Abstract
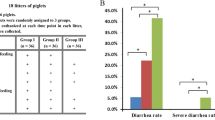
A total of 150 21-day-old weaned piglets [(Yorkshire × Landrace) × Duroc] were randomly assigned to 3 groups (CON, TRT1, TRT2) to evaluate the effects of dietary supplementation of probiotic, paraprobiotic, and hydrolyzed yeast mixture (PPY) on growth performance, nutrient digestibility, fecal bacterial counts, fecal calprotectin contents, and diarrhea rate in a 42-day experiment (phase 1: days 1–14; phase 2: days 15–42). There were 10 replicate pens per treatment with 5 pigs per pen (three gilts and two barrows). Pigs in CON were only provided with a basal diet. Pigs in TRT1 were provided with a basal diet + 3000 mg/kg zinc oxide during phase 1 and a basal diet during phase 2. Pigs in TRT2 were provided with a basal diet + 200 mg/kg probiotic (Saccharomyces cerevisiae boulardii) + 800 mg/kg paraprobiotic (inactivated yeast strains of Saccharomyces cerevisiae and Cyberlindnera jadinii) + 10 g/kg hydrolyzed yeast mixture during phase 1, and a basal diet + 100 mg/kg probiotic + 400 mg/kg paraprobiotic mixture during phase 2. Pigs in TRT1 and TRT2 were significantly heavier at day 14 and 42 than CON pigs. Growth rate during days 1–14, 15–42, and 1–42 and feed efficiency during days 1–14 were similarly affected by treatment while feed efficiency was significantly higher for TRT2 pigs between 15–42 and 1–42 days. Moreover, nitrogen and energy digestibility in both TRT1 and TRT2 were higher than that in CON. During experimental periods, diarrhea rate in TRT1 and TRT2 was lower than that in CON. Therefore, we demonstrated that PPY supplementation had comparable effects as ZnO in improving growth performance and nutrient digestibility as well as ameliorating post-weaning diarrhea in weaned piglets.
Similar content being viewed by others
Abbreviations
- ADG:
-
Average daily gain
- ADFI:
-
Average daily feed intake
- ATTD:
-
Apparent total tract digestibility
- DM:
-
Dry matter
- PPY:
-
Probiotic, paraprobiotic, and hydrolyzed yeast mixture
- ZnO:
-
Zinc oxide
References
Lallès JP, Favier C, Jondreville C (2007) A diet moderately deficient in zinc induces limited intestinal alterations in weaned pigs. Livest Sci 108:153–155. https://doi.org/10.1016/j.livsci.2007.01.033
Wang W, Chen J, Zhou H, Wang L, Ding S, Wang Y, Song D, Li A (2018) Effects of microencapsulated Lactobacillus plantarum and fructooligosaccharide on growth performance, blood immune parameters, and intestinal morphology in weaned piglets. Food Agr Immunol 29:84–94. https://doi.org/10.1080/09540105.2017.1360254
Pié S, Lallès JP, Blazy F, Laffitte J, Sève B, Oswald IP (2004) Weaning is associated with an upregulation of expression of inflammatory cytokines in the intestine of piglets. J Nutr 134:641–647. https://doi.org/10.1093/jn/134.3.641
Hung YT, Hu Q, Faris RJ, Guo J, Urriola PE, Shurson GC, Chen C, Saqui-Salces M (2020) Analysis of gastrointestinal responses revealed both shared and specific targets of zinc oxide and carbadox in weaned pigs. Antibiotics 9:463. https://doi.org/10.3390/antibiotics9080463
Kim JC, Hansen CF, Mullan BP, Pluske JR (2012) Nutrition and pathology of weaner pigs: nutritional strategies to support barrier function in the gastrointestinal tract. Anim Feed Sci Tech 173:3–16. https://doi.org/10.1016/j.anifeedsci.2011.12.022
