Abstract
Microwave energy is an efficient form of energy used to speed up the synthesis of nanoparticles. Herein, we report the use of an unmodified domestic microwave oven to prepare magnetic spherical Fe3O4 (magnetite) nanoparticles (IONPs) supported on carbonized cellulose nanocrystals, forming a nanocomposite, in an expeditious and facile one-step reaction. This was achieved using the readily available precursors of FeCl3 as an iron source, and sugarcane bagasse, using activated charcoal as a microwave absorber. The nanocomposite was characterized using Raman spectroscopy which suggested a degree of crystallinity based on a G/D ratio of 1.25. The morphology of the Fe3O4/carbonized cellulose nanocrystal nanocomposite was characterized as spherical metallic nanoparticles supported on carbonized cellulose nanocrystals using Transmission electron microscopy (TEM), SEM, and EDX, while the identity of the Fe3O4 nanoparticles was confirmed with powder XRD.
Graphical Abstract

Similar content being viewed by others
Introduction
The concept of industrial metabolism and industrial ecology inspired the circular economy (CE) (Frosch and Gallopoulos 1989). It is frequently associated with reducing, reusing, and recycling activities, under the “reduce, reuse, and recycle” mantra (Kirchherr et al. 2018), to encourage economically sustainable consumption and production trends (Carus and Dammer 2018; Ubando et al. 2020). This includes the use of new technologies that are in line with this initiative, incorporating nanotechnology with ultrasound (Maleki 2018; Pérez-Beltrán et al. 2021) microwave-assisted heating(Hojati-Talemi et al. 2010), or ultraviolet irradiation (Maleki et al. 2016), among others.
Nanotechnology is an important factor behind a new industrial revolution in various interdisciplinary sectors. Nanomaterials have been widely observed to have different chemical and physical characteristics when compared to bulk material (Pires et al. 2019; Pradhan et al. 2022). The abundance of lignocellulose globally from where cellulose nanocrystals (CNCs) can be derived gave rise to CNCs as exceptional contenders for multi-functional biomaterials development and design. The abundance of hydroxyl functional groups on CNC surfaces is convenient for the tethering of metallic nanoparticles. Specific components and polymer matrices can be imparted on CNCs to modify and functionalize their electronic, magnetic, catalytic, fluorescence, and optical properties as nanocomposites (Kvien and Oksman 2007; Pranger and Tannenbaum 2008; Li et al. 2010; Pires et al. 2019; Chen et al. 2020).
Tertiary lignocellulosic biomass such as sugarcane bagasse is a leading source of cellulose and nanocellulose (Nechyporchuk et al. 2016; Pennells et al. 2020; Pradhan et al. 2022). Sugarcane bagasse is produced in copious amounts by the sugar industry—it is a globally abundant, uniform, and stable material that can be isolated in a high yield and a relatively facile manner (Westerhoff et al. 2001; Bai et al. 2021). Currently, approximately 75% of sugarcane bagasse is used as boiler fuel, while some is used for cellulosic ethanol, bioplastic, and animal feed. The material’s significant potential for application in higher-value applications is derived from its substantial lignin and cellulose content (Bai et al. 2021; Laranja et al. 2022).
Various sugarcane bagasse applications have been reported over the years. For instance, adsorption studies using sugarcane bagasse-derived materials have been conducted to remove heavy metals (Pehlivan et al. 2013; Wang et al. 2017; Bai et al. 2021), tetracycline (Rattanachueskul et al. 2017) and methylene blue (Anh et al. 2020) from aqueous solutions, while other reports had prepared nanomaterials (Alves et al. 2012) and nanocatalysts for transesterification of waste cooking oil to bio-diesel (Nazir et al. 2022). Graphene oxide has also been produced from sugarcane bagasse-derived material (Somanathan et al. 2015).
