Abstract
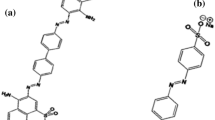
In this study, zeolite clay was modified with Fe-Al LDH (layered double hydroxide or hydrotalcite) under ultrasonic process and was then used to remove methyl violet (MV) and Nile blue (NB) dyes from aqueous media. Various analyses such as FT-IR, EDX, SEM, and XRD were done to evaluate the adsorbent properties. The highest sorption efficiency of MV and NB dyes was 99.15% and 98.16%, respectively, which indicate significant adsorption efficiencies. The highest adsorption efficiency of MV dye was obtained at pH 8, adsorbent dosage of 1 g/L, contact time of 40 min, dye concentration of 20 mg/L and temperature of 25 °C. Also, the highest adsorption efficiency of NB dye was obtained at pH 9, adsorbent dosage of 1.25 g/L, contact time of 40 min, dye concentration of 20 mg/L and temperature of 25 °C. In addition, the maximum adsorption capacity of MV and NB dyes was found to be 81.98 and 60.61 mg/g, respectively, which are significant values. Moreover, the maximum desorption efficiencies of MV and NB were achieved 99.14% and 98.67%, respectively. Furthermore, the sorption mechanism showed that the intraparticle and film diffusion processes are important in the adsorption process. The aforementioned adsorbent can be effectively reused for five consecutive cycles. Equilibrium and kinetic studies indicated that the Freundlich isotherm model and pseudo-second-order kinetic model fit the experimental data well.










Similar content being viewed by others
References
Basheer, A.A.: New generation nano-adsorbents for the removal of emerging contaminants in water. J. Mol. Liq. 261, 583–593 (2018). https://doi.org/10.1016/j.molliq.2018.04.021
Boushehrian, M.M.; Esmaeili, H.; Foroutan, R.: Ultrasonic assisted synthesis of Kaolin/CuFe2O4 nanocomposite for removing cationic dyes from aqueous media. J. Environ. Chem. Eng. 8, 103869 (2020). https://doi.org/10.1016/j.jece.2020.103869
Al-Zoubi, H.; Zubair, M.; Manzar, M.S.; Manda, A.A.; Blaisi, N.I.; Qureshi, A.; Matani, A.: Comparative adsorption of anionic dyes (eriochrome black t and Congo red) onto jojoba residues: isotherm, kinetics and thermodynamic studies. Arab. J. Sci. Eng. 45, 7275–7287 (2020). https://doi.org/10.1007/s13369-020-04418-5
Halim, K.A.; Yong, E.L.: Integrating two-stage up-flow anaerobic sludge blanket with a single-stage aerobic packed-bed reactorfor raw palm oil mill effluent treatment. Water Conserv. Manag. 2, 1–4 (2018). https://doi.org/10.26480/wcm.01.2018.01.04
Tahir, U.; Yasmin, A.; Khan, U.H.: Phytoremediation: potential flora for synthetic dyestuff metabolism. J. King Saud Univ. Sci. 28, 119–130 (2016). https://doi.org/10.1016/j.jksus.2015.05.009
Wang, W.-Y.; Ku, Y.: Photocatalytic degradation of Reactive Red 22 in aqueous solution by UV-LED radiation. Water Res. 40, 2249–2258 (2006). https://doi.org/10.1016/j.watres.2006.04.041
Jones, J.J.; Falkinham, J.O.: Decolorization of malachite green and crystal violet by waterborne pathogenic mycobacteria. Antimicrob. Agents Chemother. 47, 2323–2326 (2003). https://doi.org/10.1128/AAC.47.7.2323-2326.2003
Sarwan, B.; Pare, B.; Acharya, A.: Heterogeneous photocatalytic degradation of nile blue dye in aqueous BiOCl suspensions. Appl. Surf. Sci. 301, 99–106 (2014). https://doi.org/10.1016/j.apsusc.2014.01.136
Bansal, R.C.; Goyal, M.: Activated Carbon Adsorption. CRC Press, Boca Raton (2005)
