Abstract
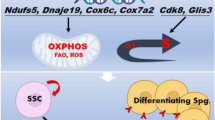
The differentiation of adult stem cells involves extensive chromatin remodeling, mediated in part by the gene products of histone deacetylase (HDAC) family members. While the transcriptional downregulation of HDACs can impede stem cell self-renewal in certain contexts, it may also promote stem cell maintenance under other circumstances. In self-renewing, differentiating, and aging spermatogonial stem cells (SSCs), the gene expression dynamics of HDACs have not yet been characterized. To gain further insight with these studies, we analyzed the transcriptional profiles of six HDAC family members, previously identified to be the most highly expressed in self-renewing SSCs, during stem cell differentiation and aging. Here we discovered that in both differentiating and aging SSCs the expression of Sirt4 increases, while the expression of Hdac2, Hdac6, and Sirt1 decreases. When SSCs are exposed to the lifespan-enhancing drug rapamycin in vivo, the resultant HDAC gene expression patterns are opposite of those seen in the differentiating and aging SSCs, with increased Hdac2, Hdac6, and Sirt1 and decreased Hdac8, Hdac9, and Sirt4. Our findings suggest that HDACs important for stem cell maintenance and oxidative capacity are downregulated as adult stem cells differentiate or age. These results provide important insights into the epigenetic regulation of stem cell differentiation and aging in mammals.



Similar content being viewed by others
References
McCool, K. W., Xu, X., Singer, D. B., Murdoch, F. E., & Fritsch, M. K. (2007). The role of histone acetylation in regulating early gene expression patterns during early embryonic stem cell differentiation. Journal of Biological Chemistry, 282, 6696–6706.
Lee, S., Park, J. R., Seo, M. S., Roh, K. H., Park, S. B., Hwang, J. W., Sun, B., et al. (2009). Histone deacetylase inhibitors decrease proliferation potential and multilineage differentiation capability of human mesenchymal stem cells. Cell Proliferation, 42, 711–720.
Bug, G., Schwarz, K., Schoch, C., Kampfmann, M., Henschler, R., Hoelzer, D., Ottmann, O. G., et al. (2007). Effect of histone deacetylase inhibitor valproic acid on progenitor cells of acute myeloid leukemia. Haematologica, 92, 542–545.
Yang, X. J., & Seto, E. (2008). The rpd3/hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nature Reviews Molecular Cell Biology, 9, 206–218.
Chalkiadaki, A., & Guarente, L. (2012). Sirtuins mediate mammalian metabolic responses to nutrient availability. Nature Reviews Endocrinology.
Montgomery, R. L., Hsieh, J., Barbosa, A. C., Richardson, J. A., & Olson, E. N. (2009). Histone deacetylases 1 and 2 control the progression of neural precursors to neurons during brain development. Proceedings of the National Academy of Sciences of the United States of America, 106, 7876–7881.
Ye, F., Chen, Y., Hoang, T., Montgomery, R. L., Zhao, X. H., Bu, H., Hu, T., et al. (2009). Hdac1 and hdac2 regulate oligodendrocyte differentiation by disrupting the beta-catenin-tcf interaction. Nature Neuroscience, 12, 829–838.
Spallotta, F., Rosati, J., Straino, S., Nanni, S., Grasselli, A., Ambrosino, V., Rotili, D., et al. (2010). Nitric oxide determines mesodermic differentiation of mouse embryonic stem cells by activating class iia histone deacetylases: potential therapeutic implications in a mouse model of hindlimb ischemia. Stem Cells, 28, 431–442.
Snyder, E. Y., & Loring, J. F. (2005). A role for stem cell biology in the physiological and pathological aspects of aging. Journal of the American Geriatrics Society, 53, S287–S291.
Jung, J. W., Lee, S., Seo, M. S., Park, S. B., Kurtz, A., Kang, S. K., & Kang, K. S. (2010). Histone deacetylase controls adult stem cell aging by balancing the expression of polycomb genes and jumonji domain containing 3. Cellular and Molecular Life Sciences, 67, 1165–1176.
Lee, S., Jung, J. W., Park, S. B., Roh, K., Lee, S. Y., Kim, J. H., Kang, S. K., et al. (2011). Histone deacetylase regulates high mobility group a2-targeting micrornas in human cord blood-derived multipotent stem cell aging. Cellular and Molecular Life Sciences, 68, 325–336.
Buageaw, A., Sukhwani, M., Ben-Yehudah, A., Ehmcke, J., Rawe, V. Y., Pholpramool, C., Orwig, K. E., et al. (2005). Gdnf family receptor alpha1 phenotype of spermatogonial stem cells in immature mouse testes. Biology of Reproduction, 73, 1011–1016.
Gassei, K., Ehmcke, J., & Schlatt, S. (2009). Efficient enrichment of undifferentiated gfr alpha 1+ spermatogonia from immature rat testis by magnetic activated cell sorting. Cell and Tissue Research, 337, 177–183.
Kubota, H., Avarbock, M. R., & Brinster, R. L. (2003). Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proceedings of the National Academy of Sciences of the United States of America, 100, 6487–6492.
Shima, J. E., McLean, D. J., McCarrey, J. R., & Griswold, M. D. (2004). The murine testicular transcriptome: characterizing gene expression in the testis during the progression of spermatogenesis. Biology of Reproduction, 71, 319–330.