Microwave-assisted heating has been dubbed the “Bunsen burner” of the twenty-first century by some observers, this technology has enabled chemists to prepare desired materials and compounds much quicker and with better selectivity and control compared to conventional heating (Gawande et al. 2014). This technology has been adopted to encourage sustainable and energy-efficient production of nanomaterials (Chen et al. 2008a; Liang et al. 2008; Hojati-Talemi et al. 2010), to mitigate energy and time constraints involved with using slower conventional heating techniques (Bai et al. 2021). Microwave-assisted processes have a volumetric heating mechanism that involves the direct conversion of electromagnetic energy to heat (Hassan et al. 2018), thereby enabling efficient heat dissipation and in turn faster reaction speeds (Barbera et al. 2014). In some cases, microwave absorbers have also been coupled with less microwavable samples. These absorbers include activated charcoal, biochar, SiC, metallic oxides, ionic liquids, and sulfuric acid, among others (Chen et al. 2008b; Salema and Ani 2012; Zhao et al. 2012; Barbera et al. 2014; Lin and Chen 2015).
Fe3O4 nanoparticles (IONPs) are relatively cheap and useful nanoparticles (Chen et al. 2020), these particles have considerable antibacterial (Abid and Kadhim 2022), catalytic (Jung et al. 2007) and adsorption properties (Hajizadeh et al. 2020; Abid et al. 2021), among other attributes. Their magnetic nature makes is convenient in their magnetic recovery during recycling. Numerous studies have synthesized IONPs using various methods as bare nanoparticles and on various supports.
Leveraging process intensification techniques, such as microwave heating, in conjunction with sustainable materials such as lignocellulosic biomass is prudent to efficiently fabricate sustainable supported IONPs. In particular the synthesis of IONPs bearing composites with sugarcane bagasse or its carbonized forms as support material using microwave heating methods, as achieved by others (Bai et al. 2021). However, the use of specialized equipment in the study, numerous chemicals, and time-consuming steps (including a soaking step for over 24 h) were significant aspects that appeared to defeat the very essence of using the microwave approach as a fast and easy method of IONPs synthesis. In this study, we report on sugarcane bagasse as a readily available feedstock in the facile synthesis of a nanocomposite of IONPs on carbonized cellulose nanocrystals (cCNCs), using an ordinary unmodified domestic microwave in only 3 min, simultaneously straightforwardly producing two types of nanoparticles. This was achieved using a setup with an activated charcoal jacket as the microwave absorber to induce pyrolysis of the sugarcane bagasse. We are not aware of previous reports that achieved the same using sugarcane bagasse.
Experimental
Synthesis equipment, chemicals, and materials
FeCl3·6H2O and activated charcoal were sourced from Sigma Aldrich at analytical grade and used as received. Sugarcane bagasse was produced from sugarcane obtained from a local market. A 28 L Defy (South Africa) domestic microwave (MW) oven (model DMO351) was used for the MW-assisted pyrolysis.
IONP/carbonized CNC nanocomposite material preparation
FeCl3·6H2O (0.0145 g) was weighed and placed into a beaker, followed by the addition of deionized water (0.2 mL) and stirred with a magnetic stirrer for 5 min to ensure complete dissolution. Thereafter dry sugarcane bagasse (SB) (0.1 g) was weighed and placed into a separate beaker. The FeCl3·6H2O solution was then poured onto the sugarcane bagasse and mixed well to wet all the solids. The wet SB was placed inside an oven at 105 °C for 3 h to ensure complete evaporation of the water. The dry material was placed into a small glass vial placed inside the crucible with activated charcoal, such that the vial was surrounded by activated charcoal to a depth of about 1 cm. The setup was placed on the MW turntable and the unit closed, see Fig. 1. The MW oven was set at 900W for 3 min and started. The activated charcoal turned red hot a few seconds after turning on the MW unit. Some smoke was also liberated from the vial as the reaction proceeded (Viswanathan 2014). After the reaction, the setup was allowed to cool down, thereafter the magnetic black charred product was collected, weighed, and stored, yielding 27 mg of the IONP/carbonized CNC nanocomposite. The experiment was conducted in triplicate.