Mota, J.P.; Lyubchik, S.: Recent advances in adsorption processes for environmental protection and security. Springer, Amsterdam (2008)
Gözmen, B.; Kayan, B.; Gizir, A.M.; Hesenov, A.: Oxidative degradations of reactive blue 4 dye by different advanced oxidation methods. J. Hazard. Mater. 168, 129–136 (2009). https://doi.org/10.1016/j.jhazmat.2009.02.011
Bessaim, M.M.; Missoum, H.; Bendani, K.; Bekkouche, M.S.; Laredj, N.: Removal of hazardous cationic salt pollutants during electrochemical treatment from contaminated mixed heterogeneous saline soil. Arab. J. Sci. Eng. 44, 4783–4794 (2019). https://doi.org/10.1007/s13369-018-3551-1
He, L.; Li, M.X.; Chen, F.; Yang, S.S.; Ding, J.; Ding, L.; Ren, N.Q.: Novel coagulation waste-based Fe-containing carbonaceous catalyst as peroxymonosulfate activator for pollutants degradation: role of ROS and electron transfer pathway. J. Hazard. Mater. 417, 126113 (2021). https://doi.org/10.1016/j.jhazmat.2021.126113
Zhang, X.; Sun, X.; Lv, T.; Weng, L.; Chi, M.; Shi, J.; Zhang, S.: Preparation of PI porous fiber membrane for recovering oil-paper insulation structure. J. Mater. Sci. Mater. Electron. 31, 13344–13351 (2020). https://doi.org/10.1007/s10854-020-03888-5
Ali, I.; Burakov, A.E.; Melezhik, A.V.; Babkin, A.V.; Burakova, I.V.; Neskomornaya, M.E.A.; Galunin, E.V.; Tkachev, A.G.; Kuznetsov, D.V.: Removal of copper (II) and zinc (II) ions in water on a newly synthesized polyhydroquinone/graphene nanocomposite material: kinetics, thermodynamics and mechanism. ChemistrySelect 4, 12708–12718 (2019). https://doi.org/10.1002/slct.201902657
Ali, I.; Babkin, A.V.; Burakova, I.V.; Burakov, A.E.; Neskoromnaya, E.A.; Tkachev, A.G.; Panglisch, S.; AlMasoud, N.; Alomar, T.S.: Fast removal of samarium ions in water on highly efficient nanocomposite based graphene oxide modified with polyhydroquinone: isotherms, kinetics, thermodynamics and desorption. J. Mol. Liq. 329, 115584 (2021). https://doi.org/10.1016/j.molliq.2021.115584
Ali, I.; Kon’kova, T.; Kasianov, V.; Rysev, A.; Panglisch, S.; Mbianda, X.Y.; Habila, M.A.; AlMasoud, N.: Preparation and characterization of nano-structured modified montmorillonite for dioxidine antibacterial drug removal in water. J. Mol. Liq. 331, 115770 (2021). https://doi.org/10.1016/j.molliq.2021.115770
Ali, I.; Afshinb, S.; Poureshgh, Y.; Azari, A.; Rashtbari, Y.; Feizizadeh, A.; Hamzezadeh, A.; Fazlzadeh, M.: Green preparation of activated carbon from pomegranate peel coated with zero-valent iron nanoparticles (nZVI) and isotherm and kinetic studies of amoxicillin removal in water. Environ. Sci. Pollut. Res. 27, 36732–36743 (2020). https://doi.org/10.1007/s11356-020-09310-1
Ai, L.; Huang, H.; Chen, Z.; Wei, X.; Jiang, J.: Activated carbon/CoFe2O4 composites: facile synthesis, magnetic performance and their potential application for the removal of malachite green from water. Chem. Eng. J. 156, 243–249 (2010). https://doi.org/10.1016/j.cej.2009.08.028
Mahini, R.; Esmaeili, H.; Foroutan, R.: Adsorption of methyl violet from aqueous solution using brown algae Padina sanctae-crucis. Turk. J. Biochem. 43, 623–631 (2018). https://doi.org/10.1515/tjb-2017-0333
Esmaeili, H.; Foroutan, R.: Adsorptive behavior of methylene blue onto sawdust of sour lemon, date palm, and eucalyptus as agricultural wastes. J. Dispers. Sci. Technol. 40, 990–999 (2019). https://doi.org/10.1080/01932691.2018.1489828