Liu P (2014) Influence of dietary zinc oxide on morphological and immunological characteristics in the jejunum and colon of weaned piglets [PhD dissertation]. Freie Universität Berlin, Berlin
Boontiam W, Wachirapakorn C, Phaengphairee P (2020) Effects of hydrolyzed yeast supplementation on growth performance, immunity, antioxidant capacity, and microbial shedding in weaning pigs. Vet World 13:1902–1909. https://doi.org/10.14202/2Fvetworld.2020.1902-1909
Yu T, Zhu C, Chen S, Gao L, Lv H, Feng R, Zhu Q, Xu J, Chen Z, Jiang Z (2017) Dietary high zinc oxide modulates the microbiome of ileum and colon in weaned piglets. Front Microbiol 8:825. https://doi.org/10.3389/fmicb.2017.00825
Zhu C, Lv H, Chen Z, Wang L, Wu X, Chen Z, Zhang W, Liang R, Jiang Z (2017) Dietary zinc oxide modulates antioxidant capacity, small intestine development, and jejunal gene expression in weaned piglets. Biol Trace Elem Res 175:331–338. https://doi.org/10.1007/s12011-016-0767-3
Zhang Q, Wu T, Li S, Meng Y, Tan Z, Wu M, Yi D, Wang L, Zhao D, Hou Y (2021) Protective effect of zinc oxide and its association with neutrophil degranulation in piglets infected with porcine epidemic diarrhea virus. Oxid Med Cell Longev 2021:3055810. https://doi.org/10.1155/2021/3055810
Bonetti A, Tugnoli B, Piva A, Grilli E (2021) Towards zero zinc oxide: feeding strategies to manage post-weaning diarrhea in piglets. Animals 11:642. https://doi.org/10.3390/ani11030642
Jensen J, Larsen MM, Bak J (2016) National monitoring study in Denmark finds increased and critical levels of copper and zinc in arable soils fertilized with pig slurry. Environ Pollut 214:334–340. https://doi.org/10.1016/j.envpol.2016.03.034
Mehrabi Z, Gill M, van Wijk M, Herrero M, Ramankutty N (2020) Livestock policy for sustainable development. Nat Food 1:160–165. https://doi.org/10.1038/s43016-020-0042-9
Wang W, Van Noten N, Degroote J, Romeo A, Vermeir P, Michiels J (2019) Effect of zinc oxide sources and dosages on gut microbiota and integrity of weaned piglets. J Anim Physiol Anim Nutr 103:231–241. https://doi.org/10.1111/jpn.12999
Rafique K, Rahman A, Mahmood M (2020) Effect of dietary supplementation of different levels of saccharomyces cerevisiae on growth performance and hematology in broiler. Indian J Anim Res 54:59–64. https://doi.org/10.18805/ijar.B-695
Chu GM, Lee SJ, Jeong HS, Lee SS (2011) Efficacy of probiotics from anaerobic microflora with prebiotics on growth performance and noxious gas emission in growing pigs. Anim Sci J 82:282–290. https://doi.org/10.1111/j.1740-0929.2010.00828.x
Guerra-Ordaz AA, Molist F, Hermes RG, de Segura AG, Ragione RML, Woodward MJ, Tchorzewska MA, Collins JW, Pérez JF, Martín-Orúe SM (2013) Effect of inclusion of lactulose and Lactobacillus plantarum on the intestinal environment and performance of piglets at weaning. Anim Feed Sci Tech 185:160–168. https://doi.org/10.1016/j.anifeedsci.2013.07.009
Gaggìa F, Mattarelli P, Biavati B (2010) Probiotics and prebiotics in animal feeding for safe food production. Int J Food Microbiol 141:S15–S28. https://doi.org/10.1016/j.ijfoodmicro.2010.02.031
Hamasalim HJ (2016) Synbiotic as feed additives relating to animal health and performance. Adv Microbiol 6:288–302. https://doi.org/10.4236/aim.2016.64028
Krause DO, Bhandari SK, House JD, Nyachoti CM (2020) Response of nursery pigs to a synbiotic preparation of starch and an anti-Escherichia coli K88 probiotic. Appl Environ Microbiol 76:24. https://doi.org/10.1128/AEM.01427-10