Characterization of Fe3O4 nanoparticles (IONPs)supported on cCNC
The crystalline nature of the carbonized material was characterized using a Renishaw inVia Raman spectrometer operating at 488 nm and at 50% laser power with an exposure time of 10 s. A JEOL 1010 (Japan) transmission electron microscope (TEM) was used to characterize the morphology and size of the produced IONPs and their support. A Field Emission Gun Scanning Electron Microscope (FEG-SEM) Shimadzu Model SSX 550(Japan), with an energy dispersive spectrometer (EDS) was used to characterize the morphology of the nanoparticles. The instrument was operated at an acceleration voltage of 15 kV, with images acquired in secondary electron mode. Before analysis, the samples were coated with a thin layer of gold, using a Shimadzu Model IC-50 metallizer(Japan). Energy dispersive spectroscopy (EDS) attached to the SEM was used for elemental mapping of the nanocomposite. A Miniflex600 diffractometer(China) was used to carbonize the powder diffractograms with the X-ray generator at 40 kV, 15 mA, and a scan speed of 10.00°/min.
Results and discussion
Microwave-assisted heating process
Microwave technology was central in this study, it enabled the preparation of the composite in only 3 min when used in conjunction with activated charcoal (as a microwave absorber) to encourage pyrolysis. During the MW process, the transmission of heat occurred via conduction and radiation from the heated activated charcoal to the precursors inside the vial. The precursors and activated charcoal were not in direct contact to avoid contamination. To simplify the process, an open vessel with no inert gases or evacuation was used, unlike other reports in which an evacuated quartz tube in the MW-assisted preparation of IONPs from polystyrene was used (Hojati-Talemi et al. 2010).
The method used in this study was inspired by a 2014 patent application using lignin and tannin as carbon sources to prepare nanotubes (Viswanathan 2014) and similar work using tannin (Finlay et al. 2012). Using a modified method and sugarcane bagasse instead, we synthesized a nanocomposite comprised of two different types of nanoparticles, namely IONPs supported on carbonized cellulose nanocrystals (cCNCs) in one step.
Powder XRD
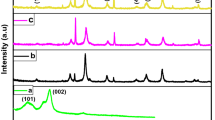
The successful synthesis of Fe3O4-containing material was confirmed by powder XRD analysis. The powder XRD spectrum is shown in Fig. 2, more information is given in the electronic supplementary information (ESI) in Table S1. The diffraction peaks at 2θ values of 30.08, 35.4, 43.1, 57.2, and 62.6° in the diffraction spectrum of the prepared composite resemble the characteristic peaks of Fe3O4 and are highly consistent with card number 1526955 on the Crystallography Open Database. The amorphous background peak in the initial 2θ range indicated a low degree of crystallization of the organic carbon in the composite (Bai et al. 2021). Subsequent tests were also conducted on the material to understand the morphology of the IONP/cCNC composite.
Raman spectroscopy
Raman spectroscopy indicated peaks at ~ 1350 cm−1 (D) and 1590 cm−1 (G) of the nanocomposite, see Fig. 3. The intensity of the D peak is associated with amorphous carbon (sp3 hybridization), and it indicates the defects and edges, while the G peak is associated with nanocrystalline carbon (sp2 hybridization) indicating material of a graphitic nature. The intensities of the peaks were used to calculate the G/D ratio of 1.25 (D/G = 0.8), indicating a degree of crystallinity/graphitization in the material as a whole; the intensity ratio of the G band compared to the D band (G/D ratio) could be obtained by fitting the spectra (Yang et al. 2020) and indicated the formation of carbonized cellulose derived material. The spectrum was similar to the spectra of carbonized cellulose nanocrystals previously reported (Bartoli et al. 2019).
