Abstract
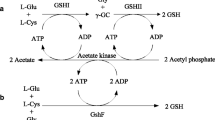
l-glutamine (Gln) is an important conditionally necessary amino acid in human body and potential demand in food or medicine industry is expected. High efficiency of l-Gln production by coupling genetic engineered bacterial glutamine synthetase (GS) with yeast alcoholic fermentation system has been developed. We report here first the application of small ubiquitin-related modifier (SUMO) fusion technology to the expression and purification of recombinant Bacillus subtilis GS. In order to obtain GS with high Gln-forming activity, safety and low cost for food and pharmaceutics industry, 0.1% (w/v) lactose was selected as inducer. The fusion protein was expressed in totally soluble form in E. coli, and expression was verified by SDS–PAGE and western blot analysis. The fusion protein was purified to 90% purity by nickel nitrilo-triacetic acid (Ni–NTA) resin chromatography with a yield of 625 mg per liter fermentation culture. After the SUMO/GS fusion protein was cleaved by the SUMO protease, the cleaved sample was reapplied to a Ni–NTA column. Finally, about 121 mg recombinant GS was obtained from 1 l fermentation culture with no less than 96% purity. The recombinant purified GS showed great transferase activity (23 U/mg), with 25 U recombinant GS in a 50 ml reaction system, a biosynthesis yield of 27.5 g/l l-Gln was detected by high pressure liquid chromatography (HPLC) or thin-layer chromatography. Thus, the application of SUMO technology to the expression and purification of GS potentially could be employed for the industrial production of l-Gln.






Similar content being viewed by others
References
Butt TR, Edavettal SC, Hall JP, Mattern MR (2005) SUMO fusion technology for difficult-to-express proteins. Protein Expr Purif 43:1–9. doi:10.1016/j.pep.2005.03.016
Castell LM (1997) The effects of oral glutamine supplementation upon athletes after prolonged, exhaustive exercise. Nutrition 13:738. doi:10.1139/cjpp-76-5-524
Castell LM (1998) Glutamine and the effects of exhaustive exercise upon the immune response. Can J Physiol Pharmacol 76:524–532. doi:10.1139/cjpp-76-5-524
Castell LM (2003) Glutamine supplementation in vitro and in vivo, in exercise and in immunodepression. SportsMed 33:323–345
Chen QY, Chen GA, Xue B, Zhang XJ, Yin ZM (2000) High efficiency of l-glutamine production by coupling genetic engineered bacterial glutamine synthetase with yeast alcoholic fermentation system. Chin J Biotechnol 20:456–460 (in Chinese)
Gardner AL, Aronson AI (1987) Expression of the Bacillus subtilis glutamine synthetase gene in Escherichia coli. J bacterial 158:967–971
Hiscock N (2002) Exercise-induced immunodepression-plasma glutamine is not the link. J Appl Physiol 93:813–822
Jonasson P, Liljeqvist S, Nygren PA, Stahl S (2002) Genetic design for facilitated production and recovery of recombinant proteins in Escherichia coli. Appl Biochem Biotechnol 35:91–105
Labow BI, Souba WW, Abcouwer SF (2001) Mechanisms governing the expression of the enzymes of glutamine metabolism-glutaminase and glutamine synthetase. J Nutr 131:2467–2474
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277:680–685
Loll PJ (2003) Membrane protein structural biology: the high throughput challenge. J Struct Biol 142:144–153. doi:10.1016/S1047-8477(03)00045-5
Ma XY, Zheng WY, Wei DZ, Ma YS, Wang TW, Wang JZ, Liu QH, Yang SL (2006) High-level expression, purification and pro-apoptosis activity of HIV-TAT-survivin (T34A) mutant to cancer cells in vitro. J Biotechnol 123:367–378. doi:10.1016/j.jbiotec.2005.11.018
Malakhova OA, Drinker M, Weeks SD, Butt TR (2004) SUMO fusions and SUMO-specific protease for efficient expression and purification of proteins. J Struct Funct Genomics 5:75–86. doi:10.1023/B:JSFG.0000029237.70316.52
Marblestone JG, Edavettal SC, Lim Y, Lim P, Zuo X, Butt TR (2006) Comparison of SUMO fusion technology with traditional gene fusion systems: enhanced expression and solubility with SUMO. Protein Sci 15:182–189. doi:10.1110/ps.051812706
