We also noted that DHA was positively associated with thrombocytopenia at baseline. Whether this could be a mechanism to explain a potential effect on progression to severe disease is a matter of speculation. DHA might dampen the procoagulant function of platelets through altered synthesis of thrombin precursor proteins( Reference Larson, Tormoen and Weaver 32 ); nevertheless, there is no evidence to suggest that it may affect platelet numbers. Additional in vitro and non-human experimentation is needed to clarify the role of n-3 PUFA in DENV infection before considering a potential therapeutic role for these nutrients.
The inverse associations of pentadecanoic acid and DGLA with progression to DHF/DSS are novel findings. Pentadecanoic acid is an odd-chain SFA that, together with heptadecanoic acid (C17 : 0), represents dairy fat intake( Reference Wolk, Furuheim and Vessby 33 ); its endogenous production is negligible( Reference Pfeuffer and Jaudszus 34 ). Epidemiological studies have consistently shown inverse associations of pentadecanoic and heptadecanoic acid biomarkers with long-term cardiometabolic disorders( Reference Riserus and Marklund 35 ), but the biological mechanisms underlying these relations are uncertain. Investigators have posited that one of the biological functions of pentadecanoic acid involves the regulation of circulating propionic acid( Reference Pfeuffer and Jaudszus 34 ), a short-chain SFA produced by intestinal bacteria through fermentation of dietary fibre. Propionic acid and other short-chain SFA may be potential mediators of the effects of microbiota on intestinal immunity and inflammation. These FA regulate leucocytes’ recruitment, migration and activation through production of cytokines, chemokines and eicosanoids( Reference Vinolo, Rodrigues and Nachbar 36 ). Whether these effects extend systemically in the event of an acute infection such as dengue remains to be elucidated. We observed a decrease in palmitic acid, the most abundant SFA, with a concomitant increase in palmitoleic acid from the acute episode to post-convalescence. Palmitic acid can be synthesised endogenously or ingested from diet, whereas palmitoleic acid can be formed from palmitic acid by the SCD. If diet remained constant, one could speculate that SCD activity might be dampened during the acute dengue episode, but the clinical implications of this potential effect are uncertain.
DGLA is a long-chain n-6 PUFA synthesised endogenously from linoleic acid through desaturation and elongation. D5D can convert DGLA into AA, a pro-inflammatory FA and other acute viral infections, including influenza, induce up-regulation of the lipoxygenase pathway increasing the synthesis of pro-inflammatory oxylipins derived from AA( Reference Cui, Fang and Ooi 37 ). Nevertheless, DGLA is generally considered anti-inflammatory because it can interfere with eicosanoid biosynthesis and can be converted to prostaglandin E1, a suppressor of chronic inflammation( Reference Wang, Lin and Gu 13 ). An increase in DGLA relative to AA could acutely attenuate the synthesis of pro-inflammatory eicosanoids derived from AA, including four-series leukotrienes, two-series prostaglandins and platelet-activating factor, which may be involved in the pathophysiology of severe dengue( Reference Voge, Perera and Mahapatra 7 ). Consistent with this possibility, increased activity of D5D, the enzyme that converts DGLA into AA, was related to higher odds of progression to DHF/DSS. We found that DGLA was inversely correlated with IL-10 at baseline, especially among cases, and elevated IL-10 seemed to predict progression to DHF/DSS in this and other studies( Reference Malavige, Gomes and Alles 38 , Reference Liao, Tang and Hu 39 ). This could indicate that a potential protective effect of DGLA on progression to severe dengue might be mediated through reduced inflammation. Another intriguing potential mechanism could be related to virucidal activity against encapsulated viruses that some long-chain n-6 PUFA have shown in vitro ( Reference Kohn, Gitelman and Inbar 40 – Reference Hilmarsson, Kristmundsdottir and Thormar 43 ). DGLA breastmilk concentrations were inversely related to cell-free and cell-associated HIV load in milk in a study of Tanzanian HIV-infected women( Reference Villamor, Koulinska and Furtado 44 ). Non-causal explanations are also plausible. We noted that DGLA concentrations were lower during the acute episode than in an apparently disease-free period in a subgroup of participants. Assuming there were no major changes in diet between the two measurements, this opens the possibility that acute inflammation during the early stages of the disease influences DGLA concentrations and that an inverse association of DGLA with progression to severe dengue be only a reflection of early inflammatory status. Notwithstanding this possibility, DGLA supplementation increases blood DGLA in a dose–response manner( Reference Tanaka, Kakutani and Horikawa 45 , Reference Teraoka, Kawashima and Shiraishi-Tateishi 46 ) and should not be discarded as a potential intervention that could be considered for testing in the treatment of acute DF.