Electron microscopy: TEM, SEM, and EDX
Electron microscopy was used to perform a morphological analysis of the nanocomposite. SEM and TEM images are shown in Fig. 4. It showed uniformly distributed IONPs of approximately 50 nm diameter supported on cCNCs with approximate widths of 50 nm and lengths of 100 nm. The EDX spectrum showed a uniform distribution of the elements C, O, and Fe in the composite material overall, see Fig. 5. The C and O atomic percentages of the material were determined to be ~ 90% carbon and 7% oxygen, the typical atomic percentages of raw sugarcane bagasse are ~ 45.5 wt% C and 45.2 wt% O, and the increase in C atomic percentage further confirmed almost complete carbonization, more information is given in the electronic supplementary information (ESI) in Table S2 and Figure S1. This corroborated the graphitic character already indicated by Raman analysis using the observed D/G ratio.
Based on the observed morphology of the IONP/cCNCs composite we hypothesize that the cellulose in the sugarcane bagasse produced the cellulose nanocrystals which were subsequently carbonized during the MW-assisted process. However, the production sequence of the CNCs and IONPs was not obvious, but we propose at least four pathways through which this could have occurred from the cellulose component in the dried sugarcane bagasse.
Firstly, it is plausible that the CNCs were formed from the rapid hydrolysis of cellulose using residual moisture in the cell walls of the sugarcane bagasse. Given that cellulose contains alternating sections of weaker amorphous and stronger crystalline regions (Bagde and Nadanathangam 2019; Raza et al. 2022), the high temperatures experienced during the MW irradiation process likely caused a significant increase in the Kw of water (1 × 10–14 mol2 L−2) producing enough H+ via autoionization to hydrolyze these weaker amorphous sections of cellulose, producing cellulose nanocrystals from the crystalline sections (Perlt et al. 2017). As illustrated in Fig. 6.
Secondly, the acidic salt FeCl3 could have used internally bound residual water molecules within sugarcane bagasse to form a slightly acidic solution, which in turn possibly hydrolyzed cellulose into CNCs. It should also be noted that upon heating hydrated FeCl3 is known to release HCl and the basic salt iron oxychloride (FeOCl) (Lussiez and Beckstead 1996), with the HCl probably participating in hydrolyzing the cellulose into nanocellulose crystals.
Thirdly, it is also conceivable that IONPs formed played a catalytic role in the formation of CNCs via cleavage of weaker amorphous sections of the cellulose leaving behind the more resilient crystalline sections producing CNCs (Hojati-Talemi et al. 2010; Raza et al. 2022). Based on Fig. 4b, c, it was observed that IONPs appeared to be tethered to extreme ends of the CNS as opposed to a more central position on the CNC, which would suggest the IONPs could have played a role in the cleavage of the cellulose amorphous sections into CNCs after which the IONPs/CNCs composite was then carbonized, turning the CNCs into their carbonized form, see Fig. 6.
Lastly, based on the properties of nanosized metal and metal oxide powders under MW heating. It was suggested that nanoparticles can be heated up under MW irradiation, unlike bulk materials which reflect MW irradiation and cannot be heated (Chen et al. 2008a; Chandrasekaran et al. 2013). We propose that once the Fe3O4 nanoparticles were produced, they became tethered to cellulose due to its large surface area, high surface energy, and abundance of surface functional groups (Chen et al. 2020). These negatively charged functional groups likely attracted the IONPs, acting as nucleating centes and allowed the IONPs to have an anchoring point on the surface of the support (Chen et al. 2020). Ultimately the effect of the MW selective heating of the IONPs on their cellulose support anchor points induced hotspots and subsequent thermal cleavage of the weaker amorphous cellulose sections into CNCs (Ano et al. 2021), which were subsequently carbonized into an IONP/cCNC nanocomposite.
IONPS have been investigated for several applications, including catalysis (Jung et al. 2007) and adsorption of dyes (Anh et al. 2020) as well as heavy metals (Bai et al. 2021) from wastewater. In these applications, the nano-size of the spherical IONPs enabled a larger surface area for better reactions or adsorption. However, the use of powdered or nanosized adsorbents or catalysts can have challenging implications on the recycling of used IONPs after application due to their small size, risking environmental contamination by bare unsupported IONPs. In this regard, the use of a cCNC support material afforded stable anchoring to IONPs and gave bulk to the IONPs enabling easier collection and recycling compared to minute bare IONPs.
