Abstract
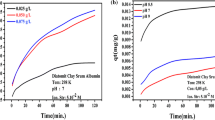
The adsorption of a model protein such as bovine serum albumin (BSA) on a magnetic material based on montmorillonite (MtMag) was studied. Kinetic data, equilibrium isotherms, and thermodynamic calculations were performed and analyzed to obtain information about the rate-limiting step of the adsorption process and propose a possible adsorption mechanism. Equilibrium studies showed optimal adsorption around pH 4.5, the maximum adsorption capacity being around 231.9 mg g-1 at 25 °C. The latter behavior may be ascribed to the most packed protein conformation at pH conditions near the BSA isoelectric point. In addition, the BSA adsorption capacity decreased with the ionic strength (10–3−10–1 M) and increased with the temperature (15–30 °C). In all cases, the Sips model yielded the best fit to the experimental results. The thermodynamic analysis revealed an isosteric heat dependence on the coverage, which allowed ruling out the Langmuir model. The kinetic data were adequately fitted by the IPD model, which suggests that the rate-limiting step of the adsorption process is the diffusion of BSA molecules into the internal sites.








Similar content being viewed by others
References
Cacciotti, I.; Lombardelli, C.; Benucci, I.; Esti, M.: Clay/chitosan biocomposite systems as novel green carriers for covalent immobilization of food enzymes. J. Mat. Res. Technol. 8, 3644–3652 (2019). https://doi.org/10.1016/j.jmrt.2019.06.002
Çalımlı, M.H.; Demirbaş, Ö.; Aygün, A.; Alma, M.H.; Nas, M.S.; Şen, F.: Immobilization kinetics and mechanism of bovine serum albumin on diatomite clay from aqueous solutions. Appl Water Sci 8, 209 (2018). https://doi.org/10.1007/s13201-018-0858-8
Schmid, M.; Merzbacher, S.; Brzoska, N.; Müller, K.; Jesdinszki, M.: Improvement of food packaging-related properties of whey protein isolate-based nanocomposite films and coatings by addition of montmorillonite nanoplatelets. Front. Mater. (2017). https://doi.org/10.3389/fmats.2017.00035
Staunton, S.; Quiquampoix, H.: Adsorption and conformation of bovine serum albumin on montmorillonite: modification of the balance between hydrophobic and electrostatic interactions by protein methylation and pH variation. J. Colloid Interface Sci. 166, 89–94 (1994). https://doi.org/10.1006/jcis.1994.1274
Servagent-Noinville, S.; Revault, M.; Quiquampoix, H.; Baron, M.-H.: Conformational changes of bovine serum albumin induced by adsorption on different clay surfaces: FTIR analysis. J. Colloid Interface Sci. 221, 273–283 (2000)
Tran, A.T.T.; James, B.J.: A study the interaction forces between the bovine serum albumin protein and montmorillonite surface. Colloids Surf. A 414, 104–114 (2012). https://doi.org/10.1016/j.colsurfa.2012.08.066
Kim, Oh.: Physico-chemical interaction between clay minerals and albumin protein according to the type of clay. Minerals. 9, 396 (2019). https://doi.org/10.3390/min9070396
Gamba, M.; Kovář, P.; Pospíšil, M.; Torres Sánchez, R.M.: Insight into thiabendazole interaction with montmorillonite and organically modified montmorillonites. Appl. Clay Sci. 137, 59–68 (2017). https://doi.org/10.1016/j.clay.2016.12.001