Guerra-Ordaz AA, González-Ortiz G, La Ragione RM, Woodward MJ, Collins JW, Pérez JF, Martín-Orúe SM (2014) Lactulose and Lactobacillus plantarum, a potential complementary synbiotic to control postweaning colibacillosis in piglets. Appl Environ Microbiol 80:16. https://doi.org/10.1128/AEM.00770-14
Badia R, Zanello G, Chevaleyre C, Lizardo R, Meurens F, Martínez P, Brufau J, Salmon H (2012) Effect of Saccharomyces cerevisiae var. boulardii and β-galactomannan oligosaccharide on porcine intestinal epithelial and dendritic cells challenged in vitro with Escherichia coli F4 (K88). Vet Res 43:4. https://doi.org/10.1186/1297-9716-43-4
Tanzer JM, Thompson A, Lang C, Cooper B, Hareng L, Gamer A, Reindl A, Pompejus M (2010) Caries inhibition by and safety of Lactobacillus paracasei DSMZ16671. J Dent Res 89:921–926. https://doi.org/10.1177/2F0022034510369460
Nakamura S, Kuda T, An C, Kanno T, Takahashi H, Kimura B (2012) Inhibitory effects of Leuconostoc mesenteroides 1RM3 isolated from narezushi, a fermented fish with rice, on Listeria monocytogenes infection to Caco-2 cells and A/J mice. Anaerobe 18:19–24. https://doi.org/10.1016/j.anaerobe.2011.11.006
Patel RM, Denning PW (2013) Therapeutic use of prebiotics, probiotics, and postbiotics to prevent necrotizing enterocolitis: what is the current evidence? Clin Perinatol 40:11–25. https://doi.org/10.1016/j.clp.2012.12.002
Taverniti V, Guglielmetti S (2011) The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept). Genes Nutr 6:261–274. https://doi.org/10.1007/s12263-011-0218-x
Yang HL, Xia HQ, Ye YD, Zou WC, Sun YZ (2014) Probiotic Bacillus pumilus SE5 shapes the intestinal microbiota and mucosal immunity in grouper Epinephelus coioides. Dis Aquat Org 111:119–127. https://doi.org/10.3354/dao02772
Grześkowiak Ł, Collado MC, Beasley S, Salminen S (2014) Pathogen exclusion properties of canine probiotics are influenced by the growth media and physical treatments simulating industrial processes. J Appl Microbiol 116:1308–1314. https://doi.org/10.1111/jam.12477
Biswas G, Korenaga H, Nagamine R, Takayama H, Kawahara S, Takeda S, Kikuchi Y, Dashnyam B, Kono T, Sakai M (2013) Cytokine responses in the Japanese pufferfish (Takifugu rubripes) head kidney cells induced with heat-killed probiotics isolated from the Mongolian dairy products. Fish Shellfish Immunol 34:1170–1177. https://doi.org/10.1016/j.fsi.2013.01.024
Fu R, Liang C, Chen D, Yan H, Tian G, Zheng P, He J, Yu J, Mao X, Huang Z, Luo Y (2021) Effects of dietary Bacillus coagulans and yeast hydrolysate supplementation on growth performance, immune response and intestinal barrier function in weaned piglets. J Anim Physiol Anim Nutr 105:898–907. https://doi.org/10.1111/jpn.13529
Yoon SY, Sa SJ, Cho ES, Ko HS, Choi JW, Kim JS (2020) Effects of zinc oxide and arginine on the intestinal microbiota and immune status of weaned pigs subjected to high ambient temperature. Animals 10:1537. https://doi.org/10.3390/ani10091537
Rattigan R, Sweeney T, Maher S, Ryan MT, Thornton K, O’Doherty JV (2020) Effects of reducing dietary crude protein concentration and supplementation with either laminarin or zinc oxide on the growth performance and intestinal health of newly weaned pigs. Anim Feed Sci Tech 270:114693. https://doi.org/10.1016/j.anifeedsci.2020.114693