Table 1 compares several studies in the fabrication of IONP-containing composites via MW heating, with particular emphasis on the support material used. In several instances, the IONPs were synthesized separately from their support material. For instance, pre-synthesized IONPs were supported on citric acid-modified SB (Anh et al. 2020); jackfruit biochar from microwave-assisted pyrolysis (Nayak et al. 2021) and carbon support from carbonized polystyrene (Hojati-Talemi et al. 2010).
However, other studies displayed single-step MW carbonization processes similar to the present study, while the actual MW-assisted carbonizations were short, the preparation times were significantly longer. For example, an IONP/sugarcane bagasse biochar involved the soaking of SB in a water solution of brominated hexadecyl trimethylammonium chloride (0.05 mol/L) and ferric sulfate (1 mol/L) for 24 h (Bai et al. 2021); in an IONP/tannin bio-char composite, the IONPs were synthesized after overnight stirring, and subsequent MW carbonization of the dried precipitate (Finlay et al. 2012). In the current study, the preparation was only a few hours and both nanoparticles in the composite were fabricated simultaneously from the MW step within a few minutes.
Regarding the morphology of the composites, the IONPs fabricated in previous studies shown in Table 1 were all spherical. In terms of size, our results corroborated the sizes of IONPs in a similar one-step MW pyrolysis fabrication of an IONP/sugarcane bagasse biochar composite at 400 °C (Bai et al. 2021). However, the morphology of the support appeared different from the current study as no discernible discrete CNCs were observed as we did in the current study. We speculate that differences in carbonization temperatures could explain the discrepancy, unfortunately, accurate temperature measurements in the unmodified MW unit used in this study were impractical. Regardless, some suggestions that microwaved graphite can approach temperatures of 1300 °C after just four minutes make it conceivable that the current study reached substantially higher temperatures exceeding the 400 °C used by others (Vanetsev and Tretyakov 2007).
Conclusions
A straightforward method to use sugarcane bagasse to form a magnetic Fe3O4/CNC composite was successful using an unmodified household microwave oven. This was conducted in one step using readily available sugarcane bagasse and FeCl3 as the iron source within a few minutes. We were also able to suggest several possible mechanisms grounded in the current understanding of MW technology for the production of the observed nanocomposite from the sugarcane bagasse components. We suggest that the cellulose nanocrystals were produced in situ after the cleavage of weaker amorphous sections of cellulose and subsequently carbonized. This nanocomposite material has the potential to be used as a cheap and readily prepared heterogeneous catalyst or as an adsorbent in the wastewater purification of harmful dyes and heavy metals, conveniently providing easy recovery via magnetic separation.
References
Abid MA, Kadhim DA (2022) Synthesis of iron oxide nanoparticles by mixing chilli with rust iron extract to examine antibacterial activity. Mater Technol 37:1494–1503. https://doi.org/10.1080/10667857.2021.1959189
Abid MA, Abid DA, Aziz WJ, Rashid TM (2021) Iron oxide nanoparticles synthesized using garlic and onion peel extracts rapidly degrade methylene blue dye. Phys B Condens Matter 622:413277. https://doi.org/10.1016/j.physb.2021.413277