Foroutan, R.; Mohammadi, R.; Ramavandi, B.: Elimination performance of methylene blue, methyl violet, and Nile blue from aqueous media using AC/CoFe2O4 as a recyclable magnetic composite. Environ. Sci. Pollut. Res. 26, 19523–19539 (2019). https://doi.org/10.1007/s11356-019-05282-z
Özcan, A.S.; Erdem, B.; Özcan, A.: Adsorption of Acid Blue 19 from aqueous solutions onto Na–bentonite and DTMA–bentonite. J. Colloid Interface Sci. 280, 44–54 (2004). https://doi.org/10.1016/j.jcis.2004.07.035
Bouberka, Z.; Kacha, S.; Kameche, M.; Elmaleh, S.; Derriche, Z.: Sorption study of an acid dye from an aqueous solutions using modified clays. J. Hazard. Mater. 119, 117–124 (2005). https://doi.org/10.1016/j.jhazmat.2004.11.026
Harris, R.G.; Wells, J.D.; Johnson, B.B.: Selective adsorption of dyes and other organic molecules to kaolinite and oxide surfaces. Colloids Surf. A Physicochem. Eng. Asp. 180, 131–140 (2001). https://doi.org/10.1016/S0927-7757(00)00747-0
Sajidu, S.; Persson, I.; Masamba, W.; Henry, E.: Mechanisms of heavy metal sorption on alkaline clays from Tundulu in Malawi as determined by EXAFS. J. Hazard. Mater. 158, 401–409 (2008). https://doi.org/10.1016/j.jhazmat.2008.01.087
Garcıa-Sánchez, A.; Alastuey, A.; Querol, X.: Heavy metal adsorption by different minerals: application to the remediation of polluted soils. Sci. Total Environ. 242, 179–188 (1999). https://doi.org/10.1016/S0048-9697(99)00383-6
Sanchez, L.M.; Ollier, R.P.; Gonzalez, J.S.; Alvarez, V.A.: Nanocomposite materials for dyes removal. In: Handbook of Nanomaterials for Industrial Applications: Micro and Nano Technologies, pp. 922–951. Elsevier (2018)
Barrera, K.; Briso, A.; Ide, V.; Martorana, L.; Montes, G.; Basualto, C.; Borrmann, T.; Valenzuela, F.: Treatment of acidic mine drainage in an adsorption process using calcium silicate modified with Fe (III). Hydrometallurgy 172, 19–29 (2017). https://doi.org/10.1016/j.hydromet.2017.06.016
Simpen, N.; DwiAdhiSuastuti, N.G.A.M.; Sutha Negara, I.M.; Ratnayani, O.: Adsorption of Cr(VI) using the adsorbent of hydroxyapatite extracted from Bali bovine bone waste and coated with Fe-Al oxides. Res J. Chem. Environ. Sci. 6, 23–29 (2018)
Liu, Z.; Yu, J.; Yang, L.; Dai, Y.; Wang, Y.; Zhou, L.: Preparation of Fe-loaded activated carbon and its adsorption property of U (VI) in aqueous solution. J. Radioanal. Nucl. Chem. 317, 1223–1233 (2018). https://doi.org/10.1007/s10967-018-6037-4
Ali, I.; Alharbi, O.M.; Tkachev, A.; Galunin, E.; Burakov, A.; Grachev, V.A.: Water treatment by new-generation graphene materials: hope for bright future. Environ. Sci. Pollut. Res. 25, 7315–7329 (2018). https://doi.org/10.1007/s11356-018-1315-9
Ma, Z.; Zhang, Q.; Weng, X.; Mang, C.; Si, L.; Guan, Z.; Cheng, L.: Fluoride ion adsorption from wastewater using magnesium (II), aluminum (III) and titanium (IV) modified natural zeolite: kinetics, thermodynamics, and mechanistic aspects of adsorption. J. Water Reuse Desal. 8, 479–489 (2018). https://doi.org/10.2166/wrd.2017.037
Lǚ, J.; Liu, H.; Liu, R.; Zhao, X.; Sun, L.; Qu, J.: Adsorptive removal of phosphate by a nanostructured Fe–Al–Mn trimetal oxide adsorbent. Powder Technol. 233, 146–154 (2013). https://doi.org/10.1016/j.powtec.2012.08.024
Lashanizadegan, M.; Esfandiari, Z.; Mirzazadeh, H.: Evaluation performance of Fe–Mn–Ce–O mixed metal oxides and Fe–Mn–Ce–O/Montmorillonite for adsorption of azo dyes in aqueous solution and oxidation reaction. Mater. Res. Express. 6, 125028 (2019). https://doi.org/10.1088/2053-1591/ab5550