Flores, F.M.; Undabeytia, T.; Morillo, E.; Torres Sánchez, R.M.: Technological applications of organo-montmorillonites in the removal of pyrimethanil from water: adsorption/desorption and flocculation studies. Environ. Sci. Pollut. Res.Pollut. Res. 24, 14463–14476 (2017). https://doi.org/10.1007/s11356-017-9016-3
Alkan, M.; Demirbaş, Ö.; Doğan, M.; Arslan, O.: Surface properties of bovine serum albumin – adsorbed oxides: adsorption, adsorption kinetics and electrokinetic properties. Microp. Mesop. Mater. 96, 331–340 (2006). https://doi.org/10.1016/j.micromeso.2006.07.007
Rahdar, S.; Rahdar, A.; Ahmadi, S.; Trant, J.F.: Adsorption of bovine serum albumin (BSA) by bare magnetite nanoparticles with surface oxidative impurities that prevent aggregation. Can. J. Chem. 97, 577–583 (2019). https://doi.org/10.1139/cjc-2019-0008
Shah, M.T.; Alveroglu, E.: Synthesis and characterization of magnetite nanoparticles having different cover layer and investigation of cover layer effect on the adsorption of lysozyme and bovine serum albumin. Mater. Sci. Eng. C 81, 393–399 (2017). https://doi.org/10.1016/j.msec.2017.08.033
Barraqué, F.; Montes, M.L.; Fernández, M.A.; Mercader, R.C.; Candal, R.J.; Torres Sánchez, R.M.: Synthesis and characterization of magnetic-montmorillonite and magnetic-organo-montmorillonite: surface sites involved on cobalt sorption. J. Magn. Magn. Mater.Magn. Magn. Mater. 466, 376–384 (2018)
Barraqué, F.; Montes, L.; Fernandez, M.; Candal, R.; Mercader, R.; Torres Sanchez, R.: Synthesis of high-saturation magnetization composites by montmorillonite loading with hexadecyl trimethyl ammonium ions and magnetite nucleation for improved effluent sludge handling and dye removal. Appl. Phys. A (2020). https://doi.org/10.1007/s00339-020-03834-6
Chowdhury, S.; Mishra, R.; Saha, P.; Kushwaha, P.: Adsorption thermodynamics, kinetics and isosteric heat of adsorption of malachite green onto chemically modified rice husk. Desalination 265, 159–168 (2011). https://doi.org/10.1016/j.desal.2010.07.047
Gamba, M.; Flores, F.M.; Madejová, J.; Torres Sánchez, R.M.: Comparison of imazalil removal onto montmorillonite and nanomontmorillonite and adsorption surface sites involved: an approach for agricultural wastewater treatment. Ind. Eng. Chem. Res. 54, 1529–1538 (2015). https://doi.org/10.1021/ie5035804
Yarza, F.; Morantes, C.F.; Montes, M.L.; Bellotti, N.; Salduondo, J.; Yapar, S.; Cravero, F.; Torres Sánchez, R.M.: Quantification of the distribution of cetylpyridinium chloride on the external and internal surfaces of montmorillonite: Relevance in antifungal activity assessment. Mater. Chem. Phys. (2020). https://doi.org/10.1016/j.matchemphys.2020.123390
Regazzoni, A.E.: Formación de Magnetita (Fe3O4) en Medios Acuosos y Propiedades de la Interfaz Magnetita / Solución, Dirección de Investigación y desarrollo, (1984)
Schwertmann, U.; Cornell, R.M.: Iron Oxides in the Laboratory: Preparation and Characterization, John Wiley & Sons (2008)
Reed, R.G.; Putnam, F.W.; Peters, T.: Sequence of residues 400–403 of bovine serum albumin. Biochem. J. 191, 867–868 (1980)
Hu, J.; Li, S.; Liu, B.: Adsorption of BSA onto sulfonated microspheres. Biochem. Eng. J. 23, 259–263 (2005). https://doi.org/10.1016/j.bej.2005.01.018
Tsai, T.; Hsu, C.; Chao, V.W.; Huang, S.; Chan, C.; Wu, T.; Wang, P.: Selective desorption of intercalated bovine serum albumin and lysozyme by organically modified montmorillonite. J. Chin. Chem. Soc. 62, 562–568 (2015)