Alves JO, Soares Tenório JA, Zhuo C, Levendis YA (2012) Characterization of nanomaterials produced from sugarcane bagasse. J Mater Res Technol 1:31–34. https://doi.org/10.1016/S2238-7854(12)70007-8
Anh NTH, Phuc TT, An TNM et al (2020) Microwave-assisted preparation of magnetic citric acid-sugarcane bagasse for removal of textile dyes. Indones J Chem 20:1101–1109. https://doi.org/10.22146/ijc.48713
Ano T, Tsubaki S, Fujii S, Wada Y (2021) Designing local microwave heating of metal nanoparticles/metal oxide substrate composites. J Phys Chem C 125:23720–23728. https://doi.org/10.1021/acs.jpcc.1c06650
Bagde P, Nadanathangam V (2019) Mechanical, antibacterial and biodegradable properties of starch film containing bacteriocin immobilized crystalline nanocellulose. Carbohydr Polym 222:115021. https://doi.org/10.1016/j.carbpol.2019.115021
Bai L, Su X, Feng J, Ma S (2021) Preparation of sugarcane bagasse biochar/nano-iron oxide composite and mechanism of its Cr (VI) adsorption in water. J Clean Prod 320:128723. https://doi.org/10.1016/j.jclepro.2021.128723
Barbera K, Frusteri L, Italiano G et al (2014) Low-temperature graphitization of amorphous carbon nanospheres. Cuihua Xuebao/Chinese J Catal 35:869–876. https://doi.org/10.1016/s1872-2067(14)60098-x
Bartoli M, Giorcelli M, Jagdale P et al (2019) Shape tunability of carbonized cellulose nanocrystals. SN Appl Sci 1:1–15. https://doi.org/10.1007/s42452-019-1727-2
Carus M, Dammer L (2018) The circular bioeconomy—concepts, opportunities, and limitations. Ind Biotechnol 14:83–91. https://doi.org/10.1089/ind.2018.29121.mca
Chandrasekaran S, Basak T, Srinivasan R (2013) Microwave heating characteristics of graphite based powder mixtures. Int Commun Heat Mass Transf 48:22–27. https://doi.org/10.1016/j.icheatmasstransfer.2013.09.008
Chen M-q, Wang J, Zhang M-x et al (2008b) Catalytic effects of eight inorganic additives on pyrolysis of pine wood sawdust by microwave heating. J Anal Appl Pyrolysis 82:145–150. https://doi.org/10.1016/j.jaap.2008.03.001
Chen K, Wang C, Ma D, Bao X (2008a) Graphitic carbon nanostructures via a facile microwave-induced solid-state process. ChemComm 2765–2767. https://doi.org/10.1039/b800807h
Chen L, Sharma S, Darienzo RE, Tannenbaum R (2020) Decoration of cellulose nanocrystals with iron oxide nanoparticles. Mater Res Express 7. https://doi.org/10.1088/2053-1591/ab8a82
Finlay C, Gunawan G, Biris AS et al (2012) Novel microwave-assisted synthesis of renewable-resource based carbon-magnetite nanocomposites. J Wood Chem Technol 32:268–278. https://doi.org/10.1080/02773813.2012.659320
Frosch RA, Gallopoulos NE (1989) Strategies for manufacturing the impact of industry on the environment. Sci Am 261:144–153. https://doi.org/10.1038/scientificamerican0989-144
Gawande MB, Shelke SN, Zboril R, Varma RS (2014) Microwave-assisted chemistry: synthetic applications for rapid assembly of nanomaterials and organics. Acc Chem Res 47:1338–1348. https://doi.org/10.1021/ar400309b
Hajizadeh Z, Valadi K, Taheri-Ledari R, Maleki A (2020) Convenient Cr(VI) removal from aqueous samples: executed by a promising clay-based catalytic system, magnetized by Fe3O4 nanoparticles and functionalized with humic acid. ChemistrySelect 5:2441–2448. https://doi.org/10.1002/slct.201904672
Hassan SS, Williams GA, Jaiswal AK (2018) Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresour Technol 262:310–318. https://doi.org/10.1016/j.biortech.2018.04.099
Hojati-Talemi P, Azadmanjiri J, Simon GP (2010) A simple microwave-based method for preparation of Fe3O4/carbon composite nanoparticles. Mater Lett 64:1684–1687. https://doi.org/10.1016/j.matlet.2010.04.040