Nabe K, Ujimaru T, Izuo N, Yamada S, Chibata I (1980) Production of l-glutamine by a penicillin-resistant mutant of Flavobacterium rigense. Appl Environ Microbiol 40:19–24
Nakanishi T (1978) Enzymes concerned in the conversion of l-glutamic acid fermentation to l-glutamine and N-acetyl-l-glutamine fermentation by Corynebacterium glutamicum. J Ferment Technol 56:573–585
Nilsson J, Stahl S, Lundeberg J, Uhlen M, Nygren PA (1997) Affinity fusion strategies for detection, purification, and immobilization of recombinant proteins. Protein Expr Purif 11:1–16. doi:10.1006/prep.1997.0767
Pyo SH, Lee JH, Park HB, Cho JS, Kim HR, Han BH, Park YS (2004) Expression and purification of a recombinant buforin derivative from Escherichia coli. Process Biochem 39:1731–1736. doi:10.1016/j.procbio.2003.07.007
Rao XC, Li S, Hu JC, Jin XL, Hu XM, Huang JJ, Chen ZJ, Zhu JM, Hu FQ (2004) A novel carrier molecule for high-level expression of peptide antibiotics in Escherichia coli. Protein Expr Purif 36:11–18. doi:10.1016/j.pep.2004.01.020
Skosyrev VS, Rudenko NV, Yakhnin AV, Zagranichny VE, Popova LI, Zakharov MV, Gorokhovatsky AY, Vinokurov LM (2003) EGFP as a fusion partner for the expression and organic extraction of small polypeptides. Protein Expr Purif 27:55–62. doi:10.1016/S1046-5928(02)00595-8
Souba WW (1993) The role of glutamine in maintaining a healthy gut and supporting the metabolic response to injury and infection. J Surg Res 44:383–391. doi:10.1016/0022-4804(90)90080-L
Sun Z, Chen J, Yao H, Liu L, Wang J, Zhang J, Liu JN (2005) Use of Ssp dnaB derived mini-intein as a fusion partner for production of recombinant human brain natriuretic peptide in Escherichia coli. Protein Expr Purif 43:26–32. doi:10.1016/j.pep.2005.05.005
Sun Z, Xia Z, Bi F, Liu JN (2008) Expression and purification of human urodilatin by small ubiquitin-related modifier fusion in Escherichia coli. Appl Microbiol Biotechnol 78:495–502. doi:10.1007/s00253-007-1330-0
Wagen-makers AJ (1998) Muscle amino acid metabolism at rest and during exercise: role of human physiology and metabolism. Exerc Sport Sci Rev 26:287–314
Wakisaka S, Ohshima Y, Ogawa M, Tochikura T, Tachiki T (1998) Characteristics and efficiency of glutamine production by coupling of a bacterial glutamine synthetase reaction with the alcoholic fermentation system of baker’s yeast. Appl Environ Microbiol 64:2952–2957
Weeks SD, Drinker M, Loll PJ (2007) Ligation independent cloning vectors for expression of SUMO fusions. Protein Expr Purif 53:40–50. doi:10.1016/j.pep.2006.12.006
Wu YF, Fan YM, Xue B, Luo L, Shen JY, Zhang SQ, Jiang Y, Yin ZM (2006) Human glutathione S-transferase P1–1 interacts with TRAF2 and regulates TRAF2-ASK1 signals. Oncogene 25:5787–5800. doi:10.1038/sj.onc.1209576
Xu Z, Zhong Z, Huang L, Peng L, Wang F, Cen P (2006) High-level production of bioactive human beta-defensin-4 in Escherichia coli by soluble fusion expression. Appl Microbiol Biotechnol 72:471–479. doi:10.1007/s00253-005-0287-0
Zhang F, Wang Q, Li GL, Xu JH, Yue L, Feng ZY, Yin ZM (2010) High density fed-batch culture of Escherichia coli BL21/pET28b-glnA with do feed-back control of nutrient feeding. Jo Nanjin Normal Univ 36:96–103 (In Chinese)
Zuo X, Mattern MR, Tan R, Li S, Hall J, Sterner DE, Shoo J, Tran H, Lim P, Sarafianos SG, Kazi L, Navas MS, Weiss SR, Butt TR (2005) Expression and purification of SARS coronavirus proteins using SUMO-fusions. Protein Expr Purif 42:100–110. doi:10.1016/j.pep.2005.02.004
Acknowledgments
This work was supported by the special funding from NJNU for talent faculty and Jiangsu superior discipline. We thank zhili Liu, associate professor, for performing the HPLC analysis.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, Q., Min, C., Yan, T. et al. Production of glutamine synthetase in Escherichia coli using SUMO fusion partner and application to l-glutamine synthesis. World J Microbiol Biotechnol 27, 2603–2610 (2011). https://doi.org/10.1007/s11274-011-0733-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-011-0733-3