Wang, T.; Yue, Y.; Yuan, R.; Cai, C.; Niu, C.; Guo, C.: Kinetics of adsorption of bovine serum albumin on magnetic carboxymethyl chitosan nanoparticles. Int. J. Biol. Macromol. 58, 57–65 (2013)
Wu, V.W.-K.C.; Hsu, C.-C.; Lu, W.-M.; Chen, W.-J.; Naveen, B.; Tsai, T.-Y.: Protein-concentration-dependent adsorption behaviour of inorganic layered materials. RSC Adv. 5, 10936–10943 (2015)
Umrethia, M.; Kett, V.L.; Andrews, G.P.; Malcolm, R.K.; Woolfson, A.D.: Selection of an analytical method for evaluating bovine serum albumin concentrations in pharmaceutical polymeric formulations. J. Pharm. Biomed. Anal. 51, 1175–1179 (2010)
Adamson, A.W., Gast, A.P.: Physical chemistry of surfaces, Interscience publishers New York (1967)
Foo, K.Y.; Hameed, B.H.: Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 156, 2–10 (2010)
Hiemenz, P.C., Rajagopalan, R.: Principles of Colloid and Surface Chemistry, revised and expanded, CRC press (2016)
Barreca, S.; Orecchio, S.; Pace, A.: The effect of montmorillonite clay in alginate gel beads for polychlorinated biphenyl adsorption: isothermal and kinetic studies. Appl. Clay Sci. 99, 220–228 (2014). https://doi.org/10.1016/j.clay.2014.06.037
Marco-Brown, J.L.; Barbosa-Lema, C.M.; Torres Sánchez, R.M.; Mercader, R.C.; dos Santos Afonso, M.: Adsorption of picloram herbicide on iron oxide pillared montmorillonite. Appl. Clay Sci. 58, 25–33 (2012). https://doi.org/10.1016/j.clay.2012.01.004
Wang, J.; Guo, X.: Adsorption isotherm models: classification, physical meaning, application and solving method. Chemosphere 258, 127279 (2020). https://doi.org/10.1016/j.chemosphere.2020.127279
Chen, T.; Da, T.; Ma, Y.: Reasonable calculation of the thermodynamic parameters from adsorption equilibrium constant. J. Mol. Liq. 322, 114980 (2021). https://doi.org/10.1016/j.molliq.2020.114980
Tan, K.L.; Hameed, B.H.: Insight into the adsorption kinetics models for the removal of contaminants from aqueous solutions. J. Taiwan Inst. Chem. Eng. 74, 25–48 (2017). https://doi.org/10.1016/j.jtice.2017.01.024
Mahdavinia, G.R.; Etemadi, H.: Surface modification of iron oxide nanoparticles with κ-carrageenan/carboxymethyl chitosan for effective adsorption of bovine serum albumin. Arab. J. Chem. 12, 3692–3703 (2019). https://doi.org/10.1016/j.arabjc.2015.12.002
Douven, S.; Paez, C.A.; Gommes, C.J.: The range of validity of sorption kinetic models. J. Colloid Interface Sci. 448, 437–450 (2015). https://doi.org/10.1016/j.jcis.2015.02.053
Jaber, M., Lambert, J.-F., Balme, S.: 8 - Protein adsorption on clay minerals. In: R. Schoonheydt, C.T. Johnston, F. Bergaya (Eds.), Dev Clay Sci, Elsevier, 2018: pp. 255–288. https://doi.org/10.1016/B978-0-08-102432-4.00008-1
Bhattacharyya, K.G.; Gupta, S.S.: Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv. Colloid Interface Sci. 140, 114–131 (2008). https://doi.org/10.1016/j.cis.2007.12.008
Qiu, H.; Lv, L.; Pan, B.; Zhang, Q.; Zhang, W.; Zhang, Q.: Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A. 10, 716–724 (2009). https://doi.org/10.1631/jzus.A0820524
Wu, R.-L.; Tseng, R.-S.: Juang, Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 153, 1–8 (2009). https://doi.org/10.1016/j.cej.2009.04.042
