Abstract
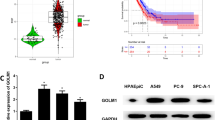
Lung adenocarcinoma (LUAD) is a predominant malignancy, and its high mortality prompts us to incessantly probe the relevant targeted treatment. This work intended to study the molecular mechanism of ESPL1 in LUAD. Bioinformatics analysis was performed for pan-cancer and prognosis analysis as well as target gene prediction. Expression of ESPL1 mRNA and let-7c-5p was determined via qRT-PCR, and western blot was employed to detect protein level of ESPL1. Dual-luciferase reporter gene method verified the interaction between ESPL1 and let-7c-5p. Thereafter, CCK-8, wound healing, Transwell, and flow cytometry assays were utilized to investigate proliferation, migration, and apoptosis of LUAD cells. The results revealed that ESPL1 was upregulated in LUAD, which was associated with poor prognosis. Overexpressed ESPL1 promoted LUAD cells to invade, proliferate, and migrate. Furthermore, ESPL1 was a target gene of let-7c-5p. Let-7c-5p was downregulated in LUAD cells, and played a suppressive role in LUAD malignant development, while reversed by ESPL1. Taken together, it was posited that let-7c-5p/ESPL1 may be underlying therapeutic targets of LUAD.




Similar content being viewed by others
Data Availability
The data used to support the findings of this study are included within the article. The data and materials in the current study are available from the corresponding author on reasonable request.
References
Henley, S. J., Ward, E. M., Scott, S., Ma, J., Anderson, R. N., Firth, A.U., Thomas, C.C., Islami, F., Weir, H. K., Lewis, D.R., Sherman, R. L., Wu, M., Benard, V. B., Richardson, L. C., Jemal, A., Cronin, K., Kohler, B. A. (2020). Annual report to the nation on the status of cancer, part I: National cancer statistics. Cancer, 126, 2225–2249. https://doi.org/10.1002/cncr.32802
Torre, L. A., Bray, F., Siegel, R. L., Ferlay, J., Lortet-Tieulent, J., Jemal, A. (2015). Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians, 65, 87–108. https://doi.org/10.3322/caac.21262
Blandin Knight, S., Crosbie, P. A., Balata, H., Chudziak, J., Hussell, T., Dive, C. (2017). Progress and prospects of early detection in lung cancer. Open Biology. https://doi.org/10.1098/rsob.170070
Pan, Z., Liu, H., & Chen, J. (2022). Lung cancer stem-like cells and drug resistance. Zhongguo fei ai za zhi = Chinese Journal of Lung Cancer, 25, 111–117. https://doi.org/10.3779/j.issn.1009-3419.2022.102.02
Heng, W. S., Gosens, R., & Kruyt, F. A. E. (2019). Lung cancer stem cells: Origin, features, maintenance mechanisms and therapeutic targeting. Biochemical pharmacology, 160, 121–133. https://doi.org/10.1016/j.bcp.2018.12.010
Nasmyth, K., & Haering, C. H. (2009). Cohesin: Its roles and mechanisms. Annual Review of Genetics, 43, 525–558. https://doi.org/10.1146/annurev-genet-102108-134233
Uhlmann, F. (2001). Secured cutting: Controlling separase at the metaphase to anaphase transition. EMBO Reports, 2, 487–492. https://doi.org/10.1093/embo-reports/kve113
Mukherjee, M., Ge, G., Zhang, N., Edwards, D. G., Sumazin, P., Sharan, S. K., Rao, P. H., Medina, D., Pati, D. (2014). MMTV-Espl1 transgenic mice develop aneuploid, estrogen receptor alpha (ERalpha)-positive mammary adenocarcinomas. Oncogene, 33, 5511–5522. https://doi.org/10.1038/onc.2013.493
Tan, K. S., Armugam, A., Sepramaniam, S., Lim, K. Y., Setyowati, K. D., Wang, C. W., Jeyaseelan, K. (2009). Expression profile of MicroRNAs in young stroke patients. PLoS ONE, 4, e7689. https://doi.org/10.1371/journal.pone.0007689
Li, J., Wang, Y., Song, Y., Fu, Z., & Yu, W. (2014). miR-27a regulates cisplatin resistance and metastasis by targeting RKIP in human lung adenocarcinoma cells. Molecular Cancer, 13, 193. https://doi.org/10.1186/1476-4598-13-193
An, J. C., Shi, H. B., Hao, W. B., Zhu, K., & Ma, B. (2019). miR-944 inhibits lung adenocarcinoma tumorigenesis by targeting STAT1 interaction. Oncology Letters, 17, 3790–3798. https://doi.org/10.3892/ol.2019.10045
Zhang, X., Zhu, J., Xing, R., Tie, Y., Fu, H., Zheng, X., Yu, B. (2012). miR-513a-3p sensitizes human lung adenocarcinoma cells to chemotherapy by targeting GSTP1. Lung Cancer, 77, 488–494. https://doi.org/10.1016/j.lungcan.2012.05.107
Roush, S., & Slack, F. J. (2008). The let-7 family of microRNAs. Trends in Cell Biology, 18, 505–516. https://doi.org/10.1016/j.tcb.2008.07.007
Meneely, P. M., & Herman, R. K. (1979). Lethals, steriles and deficiencies in a region of the X chromosome of Caenorhabditis elegans. Genetics, 92, 99–115. https://doi.org/10.1093/genetics/92.1.99