Sato Y, Kuroki Y, Oka K, Takahashi M, Rao S, Sukegawa S, Fujimura T (2019) Effects of dietary supplementation with enterococcus faecium and clostridium butyricum, either alone or in combination, on growth and fecal microbiota composition of post-weaning pigs at a commercial farm. Front Vet Sci 6:26. https://doi.org/10.3389/fvets.2019.00026
Wang H, Kim KP, Kim IH (2021) Evaluation of the combined effects of different dose levels of zinc oxide with probiotics complex supplementation on the growth performance, nutrient digestibility, faecal microbiota, noxious gas emissions and faecal score of weaning pigs. J Anim Physiol Anim Nutr 105:286–293. https://doi.org/10.1111/jpn.13493
Wang H, Ha BD, Kim IH (2021) Effects of probiotics complex supplementation in low nutrient density diet on growth performance, nutrient digestibility, faecal microbial, and faecal noxious gas emission in growing pigs. Ital J Anim Sci 20:163–170. https://doi.org/10.1080/1828051X.2020.1801358
Gómez S, Angeles ML, Mojica MC, Jalukar S (2012) Combination of an enzymatically hydrolyzed yeast and yeast culture with a direct-fed microbial in the feeds of broiler chickens. Asian-Australas J Anim Sci 25:665–673. https://doi.org/10.5713/ajas.2011.11316
NRC (2012) Nutrient requirements of swine. 11th rev. ed. Natl Acad. Press, Washington, DC
AOAC (2000) Official method of analysis. 17th ed. Assoc. Off. Anal. Chem., Arlington, VA
SAS 9.4 (2014) Applied Statistics and the SAS Programming Language. SAS Institute Inc, Cary, NC
Sommer F, Bckhed F (2013) The gut microbiota-masters of host development and physiology. Nat Rev Microbiol 11:227–238. https://doi.org/10.1038/nrmicro2974
Valdovska A, Jemeljanovs A, Pilmane M, Zitare I, Konosonoka IH, Lazdins M (2014) Alternative for improving gut microbiota: Use of Jerusalem artichoke and probiotics in diet of weaned piglets. Pol J Vet Sci 17:61–69. https://doi.org/10.2478/pjvs-2014-0008
Sun HY, Kim IH (2020) Coated omega-3 fatty acid from linseed oil positively affect sow immunoglobulin G concentration and pre-weaning performance of piglet. Anim Feed Sci Tech 269:114676. https://doi.org/10.1016/j.anifeedsci.2020.114676
Gresse R, Chaucheyras-Durand F, Fleury MA, Van de Wiele T, Forano E, Blanquet-Diot S (2017) Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol 25:851–873. https://doi.org/10.1016/j.tim.2017.05.004
Che L, Zhou Q, Liu Y, Hu L, Peng X, Wu C, Zhang R, Tang J, Wu F, Fang Z, Lin Y, Xu S, Feng B, Li J, Jiang P, Wu D, Chen D (2019) Flaxseed oil supplementation improves intestinal function and immunity, associated with altered intestinal microbiome and fatty acid profile in pigs with intrauterine growth retardation. Food Funct 10:8149–8160. https://doi.org/10.1039/C9FO01877H
Lei XJ, Kim IH (2018) Low dose of coated zinc oxide is as effective as pharmacological zinc oxide in promoting growth performance, reducing fecal scores, and improving nutrient digestibility and intestinal morphology in weaned pigs. Anim Feed Sci Technol 245:117-125. https://doi.org/10.1016/j.anifeedsci.2018.06.011
Liu JB, Cao SC, Liu J, Xie YN, Zhang HF (2018). Effect of probiotics and xylo-oligosaccharide supplementation on nutrient digestibility, intestinal health and noxious gas emission in weanling pigs. Asian-australas. J. Anim. Sci 31:1660. https://doi.org/10.5713/ajas.17.0908