Manohar, D.M.; Noeline, B.F.; Anirudhan, T.S.: Adsorption performance of Al-pillared bentonite clay for the removal of cobalt(II) from aqueous phase. Appl. Clay Sci. 31, 194–206 (2006). https://doi.org/10.1016/j.clay.2005.08.008
Hwang, Y.S.; Liu, J.; Lenhart, J.J.; Hadad, C.M.: Surface complexes of phthalic acid at the hematite/water interface. J. Colloid Interface Sci. 307, 124–134 (2007)
Fu, Q.; Wang, W.; Hu, H.; Chen, S.: Adsorption of the insecticidal protein of Bacillus thuringiensis subsp. kurstaki by minerals: effects of inorganic salts. Eur. J. Soil Sci. 59, 216–221 (2008). https://doi.org/10.1111/j.1365-2389.2007.00977.x
Lepoitevin, M.; Jaber, M.; Guégan, R.; Janot, J.-M.; Dejardin, P.; Henn, F.; Balme, S.: BSA and lysozyme adsorption on homoionic montmorillonite: influence of the interlayer cation. Appl. Clay Sci. 95, 396–402 (2014). https://doi.org/10.1016/j.clay.2014.05.003
Urano, H.; Fukuzaki, S.: Conformation of adsorbed bovine serum albumin governing its desorption behavior at alumina-water interfaces. J. Biosci. Bioeng. 90, 105–111 (2000). https://doi.org/10.1016/S1389-1723(00)80042-0
Quiquampoix, H., Abadie, J., Baron, M., Leprince, F., Matumoto-Pintro, P., Ratcliffe, R., Staunton, S.: Mechanisms and consequences of protein adsorption on soil mineral surfaces, In: ACS Publications (1995)
Barral, S.; Villa-García, M.A.; Rendueles, M.; Díaz, M.: Interactions between whey proteins and kaolinite surfaces. Acta Mater. 56, 2784–2790 (2008). https://doi.org/10.1016/j.actamat.2008.02.009
Shamim, N.; Hong, L.; Hidajat, K.; Uddin, M.S.: Thermosensitive-polymer-coated magnetic nanoparticles: adsorption and desorption of Bovine Serum Albumin. J. Colloid Interface Sci. 304, 1–8 (2006). https://doi.org/10.1016/j.jcis.2006.08.047
Tanyolaç, D.; Özdural, A.R.: BSA adsorption onto magnetic polyvinylbutyral microbeads. J. Appl. Polym. Sci. 80, 707–715 (2001). https://doi.org/10.1002/1097-4628(20010502)80:5%3c707::AID-APP1147%3e3.0.CO;2-K
Wang, Y.; Wang, X.; Luo, G.; Duo, Y.: Adsorption of bovin serum albumin (BSA) onto the magnetic chitosan nanoparticles prepared by a microemulsion system. Bioresour. Technol.. Technol. 99, 3881–3884 (2008). https://doi.org/10.1016/j.biortech.2007.08.017
Blade, W.H.; Boulton, R.: Adsorption of protein by Bentonite in a model wine solution. Am. J. Enol. Vitic. 39, 193–199 (1988)
Johnston, C.T.; Premachandra, G.S.; Szabo, T.; Lok, J.; Schoonheydt, R.A.: Interaction of biological molecules with clay minerals: a combined spectroscopic and sorption study of lysozyme on saponite. Langmuir 28, 611–619 (2012). https://doi.org/10.1021/la203161n
Perez Rodriguez, J.L.; Weiss, A.; Lagaly, G.: A natural clay organic complex from Andalusian black earth. Clays Clay Miner. 25, 243–251 (1977). https://doi.org/10.1346/CCMN.1977.0250311
Chi, Z.; Hong, B.; Ren, X.; Cheng, K.; Lu, Y.; Liu, X.: Investigation on the conformational changes of Bovine serum albumin in a wide pH range from 2 to 12. Spectrosc. Lett. 51, 279–286 (2018). https://doi.org/10.1080/00387010.2018.1471092
Salvestrini, S.; Leone, V.; Iovino, P.; Canzano, S.; Capasso, S.: Considerations about the correct evaluation of sorption thermodynamic parameters from equilibrium isotherms. J. Chem. Thermodyn. 68, 310–316 (2014). https://doi.org/10.1016/j.jct.2013.09.013