Jung H, Park H, Kim J et al (2007) Preparation of biotic and abiotic iron oxide nanoparticles (IOnPs) and their properties and applications in heterogeneous catalytic oxidation. Environ Sci Technol 41:4741–4747. https://doi.org/10.1021/es0702768
Kirchherr J, Piscicelli L, Bour R et al (2018) Barriers to the circular economy: evidence from the European Union (EU). Ecol Econ 150:264–272. https://doi.org/10.1016/j.ecolecon.2018.04.028
Kvien I, Oksman K (2007) Orientation of cellulose nanowhiskers in polyvinyl alcohol. Appl Phys A Mater Sci Process 87:641–643. https://doi.org/10.1007/s00339-007-3882-3
Laranja MJ, Júnior FHS, Nogueira GA et al (2022) Valorisation of sugar cane bagasse using hydrothermal carbonisation in the preparation of magnetic carbon nanocomposite in a single-step synthesis applied to chromium adsorption. J Chem Technol Biotechnol 97:2032–2046. https://doi.org/10.1002/jctb.7074
Li D, Liu Z, Al-Haik M et al (2010) Magnetic alignment of cellulose nanowhiskers in an all-cellulose composite. Polym Bull 65:635–642. https://doi.org/10.1007/s00289-010-0276-z
Liang Y, Hwang KC, Lo S (2008) Solid-state microwave-arcing-induced formation and surface functionalization of core/shell metal/carbon nanoparticles. Small 4:405–409. https://doi.org/10.1002/smll.200700808
Lin BJ, Chen WH (2015) Sugarcane bagasse pyrolysis in a carbon dioxide atmosphere with conventional and microwave-assisted heating. Front Energy Res 3:1–9. https://doi.org/10.3389/fenrg.2015.00004
Lussiez G, Beckstead L (1996) Hydrolysis of ferric chloride in solution. Los Alamos Report LA-13215-MS
Maleki A (2018) Green oxidation protocol: Selective conversions of alcohols and alkenes to aldehydes, ketones and epoxides by using a new multiwall carbon nanotube-based hybrid nanocatalyst via ultrasound irradiation. Ultrason Sonochem 40:460–464. https://doi.org/10.1016/j.ultsonch.2017.07.020
Maleki A, Movahed H, Ravaghi P, Kari T (2016) Facile in situ synthesis and characterization of a novel PANI/Fe3O4/Ag nanocomposite and investigation of catalytic applications. RSC Adv 6:98777–98787. https://doi.org/10.1039/C6RA18185F
Nayak A, Bhushan B, Gupta V, Kotnala S (2021) Fabrication of microwave assisted biogenic magnetite-biochar nanocomposite: a green adsorbent from jackfruit peel for removal and recovery of nutrients in water sample. J Ind Eng Chem 100:134–148. https://doi.org/10.1016/j.jiec.2021.05.028
Nazir MH, Ayoub M, Zahid I et al (2022) Waste sugarcane bagasse-derived nanocatalyst for microwave-assisted transesterification: thermal, kinetic and optimization study. Biofuels Bioprod Biorefining 16:122–141. https://doi.org/10.1002/bbb.2264
Nechyporchuk O, Belgacem MN, Bras J (2016) Production of cellulose nanofibrils: a review of recent advances. Ind Crops Prod 93:2–25. https://doi.org/10.1016/j.indcrop.2016.02.016
Pehlivan E, Tran HT, Ouédraogo WKI et al (2013) Sugarcane bagasse treated with hydrous ferric oxide as a potential adsorbent for the removal of As(V) from aqueous solutions. Food Chem 138:133–138. https://doi.org/10.1016/j.foodchem.2012.09.110
Pennells J, Godwin ID, Amiralian N, Martin DJ (2020) Trends in the production of cellulose nanofibers from non-wood sources. Cellulose 27:575–593. https://doi.org/10.1007/s10570-019-02828-9
Pérez-Beltrán CH, García-Guzmán JJ, Ferreira B et al (2021) One-minute and green synthesis of magnetic iron oxide nanoparticles assisted by design of experiments and high energy ultrasound: application to biosensing and immunoprecipitation. Mater Sci Eng C 123. https://doi.org/10.1016/j.msec.2021.112023
Perlt E, Von Domaros M, Kirchner B et al (2017) Predicting the ionic product of water. Sci Rep 7:1–10. https://doi.org/10.1038/s41598-017-10156-w