Choi, H.-J.; Yu, S.-W.; Kim, K.H.: Efficient use of Mg-modified zeolite in the treatment of aqueous solution contaminated with heavy metal toxic ions. J. Taiwan Inst. Chem. E. 63, 482–489 (2016). https://doi.org/10.1016/j.jtice.2016.03.005
Llorente, A.; Serrano, B.; Baselga, J.: The effect of polymer grafting in the dispersibility of alumina/polysulfone nanocomposites. Macromol. Res. 25, 11–20 (2017). https://doi.org/10.1007/s13233-016-4150-1
Zheng, Z.; Ma, X.; Zhang, Z.; Li, Y.: In-situ transition of amorphous gels to Na-P1 zeolite in geopolymer: mechanical and adsorption properties. Constr. Build. Mater. 202, 851–860 (2019). https://doi.org/10.1016/j.conbuildmat.2019.01.067
Pooladi, H.; Foroutan, R.; Esmaeili, H.: Synthesis of wheat bran sawdust/Fe 3 O 4 composite for the removal of methylene blue and methyl violet. Environ. Monit. Assess. 193, 276 (2021). https://doi.org/10.1007/s10661-021-09051-9
Ramesh, A.; Hasegawa, H.; Maki, T.; Ueda, K.: Adsorption of inorganic and organic arsenic from aqueous solutions by polymeric Al/Fe modified montmorillonite. Sep. Purif. Technol. 56, 90–100 (2007). https://doi.org/10.1016/j.seppur.2007.01.025
Hu, L.B.; Huang, X.Y.; Zhang, S.; Chen, X.; Dong, X.H.; Jin, H.; Jiang, Z.Y.; Gong, X.R.; Xie, Y.X.; Li, C.; Chi, Z.T.: MoO3 structures transition from nanoflowers to nanorods and their sensing performances. Mater. Electron. 32, 23728 (2021). https://doi.org/10.1007/s10854-021-06464-7
Zhang, X.; Tang, Y.; Zhang, F.; Lee, C.S.: A novel aluminum–graphite dual-ion battery. Adv. Energy Mater. 6, 1502588 (2016). https://doi.org/10.1002/aenm.201502588
Guan, Q.; Zeng, G.; Song, J.; Liu, C.; Wang, Z.; Wu, S.: Ultrasonic power combined with seed materials for recovery of phosphorus from swine wastewater via struvite crystallization process. J. Environ. Manag. 293, 112961 (2021). https://doi.org/10.1016/j.jenvman.2021.112961
Li, M.; Liu, H.; Duan, P.; Ruan, S.; Zhang, Z.; Ge, W.: The effects of lithium slag on microstructure and mechanical performance of metakaolin-based geopolymers designed by response surface method (RSM). Constr. Build Mater. 299, 123950 (2021). https://doi.org/10.1016/j.conbuildmat.2021.123950
Zhong, P.; Yu, Q.; Zhao, J.; Xu, S.; Qiu, X.; Chen, J.: Degradation of bisphenol A by Fe-Al layered double hydroxides: a new synergy of homo-and heterogeneous Fenton systems. J. Colloid Interface Sci. 552, 122–133 (2019). https://doi.org/10.1016/j.jcis.2019.05.040
Takmil, F.; Esmaeili, H.; Mousavi, S.M.; Hashemi, S.A.: Nano-magnetically modified activated carbon prepared by oak shell for treatment of wastewater containing fluoride ion. Adv. Powder Technol. 31, 3236–3245 (2020). https://doi.org/10.1016/j.apt.2020.06.015
Bind, A.; Goswami, L.; Prakash, V.: Comparative analysis of floating and submerged macrophytes for heavy metal (copper, chromium, arsenic and lead) removal: sorbent preparation, characterization, regeneration and cost estimation. Geol. Ecol. Landsc. 2, 61–72 (2018). https://doi.org/10.1080/24749508.2018.1452460
Hameed, B.H.: Spent tea leaves: a new non-conventional and low-cost adsorbent for removal of basic dye from aqueous solutions. J. Hazard. Mater. 161, 753–759 (2009). https://doi.org/10.1016/j.jhazmat.2008.04.019