Anirudhan, T.S.; Radhakrishnan, P.G.: Thermodynamics and kinetics of adsorption of Cu(II) from aqueous solutions onto a new cation exchanger derived from tamarind fruit shell. J. Chem. Thermodyn. 40, 702–709 (2008). https://doi.org/10.1016/j.jct.2007.10.005
Arai, T.; Norde, W.: The behavior of some model proteins at solid-liquid interfaces 1. Adsorption from single protein solutions. Colloids Surf. 51, 1–15 (1990)
Garland, A.; Shen, L.; Zhu, X.: Mobile precursor mediated protein adsorption on solid surfaces. Prog. Surf. Sci.. Surf. Sci. 87, 1–22 (2012). https://doi.org/10.1016/j.progsurf.2012.02.001
Norde, W.: Energy and entropy of protein adsorption. J. Dispersion Sci. Technol. 13, 363–377 (1992). https://doi.org/10.1080/01932699208943322
Schmidt, M.P.; Martínez, C.E.: Kinetic and conformational insights of protein adsorption onto montmorillonite revealed using in situ ATR-FTIR/2D-COS. Langmuir 32, 7719–7729 (2016). https://doi.org/10.1021/acs.langmuir.6b00786
Barraqué, F.; Montes, M.L.; Fernández, M.A.; Candal, R.; Torres Sánchez, R.M.; Marco-Brown, J.L.: Arsenate removal from aqueous solution by montmorillonite and organo-montmorillonite magnetic materials. Environ. Res. 192, 110247 (2021). https://doi.org/10.1016/j.envres.2020.110247
Abramian, L.; El-Rassy, H.: Adsorption kinetics and thermodynamics of azo-dye Orange II onto highly porous titania aerogel. Chem. Eng. J. 150, 403–410 (2009). https://doi.org/10.1016/j.cej.2009.01.019
Ho, Y.S.; Ng, J.C.Y.; McKay, G.: Kinetics of pollutant sorption by biosorbents: review. Sep. Purif. Methods 29, 189–232 (2000). https://doi.org/10.1081/SPM-100100009
Kopac, T.; Bozgeyik, K.: Equilibrium, kinetics, and thermodynamics of Bovine serum albumin adsorption on single-walled carbon nanotubes. Chem. Eng. Commun. 203, 1198–1206 (2016). https://doi.org/10.1080/00986445.2016.1160225
Zhu, Q.; Moggridge, G.D.; D’Agostino, C.: Adsorption of pyridine from aqueous solutions by polymeric adsorbents MN 200 and MN 500. Part 2: Kinetics and diffusion analysis. Chem. Eng. J.Eng J. 306, 1223–1233 (2016). https://doi.org/10.1016/j.cej.2016.07.087
Roca Jalil, M.E.; Baschini, M.; Sapag, K.: Removal of ciprofloxacin from aqueous solutions using pillared clays. Materials. 10, 1345 (2017). https://doi.org/10.3390/ma10121345
Igwe, J.C.; Abia, A.A.: Sorption kinetics and intrapaticulate diffusivity of As(III) bioremediation from aqueous solution, using modified and unmodified coconut fiber. Eclet. Quím. 31, 23–29 (2006). https://doi.org/10.1590/S0100-46702006000300003
Acknowledgements
The authors Facundo Barraqué, Rosa M. Torres Sánchez, Mariela A. Fernández, Fernando S. García Einschlag, and F. Manuel Floresa are members of the National Council for Scientific and Technological Research (CONICET). The author Martina Ormaechea is member of the Scientific Investigations Commission (CIC).
Funding
This work was supported by the National Council for Scientific and Technological Research (CONICET) (PIP: 12-2013-01-00236CO).
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barraqué, F., Fernández, M.A., García Einschlag, F.S. et al. Adsorption of Bovine Serum Albumin on Magnetic Material Montmorillonite: Isotherms, Kinetic, Thermodynamic, and Mechanism Studies. Arab J Sci Eng (2024). https://doi.org/10.1007/s13369-023-08649-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13369-023-08649-0