Reinhart, B. J., Slack, F. J., Basson, M., Pasquinelli, A. E., Bettinger, J. C., Rougvie, A. E., Horvitz, H. R., Ruvkun, G. (2000). The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature, 403, 901–906. https://doi.org/10.1038/35002607
Chen, S., Xie, C., & Hu, X. (2019). lncRNA SNHG6 functions as a ceRNA to up-regulate c-Myc expression via sponging let-7c-5p in hepatocellular carcinoma. Biochemical and Biophysical Research Communications, 519, 901–908. https://doi.org/10.1016/j.bbrc.2019.09.091
Jilek, J. L., Tu, M. J., Zhang, C., & Yu, A. M. (2020). Pharmacokinetic and pharmacodynamic factors contribute to synergism between Let-7c-5p and 5-fluorouracil in inhibiting hepatocellular carcinoma cell viability. Drug Metabolism and Disposition: The Biological Fate of Chemicals, 48, 1257–1263. https://doi.org/10.1124/dmd.120.000207
Jilek, J. L., Zhang, Q. Y., Tu, M. J., Ho, P. Y., Duan, Z., Qiu, J. X., Yu, A. M. (2019). Bioengineered Let-7c inhibits orthotopic hepatocellular carcinoma and improves overall survival with minimal immunogenicity. Molecular Therapy-Nucleic Acids, 14, 498–508. https://doi.org/10.1016/j.omtn.2019.01.007
Zhao, B., Han, H., Chen, J., Zhang, Z., Li, S., Fang, F., Zheng, Q., Ma, Y., Zhang, J., Wu, N., Yang, Y. (2014). MicroRNA let-7c inhibits migration and invasion of human non-small cell lung cancer by targeting ITGB3 and MAP4K3. Cancer Letters, 342, 43–51. https://doi.org/10.1016/j.canlet.2013.08.030
Wang, L., Xiao, X., & Du, H. (2022). The regulation of let-7c-5p on the biological characteristics of lung adenocarcinoma cells by targeting AURKB. Molecular Biotechnology, 64, 526–534. https://doi.org/10.1007/s12033-021-00446-0
Wang, Y., Zhou, Z., Chen, L., Li, Y., Zhou, Z., Chu, X. (2021). Identification of key genes and biological pathways in lung adenocarcinoma via bioinformatics analysis. Molecular and Cellular Biochemistry, 476, 931–939. https://doi.org/10.1007/s11010-020-03959-5
Travis, W. D. (2011). Pathology of lung cancer. Clinics in Chest Medicine, 32, 669–692. https://doi.org/10.1016/j.ccm.2011.08.005
Mukherjee, M., Ge, G., Zhang, N., Huang, E., Nakamura, L. V., Minor, M., Fofanov, V., Rao, P. H., Herron, A., Pati, D. (2011). Separase loss of function cooperates with the loss of p53 in the initiation and progression of T-and B-cell lymphoma, leukemia and aneuploidy in mice. PLoS ONE, 6, e22167. https://doi.org/10.1371/journal.pone.0022167
Rao, Z. L., Dong, J., Zhu, Y. Y., Chen, J., You, J., Zheng, Q., Jiang, J. J. (2013). Hepatitis B surface antigen affects the expression of lipid metabolism-related genes in HepG2 cells. Zhonghua Gan Zang Bing Za Zhi, 21, 624–630. https://doi.org/10.3760/cma.j.issn.1007-3418.2013.08.014
Finetti, P., Guille, A., Adelaide, J., Birnbaum, D., Chaffanet, M,. Bertucci, F. (2014). ESPL1 is a candidate oncogene of luminal B breast cancers. Breast Cancer Research and Treatment, 147, 51–59. https://doi.org/10.1007/s10549-014-3070-z
Sak, A., Fegers, I., Groneberg, M., & Stuschke, M. (2008). Effect of separase depletion on ionizing radiation-induced cell cycle checkpoints and survival in human lung cancer cell lines. Cell Proliferation, 41, 660–670. https://doi.org/10.1111/j.1365-2184.2008.00540.x
Fu, X., Mao, X., Wang, Y., Ding, X., & Li, Y. (2017). Let-7c-5p inhibits cell proliferation and induces cell apoptosis by targeting ERCC6 in breast cancer. Oncology Reports, 38, 1851–1856. https://doi.org/10.3892/or.2017.5839
Nwaeburu, C. C., Bauer, N., Zhao, Z., Abukiwan, A., Gladkich, J., Benner, A., Herr, I. (2016). Up-regulation of microRNA let-7c by quercetin inhibits pancreatic cancer progression by activation of Numbl. Oncotarget, 7, 58367–58380. https://doi.org/10.18632/oncotarget.11122
Funding
The article did not receive any funding.
Author information
Authors and Affiliations
Contributions
All authors contributed to data analysis, drafting, and revising the article, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflicts of interest.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors consent to submit the manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, X., Zeng, W., Zheng, D. et al. Let-7c-5p Restrains Cell Growth and Induces Apoptosis of Lung Adenocarcinoma Cells via Targeting ESPL1. Mol Biotechnol 64, 1367–1375 (2022). https://doi.org/10.1007/s12033-022-00511-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-022-00511-2