Yang, X.; Li, Y.; Du, Q.; Sun, J.; Chen, L.; Hu, S.; Wang, Z.; Xia, Y.; Xia, L.: Highly effective removal of basic fuchsin from aqueous solutions by anionic polyacrylamide/graphene oxide aerogels. J. Colloid Interface Sci. 453, 107–114 (2015). https://doi.org/10.1016/j.jcis.2015.04.042
Basheer, A.A.; Ali, I.: Stereoselective uptake and degradation of (±)-o, p-DDD pesticide stereomers in water-sediment system. Chirality 30, 1088–1095 (2018). https://doi.org/10.1002/chir.22989
Sen, B.; Goswami, S.; Devi, G.; Sarma, H.P.; Bind, A.: Valorization of Adenanthera pavonina seeds as a potential biosorbent for lead and cadmium removal from single and binary contaminated system. Geol. Ecol. Landsc. 2, 275–287 (2018). https://doi.org/10.1080/24749508.2018.1464266
Ali, I.; Alharbi, O.M.; ALOthman, Z.A.; Alwarthan, A.; Al-Mohaimeed, A.M.: Preparation of a carboxymethylcellulose-iron composite for uptake of atorvastatin in water. Int. J. Biol. Macromol. 132, 244–253 (2019). https://doi.org/10.1016/j.ijbiomac.2019.03.211
Abetua, A.G.; Kebedeb, A.B.: Crushed concrete as adsorptive material for removal of phosphate ions from aqueous solutions. Water Conserv. Manag. 2, 40–46 (2021). https://doi.org/10.26480/wcm.02.2021.40.46
Fanta, A.B.; Nair, A.M.; Sægrov, S.; Østerhus, S.W.: Phosphorus removal from industrial discharge impacted municipal wastewater using sequencing batch moving bed biofilm reactor. J. Water Process Eng. 41, 102034 (2021). https://doi.org/10.1016/j.jwpe.2021.102034
Zafisah, N.S.; Ang, W.L.; Mohammad, A.W.: Cake filtration for suspended solids removal in digestate from anaerobic digested palm oil mill effluent (pome). Water Conserv. Manag. 2, 5–9 (2018). https://doi.org/10.26480/wcm.01.2018.05.09
Chen, Y.; He, L.; Li, J.; Zhang, S.: Multi-criteria design of shale-gas-water supply chains and production systems towards optimal life cycle economics and greenhouse gas emissions under uncertainty. Comput. Chem. Eng. 109, 216–235 (2018). https://doi.org/10.1016/j.compchemeng.2017.11.014
Vitela-Rodriguez, A.V.; Rangel-Mendez, J.R.: Arsenic removal by modified activated carbons with iron hydro (oxide) nanoparticles. J. Environ. Manag. 114, 225–231 (2013). https://doi.org/10.1016/j.jenvman.2012.10.004
Anayurt, R.A.; Sari, A.; Tuzen, M.: Equilibrium, thermodynamic and kinetic studies on biosorption of Pb (II) and Cd (II) from aqueous solution by macrofungus (Lactarius scrobiculatus) biomass. Chem. Eng. J. 151, 255–261 (2009). https://doi.org/10.1016/j.cej.2009.03.002
Twang, S.M.; Zaini, M.A.A.; Salleh, L.M.; Azizi, M.; Yunus, C.; Naushad, M.: Potassium hydroxide-treated palm kernel shell sorbents for the efficient removal of methyl violet dye. Desalin. Water Treat. 84, 262–270 (2017). https://doi.org/10.5004/dwt.2017.21206
Abbasi, S.; Noorizadeh, H.: Adsorption of Nile Blue A from aqueous solution by different nanostructured carbon adsorbents. Carbon Lett. 23, 30–37 (2017). https://doi.org/10.5714/CL.2017.23.030
Korkmaz, M.; Özmetin, C.; Fil, B.A.; Özmetin, E.; Yaşar, Y.: Methyl violet dye adsorption onto clinoptilolite (natural zeolite): isotherm and kinetic study. Fresen. Environ. Bull. 22, 1526–1536 (2013)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamali, M., Esmaeili, H. & Tamjidi, S. Synthesis of Zeolite Clay/Fe-Al Hydrotalcite Composite as a Reusable Adsorbent for Adsorption/Desorption of Cationic Dyes. Arab J Sci Eng 47, 6651–6665 (2022). https://doi.org/10.1007/s13369-022-06580-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-022-06580-4