Rhouma M, Fairbrother JM, Beaudry F, Letellier A (2017) Post weaning diarrhea in pigs: risk factors and non-colistin-based control strategies. Acta Vet Scand 59:1–19. https://doi.org/10.1186/s13028-017-0299-7
Fouhse JM, Fouhse RT, Willing BP (2016) The role of gut microbiota in the health and disease of pigs. Anim Front 6:30–36. https://doi.org/10.2527/af.2016-0031
Huang C, Song P, Fan P, Hou C, Thacker P, Ma X (2015) Dietary sodium butyrate decreases postweaning diarrhea by modulating intestinal permeability and changing the bacterial communities in weaned piglets. J Nutr 145:2774–2780. https://doi.org/10.3945/jn.115.217406
Sargeant HR, Miller HM, Shaw MA (2011) Inflammatory response of porcine epithelial IPEC J2 cells to enterotoxigenic E. coli infection is modulated by zinc supplementation. Mol Immunol 48:2113–2121. https://doi.org/10.1016/j.molimm.2011.07.002
Tarr PI, Gordon CA, Chandler WL (2005) Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. The Lancet 365:19–25. https://doi.org/10.1016/S0140-6736(05)71144-2
De UK, Mukherjee R, Prakash C, Hanumanthagouda B, Patel M, Nandi S, Dimri U, Verma AK, Verma MR (2019) Adding a bio-response modifier and zinc oxide to piglet weaner diets influences immunological responses to weaning. Anim Prod Sci 59:140–147. https://doi.org/10.1071/AN16332
Xiang Q, Wu X, Pan Y, Wang L, Cui C, Guo Y, Zhu L, Peng J, Wei H (2020) Early-life intervention using fecal microbiota combined with probiotics promotes gut microbiota maturation, regulates immune system development, and alleviates weaning stress in piglets. Int J Mol Sci 21:503. https://doi.org/10.3390/ijms21020503
Liu PPXS, Piao XS, Thacker PA, Zeng ZK, Li PF, Wang D, Kim SW (2010) Chito-oligosaccharide reduces diarrhea incidence and attenuates the immune response of weaned pigs challenged with Escherichia coli K88. J Anim Sci 88:3871–3879. https://doi.org/10.2527/jas.2009-2771
Sun YB, Xia T, Wu H, Zhang WJ, Zhu YH, Xue JX, He DT, Zhang LY (2019) Effects of nano zinc oxide as an alternative to pharmacological dose of zinc oxide on growth performance, diarrhea, immune responses, and intestinal microflora profile in weaned piglets. Anim Feed Sci Technol 258:114312. https://doi.org/10.1016/j.anifeedsci.2019.114312
Author information
Authors and Affiliations
Contributions
Conceptualization: Mathieu Castex, Eric Chevaux, David Saornil, and Fernando Bravo de Laguna; data curation: De Xin Dang and In Ho Kim; formal analysis: Si Young Choi, Young Jae Choi, and Jong Hwa Lee; investigation: De Xin Dang, Si Young Choi, Young Jae Choi, and Jong Hwa Lee; methodology: Mathieu Castex, Eric Chevaux, David Saornil, Fernando Bravo de Laguna, and In Ho Kim; software: De Xin Dang, Mathieu Castex, Eric Chevaux, David Saornil, Fernando Bravo de Laguna, and Guillermo Jimenez; supervision: In Ho Kim; roles/writing original draft: De Xin Dang; writing, review, and editing: De Xin Dang.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dang, D.X., Choi, S.Y., Choi, Y.J. et al. Probiotic, Paraprobiotic, and Hydrolyzed Yeast Mixture Supplementation Has Comparable Effects to Zinc Oxide in Improving Growth Performance and Ameliorating Post-weaning Diarrhea in Weaned Piglets. Probiotics & Antimicro. Prot. 16, 249–258 (2024). https://doi.org/10.1007/s12602-022-10008-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-022-10008-8