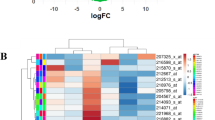
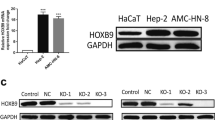
Abstract
To study the modulatory mechanism of let-7c-5p on the biological characteristics of lung adenocarcinoma (LUAD) cells by targeting AURKB. Differentially expressed genes (DEGs) were screened by bioinformatics analysis. CCK-8, colony formation, scratch healing, Transwell, and flow cytometry assays were employed to test biological functions of LUAD cells. Western Blot was undertaken to assay the protein level of AURKB, and qRT-PCR was undertaken to test AURKB mRNA and let-7c-5p expression. Dual-luciferase reporter gene method was applied to detect the interaction between AURKB and let-7c-5p. Let-7c-5p was much likely to target AURKB expression. Let-7c-5p was poorly expressed in LUAD cells and suppressed AURKB. Silencing AURKB or overexpressing let-7c-5p both could suppress proliferation, migration, and invasion and stimulate apoptosis, while overexpressing the two simultaneously could reverse such effect. Forced expression of let-7c-5p inhibited proliferation, migration, and invasion and accelerated apoptosis of LUAD cells by inhibiting AURKB, which may provide a new way to understand the malignant progression of LUAD.




Similar content being viewed by others
Data Availability
The data used to support the findings of this study are included within the article. The data and materials in the current study are available from the corresponding author on reasonable request.
References
Sung, H., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71, 209–249. https://doi.org/10.3322/caac.21660
Siegel, R. L., Miller, K. D., & Jemal, A. (2018). Cancer statistics. CA: A Cancer Journal for Clinicians, 68, 7–30. https://doi.org/10.3322/caac.21442
Devarakonda, S., Morgensztern, D., & Govindan, R. (2015). Genomic alterations in lung adenocarcinoma. The Lancet. Oncology, 16, e342-351. https://doi.org/10.1016/s1470-2045(15)00077-7
Ma, D., et al. (2020). Circ_0007142/miR-186/FOXK1 axis promoted lung adenocarcinoma progression. American Journal of Translational Research, 12, 4728–4738.
Dong, H. X., Wang, R., Jin, X. Y., Zeng, J., & Pan, J. (2018). LncRNA DGCR5 promotes lung adenocarcinoma (LUAD) progression via inhibiting hsa-mir-22-3p. Journal of Cellular Physiology, 233, 4126–4136. https://doi.org/10.1002/jcp.26215
Nie, M., et al. (2020). AURKB promotes gastric cancer progression via activation of CCND1 expression. Aging, 12, 1304–1321. https://doi.org/10.18632/aging.102684
Carmena, M., & Earnshaw, W. C. (2003). The cellular geography of aurora kinases. Nature reviews. Molecular Cell Biology, 4, 842–854. https://doi.org/10.1038/nrm1245
Goldenson, B., & Crispino, J. D. (2015). The aurora kinases in cell cycle and leukemia. Oncogene, 34, 537–545. https://doi.org/10.1038/onc.2014.14
Huang, D., et al. (2019). Relation of AURKB over-expression to low survival rate in BCRA and reversine-modulated aurora B kinase in breast cancer cell lines. Cancer Cell International, 19, 166. https://doi.org/10.1186/s12935-019-0885-z
Bertran-Alamillo, J., et al. (2019). AURKB as a target in non-small cell lung cancer with acquired resistance to anti-EGFR therapy. Nature Communications, 10, 1812. https://doi.org/10.1038/s41467-019-09734-5
Boyerinas, B., Park, S. M., Hau, A., Murmann, A. E., & Peter, M. E. (2010). The role of let-7 in cell differentiation and cancer. Endocrine-Related Cancer, 17, F19-36. https://doi.org/10.1677/erc-09-0184
Tang, H., et al. (2019). miR-let-7b and miR-let-7c suppress tumourigenesis of human mucosal melanoma and enhance the sensitivity to chemotherapy. Journal of Experimental & Clinical Cancer Research : CR, 38, 212. https://doi.org/10.1186/s13046-019-1190-3
Nadiminty, N., et al. (2012). MicroRNA let-7c is downregulated in prostate cancer and suppresses prostate cancer growth. PLoS ONE, 7, e32832. https://doi.org/10.1371/journal.pone.0032832