Sekeri-Pataryas, K. E., & Sourlingas, T. G. (2007). The differentiation-associated linker histone, h1.0, during the in vitro aging and senescence of human diploid fibroblasts. Annals of the New York Academy of Sciences, 1100, 361–367.
Zhang, X., Ebata, K. T., Robaire, B., & Nagano, M. C. (2006). Aging of male germ line stem cells in mice. Biology of Reproduction, 74, 119–124.
Ryu, B. Y., Orwig, K. E., Oatley, J. M., Avarbock, M. R., & Brinster, R. L. (2006). Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells, 24, 1505–1511.
Kokkinaki, M., Lee, T. L., He, Z., Jiang, J., Golestaneh, N., Hofmann, M. C., Chan, W. Y., et al. (2010). Age affects gene expression in mouse spermatogonial stem/progenitor cells. Reproduction, 139, 1011–1020.
Schmidt, J. A., Abramowitz, L. K., Kubota, H., Wu, X., Niu, Z., Avarbock, M. R., Tobias, J. W., et al. (2011). In vivo and in vitro aging is detrimental to mouse spermatogonial stem cell function. Biology of Reproduction, 84, 698–706.
Hobbs, R. M., Seandel, M., Falciatori, I., Rafii, S., & Pandolfi, P. P. (2010). Plzf regulates germline progenitor self-renewal by opposing mtorc1. Cell, 142, 468–479.
Hansen, M., Taubert, S., Crawford, D., Libina, N., Lee, S. J., & Kenyon, C. (2007). Lifespan extension by conditions that inhibit translation in caenorhabditis elegans. Aging Cell, 6, 95–110.
Bjedov, I., Toivonen, J. M., Kerr, F., Slack, C., Jacobson, J., Foley, A., & Partridge, L. (2010). Mechanisms of life span extension by rapamycin in the fruit fly drosophila melanogaster. Cell Metabolism, 11, 35–46.
Harrison, D. E., Strong, R., Sharp, Z. D., Nelson, J. F., Astle, C. M., Flurkey, K., Nadon, N. L., et al. (2009). Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature, 460, 392–395.
Feng, L. X., Ravindranath, N., & Dym, M. (2000). Stem cell factor/c-kit up-regulates cyclin d3 and promotes cell cycle progression via the phosphoinositide 3-kinase/p70 s6 kinase pathway in spermatogonia. Journal of Biological Chemistry, 275, 25572–25576.
Yilmaz, O. H., Valdez, R., Theisen, B. K., Guo, W., Ferguson, D. O., Wu, H., & Morrison, S. J. (2006). Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature, 441, 475–482.
Castilho, R. M., Squarize, C. H., Chodosh, L. A., Williams, B. O., & Gutkind, J. S. (2009). Mtor mediates wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell, 5, 279–289.
Kofman, A. E., McGraw, M. R., & Payne, C. J. (2012). Rapamycin increases oxidative stress response gene expression in adult stem cells. Aging, 4, 279–289.
Luzzani, C., Solari, C., Losino, N., Ariel, W., Romorini, L., Bluguermann, C., Sevlever, G., et al. (2011). Modulation of chromatin modifying factors’ gene expression in embryonic and induced pluripotent stem cells. Biochemical and Biophysical Research Communications, 410, 816–822.
Dovey, O. M., Foster, C. T., & Cowley, S. M. (2010). Histone deacetylase 1 (hdac1), but not hdac2, controls embryonic stem cell differentiation. Proceedings of the National Academy of Sciences of the United States of America, 107, 8242–8247.
Haigis, M. C., Mostoslavsky, R., Haigis, K. M., Fahie, K., Christodoulou, D. C., Murphy, A. J., Valenzuela, D. M., et al. (2006). Sirt4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic beta cells. Cell, 126, 941–954.
Yu, W., Fu, Y. C., Zhou, X. H., Chen, C. J., Wang, X., Lin, R. B., & Wang, W. (2009). Effects of resveratrol on h(2)o(2)-induced apoptosis and expression of sirts in h9c2 cells. Journal of Cellular Biochemistry, 107, 741–747.
Qian, D. Z., Kachhap, S. K., Collis, S. J., Verheul, H. M., Carducci, M. A., Atadja, P., & Pili, R. (2006). Class ii histone deacetylases are associated with vhl-independent regulation of hypoxia-inducible factor 1 alpha. Cancer Research, 66, 8814–8821.
Acknowledgments
We thank Shannon Gallagher, Rachel Anderson, and Kristin Kalita for their assistance with these experiments, and the Medical Research Institute Council at Children’s Memorial Research Center for their generous financial support. C.J.P. is the recipient of an NIH Pathway-to-Independence Award from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. This work was supported by an NIH grant to C.J.P. (5R00 HD055330-5).
Disclosures
The authors declare no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplemental Table 1
(DOC 44 kb)
Rights and permissions
About this article
Cite this article
Kofman, A.E., Huszar, J.M. & Payne, C.J. Transcriptional Analysis of Histone Deacetylase Family Members Reveal Similarities Between Differentiating and Aging Spermatogonial Stem Cells. Stem Cell Rev and Rep 9, 59–64 (2013). https://doi.org/10.1007/s12015-012-9392-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-012-9392-5