This study has several strengths. Reverse causation bias was minimised by the prospective nature of the design. Recall bias was avoided with the use of objective measures of exposure. We had an opportunity to control for important potential confounders, including inflammatory biomarkers that predict progression of the disease to severe forms. We compared exposure status between the acute episode and an apparently healthy period in a subsample of participants; this approach is stronger than using a different set of healthy controls. Some limitations are also worth noting. Selection bias could occur if the selection of controls is not independent of exposure status. We noted that selected controls differed from the cohort’s non-cases in some characteristics that could be related to FA status, including body weight; thus, it is not possible to completely rule out selection bias. Second, confounding could have occurred if an unmeasured predisposing factor for progression was related to FA concentrations at the onset of the febrile episode. Third, because FA concentrations are conventionally expressed as percent of total FA in the sample, associations observed with higher levels of a given FA could also represent those due to lower levels of another and vice versa. Fourth, pentadecanoic acid is present at low concentrations which are prone to measurement error. The confidence bounds around the OR were wide. Nevertheless, the mean serum C15 : 0 concentration in this population, 0·16 %, was the same or very close as that reported in other settings including Costa Rica( Reference Baylin, Kim and Donovan-Palmer 47 ) and the United States( Reference de Oliveira Otto, Nettleton and Lemaitre 48 – Reference Yakoob, Shi and Willett 50 ), lending support to the external validity of this finding. Fifth, random error may have occurred due to differences in the timing of the last meal since the acute serum sample in this study was not always a fasting sample. Serum FA concentrations do not necessarily reflect long-term intake as some may change from day to day depending on the FA composition of recent meals. Correlations between long-term intake and serum concentrations vary for different FA. They are moderate to high for exogenous FA including odd chain SFA and essential PUFA (ALA and LA) or for endogenous FA that may nevertheless be abundant in diet such as oleic acid, EPA and DHA( Reference Baylin, Kim and Donovan-Palmer 47 ). By contrast, serum FA that are not common in the diet and mostly reflect endogenous hepatic metabolism, such as GLA and DGLA, may not represent long-term intake( Reference Baylin, Kim and Donovan-Palmer 47 ). Sixth, the subsample of patients with postconvalescence samples was not comparable with the rest of participants with regard to some characteristics. Finally, lack of sample volume and funding constraints prevented us from determining serotype in all study subjects. DENV serotype is a strong predictor of progression to severe disease and could have confounded the associations if it were also related to exposure status. Nonetheless, the data do not support this notion because only DHA was related to both progression and serotype in the subset with available information, and the directions of the serotype-DHA and serotype-DHF/DSS associations would have caused an attenuation rather than an exaggeration of a positive DHA-DHF/DSS relation.
In sum, serum DHA concentrations at the early stages of DF are positively associated with progression to DHF/DSS whereas pentadecanoic acid and DGLA concentrations are inversely related to progression. Serum FA concentrations differ between an acute dengue episode and an apparently healthy period. These results should be confirmed in other populations as the next step in identifying FA as eventual therapeutic targets in patients with DF.
Acknowledgements
This study was supported by the National Institute of Allergy and Infectious Diseases (R21AI103364) and the Colombian Administrative Department of Science, Technology and Innovation – Colciencias (1102-04-12919, 1102-04-18205, 1102-459-21561 and BPIN 2013000100011). The funding source had no role in the design, analysis or writing of this article.
E. V. and L. A. V. designed the research. L. A. V., A. L.-P. and O. F. H. conducted the research. V. M. H. conducted data management and quality assurance. E. V. performed the statistical analyses, wrote the manuscript and had primary responsibility for the final content. All authors have read and approved the final version of the manuscript.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114518002039