Wang, L., Li, J., Li, Y., & Pang, L. B. (2020). Hsa-let-7c exerts an anti-tumor function by negatively regulating ANP32E in lung adenocarcinoma. Tissue & Cell, 65, 101372. https://doi.org/10.1016/j.tice.2020.101372
Han, X., Zhang, J. J., Han, Z. Q., Zhang, H. B., & Wang, Z. A. (2018). Let-7b attenuates cisplatin resistance and tumor growth in gastric cancer by targeting AURKB. Cancer Gene Therapy, 25, 300–308. https://doi.org/10.1038/s41417-018-0048-8
Cai, L., Wang, Z., Zheng, H., & Xu, L. (2020). The let-7c/HoxB7 axis regulates the cell proliferation, migration and apoptosis in hepatocellular carcinoma. Anti-Cancer Drugs, 31, 6–18. https://doi.org/10.1097/cad.0000000000000843
Nadiminty, N., et al. (2012). MicroRNA let-7c suppresses androgen receptor expression and activity via regulation of Myc expression in prostate cancer cells. Journal of Biological Chemistry, 287, 1527–1537. https://doi.org/10.1074/jbc.M111.278705
Fu, X., Mao, X., Wang, Y., Ding, X., & Li, Y. (2017). Let-7c-5p inhibits cell proliferation and induces cell apoptosis by targeting ERCC6 in breast cancer. Oncology Reports, 38, 1851–1856. https://doi.org/10.3892/or.2017.5839
Wan, B., Huang, Y., Liu, B., Lu, L., & Lv, C. (2019). AURKB: A promising biomarker in clear cell renal cell carcinoma. PeerJ, 7, e7718. https://doi.org/10.7717/peerj.7718
Smith, S. L., et al. (2005). Overexpression of aurora B kinase (AURKB) in primary non-small cell lung carcinoma is frequent, generally driven from one allele, and correlates with the level of genetic instability. British Journal of Cancer, 93, 719–729. https://doi.org/10.1038/sj.bjc.6602779
Chieffi, P., et al. (2004). Aurora B expression in normal testis and seminomas. Journal of Endocrinology, 181, 263–270. https://doi.org/10.1677/joe.0.1810263
Sorrentino, R., et al. (2005). Aurora B overexpression associates with the thyroid carcinoma undifferentiated phenotype and is required for thyroid carcinoma cell proliferation. Journal of Clinical Endocrinology and Metabolism, 90, 928–935. https://doi.org/10.1210/jc.2004-1518
Hegyi, K., Egervari, K., Sandor, Z., & Mehes, G. (2012). Aurora kinase B expression in breast carcinoma: Cell kinetic and genetic aspects. Pathobiology, 79, 314–322. https://doi.org/10.1159/000338082
Tanaka, S., et al. (2008). Aurora kinase B is a predictive factor for the aggressive recurrence of hepatocellular carcinoma after curative hepatectomy. British Journal of Surgery, 95, 611–619. https://doi.org/10.1002/bjs.6011
Yu, D. H., et al. (2020). Analysis of the interaction network of hub miRNAs-hub genes, being involved in idiopathic pulmonary fibers and its emerging role in non-small cell lung cancer. Frontiers in Genetics, 11, 302. https://doi.org/10.3389/fgene.2020.00302
Wu, Y., et al. (2020). Circular RNA circCORO1C promotes laryngeal squamous cell carcinoma progression by modulating the let-7c-5p/PBX3 axis. Molecular cancer, 19, 99. https://doi.org/10.1186/s12943-020-01215-4
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
LW and HD contributed to the study design. XX conducted the literature search. LW acquired the data. LW wrote the article. XX performed data analysis and drafted. HD revised the article. All the authors gave the final approval of the version to be submitted.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical Approval
Not applicable.
Consent for Publication
All authors consent to submit the manuscript for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Xiao, X. & Du, H. The Regulation of let-7c-5p on the Biological Characteristics of Lung Adenocarcinoma Cells by Targeting AURKB. Mol Biotechnol 64, 526–534 (2022). https://doi.org/10.1007/s12033-021-00446-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-021-00446-0