Pires JRA, Souza VGL, Fernando AL (2019) Valorization of energy crops as a source for nanocellulose production—current knowledge and future prospects. Ind Crops Prod 140:111642. https://doi.org/10.1016/j.indcrop.2019.111642
Pradhan D, Jaiswal AK, Jaiswal S (2022) Emerging technologies for the production of nanocellulose from lignocellulosic biomass. Carbohydr Polym 285:119258. https://doi.org/10.1016/j.carbpol.2022.119258
Pranger L, Tannenbaum R (2008) Biobased nanocomposites prepared by in situ polymerization of furfuryl alcohol with cellulose whiskers or montmorillonite clay. Macromolecules 41:8682–8687. https://doi.org/10.1021/ma8020213
Rattanachueskul N, Saning A, Kaowphong S et al (2017) Magnetic carbon composites with a hierarchical structure for adsorption of tetracycline, prepared from sugarcane bagasse via hydrothermal carbonization coupled with simple heat treatment process. Bioresour Technol 226:164–172. https://doi.org/10.1016/j.biortech.2016.12.024
Raza M, Abu-Jdayil B, Banat F, Al-Marzouqi AH (2022) Isolation and characterization of cellulose nanocrystals from date palm waste. ACS Omega 7:25366–25379. https://doi.org/10.1021/acsomega.2c02333
Salema AA, Ani FN (2012) Microwave-assisted pyrolysis of oil palm shell biomass using an overhead stirrer. J Anal Appl Pyrolysis 96:162–172. https://doi.org/10.1016/j.jaap.2012.03.018
Somanathan T, Prasad K, Ostrikov KK et al (2015) Graphene oxide synthesis from agro waste. Nanomaterials 5:826–834. https://doi.org/10.3390/nano5020826
Ubando AT, Felix CB, Chen WH (2020) Biorefineries in circular bioeconomy: a comprehensive review. Bioresour Technol 299:122585. https://doi.org/10.1016/j.biortech.2019.122585
Vanetsev AS, Tretyakov YD (2007) Microwave-assisted synthesis of individual and multicomponent oxides. Russ Chem Rev 76:397–413. https://doi.org/10.1070/rc2007v076n05abeh003650
Viswanathan T (2014) Microwave-assisted synthesis of carbon nanotubes from tannin, lignin, and derivatives. US patent 8,753,603 B2 June 27, 2014
Wang F, Liu LY, Liu F et al (2017) Facile one-step synthesis of magnetically modified biochar with enhanced removal capacity for hexavalent chromium from aqueous solution. J Taiwan Inst Chem Eng 81:414–418. https://doi.org/10.1016/j.jtice.2017.09.035
Westerhoff P, Chen W, Esparza M (2001) Fluorescence analysis of a standard fulvic acid and tertiary treated wastewater. J Environ Qual 30:2037–2046. https://doi.org/10.2134/jeq2001.2037
Yang Z, Guo H, Yan G et al (2020) High-value utilization of lignin to prepare functional carbons toward advanced lithium ion capacitors. ACS Sustain Chem Eng 8:11522–11531. https://doi.org/10.1021/acssuschemeng.0c01949
Zhao X, Wang M, Liu H et al (2012) A microwave reactor for characterization of pyrolyzed biomass. Bioresour Technol 104:673–678. https://doi.org/10.1016/j.biortech.2011.09.137
Acknowledgements
This work was funded by the National Research Foundation (NRF), South Africa (Grant
#95799), and the Eskom TESP Program (Grant #P677). Microscopy images were obtained from the Microscopy and Microanalysis Unit (MMU) at the University of KwaZulu-Natal.
Funding
Open access funding provided by University of KwaZulu-Natal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mkumbuzi, E., van Zyl, W.E. Facile microwave-assisted preparation of Fe3O4 nanoparticles supported on carbonized cellulose nanocrystals derived from sugarcane bagasse. Chem. Pap. 78, 2933–2941 (2024). https://doi.org/10.1007/s11696-023-03282-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11696-023-03282-5