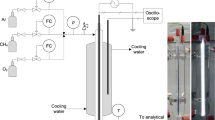
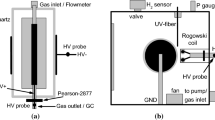
Abstract
Low-temperature non-thermal plasmas produce highly reactive chemical environments made up of electrons, ions, radicals, and vibrationally excited molecules. These reactive species, when combined with catalysts, can help drive thermodynamically unfavorable chemical reactions at low temperatures and atmospheric pressure. The conversion of methane (CH4) to produce other value-added chemicals is a good model system because of its applicability to a wide range of industries. To effectively create these plasma catalytic systems, a fundamental understanding of the plasma-phase chemistry alone is imperative. While there have been many studies on methane plasmas and how certain operating conditions (i.e., gas composition and power) affect the plasma, there is limited understanding on how changing bulk reaction temperature affects the plasma properties and ensuing plasma chemistry. In this work, we use a dielectric barrier discharge to investigate the effects of temperature on the reaction chemistry and the plasma’s electrical properties in various methane-gas mixtures. Results show that increasing temperature leads to a reduction in methane conversion as well as changes to both the gas and dielectric material pre-breakdown, which manifests itself in temperature-dependent electrical properties of the plasma. Experiments at various temperatures and power show a positive correlation between key electrical plasma properties (average charge and lifetime per filament) and the measured methane conversion as a function of temperature.










Similar content being viewed by others
Data Availability
All data generated during this work are available from the corresponding author upon request.
References
Emam EA (2015) Gas flaring in industry: an overview. Pet Coal 57(5):532–555
Ajugwo AO (2013) Negative effects of gas flaring: the Nigerian experience. Environ Pollut Hum Health 6:6–8
Alvarez-Galvan MC, Mota N, Ojeda M et al (2011) Direct methane conversion routes to chemicals and fuels. Catal Today 171:15–23. https://doi.org/10.1016/j.cattod.2011.02.028
Bariwal J, der Eycken EV (2013) C-N bond forming cross-coupling reactions: an overview. Chem Soc Rev 42:9283–9303. https://doi.org/10.1039/C3CS60228A
Mehta P, Barboun P, Go DB et al (2019) Catalysis enabled by plasma activation of strong chemical bonds: a review. ACS Energy Lett 4:1115–1133. https://doi.org/10.1021/acsenergylett.9b00263
Mehta P, Barboun PM, Engelmann Y et al (2020) Plasma-catalytic ammonia synthesis beyond the equilibrium limit. ACS Catal 10:6726–6734. https://doi.org/10.1021/acscatal.0c00684
Turan N, Saeidi-Javash M, Zhang Y, Go DB (2022) Does plasma jet sintering follow an Arrhenius-type expression? Plasma Process Polym 19:2200011. https://doi.org/10.1002/ppap.202200011
Martin-del-Campo J, Coulombe S, Kopyscinski J (2020) Influence of operating parameters on plasma-assisted dry reforming of methane in a rotating gliding arc reactor. Plasma Chem Plasma Process 40:857–881. https://doi.org/10.1007/s11090-020-10074-2
Kim J, Abbott MS, Go DB, Hicks JC (2016) Enhancing C–H bond activation of methane via temperature-controlled, catalyst-plasma interactions. ACS Energy Lett 1:94–99. https://doi.org/10.1021/acsenergylett.6b00051
Bogaerts A, Kozák T, van Laer K, Snoeckx R (2015) Plasma-based conversion of CO2: current status and future challenges. Faraday Discuss 183:217–232. https://doi.org/10.1039/C5FD00053J
Snoeckx R, Setareh M, Aerts R et al (2013) Influence of N2 concentration in a CH4/N2 dielectric barrier discharge used for CH4 conversion into H2. Int J Hydrogen Energy 38:16098–16120. https://doi.org/10.1016/j.ijhydene.2013.09.136
Brune L, Ozkan A, Genty E et al (2018) Dry reforming of methane via plasma-catalysis: influence of the catalyst nature supported on alumina in a packed-bed DBD configuration. J Phys D Appl Phys 51:234002. https://doi.org/10.1088/1361-6463/aac047
Zhang H, Wang W, Li X et al (2018) Plasma activation of methane for hydrogen production in a N2 rotating gliding arc warm plasma: a chemical kinetics study. Chem Eng J 345:67–78. https://doi.org/10.1016/j.cej.2018.03.123
Ozkan A, Dufour T, Silva T et al (2016) The influence of power and frequency on the filamentary behavior of a flowing DBD—application to the splitting of CO2. Plasma Sources Sci Technol 25:025013. https://doi.org/10.1088/0963-0252/25/2/025013
Istadi I, Amin NAS (2007) Modelling and optimization of catalytic–dielectric barrier discharge plasma reactor for methane and carbon dioxide conversion using hybrid artificial neural network—genetic algorithm technique. Chem Eng Sci 62:6568–6581. https://doi.org/10.1016/j.ces.2007.07.066
Hu X, Liu Y, Dou L et al (2021) Plasma enhanced anti-coking performance of Pd/CeO2 catalysts for the conversion of methane. Sustain Energy Fuels 6:98–109. https://doi.org/10.1039/D1SE01441B
Zhang X, Cha MS (2013) Electron-induced dry reforming of methane in a temperature-controlled dielectric barrier discharge reactor. J Phys D Appl Phys 46:415205. https://doi.org/10.1088/0022-3727/46/41/415205
Zhang X, Cha MS (2015) Partial oxidation of methane in a temperature-controlled dielectric barrier discharge reactor. Proc Combust Inst 35:3447–3454. https://doi.org/10.1016/j.proci.2014.05.089
Jans ER, Jones IW, Yang X et al (2022) Time-resolved measurements of HO2 radical in a heated plasma flow reactor. Combust Flame 241:112097. https://doi.org/10.1016/j.combustflame.2022.112097
Herrera FA, Brown GH, Barboun P et al (2019) The impact of transition metal catalysts on macroscopic dielectric barrier discharge (DBD) characteristics in an ammonia synthesis plasma catalysis reactor. J Phys D Appl Phys 52:224002. https://doi.org/10.1088/1361-6463/ab0c58
Peeters F, Butterworth T (2019) Electrical diagnostics of dielectric barrier discharges. In: Nikiforov A, Chen Z (eds) Atmospheric pressure plasma—from diagnostics to applications. IntechOpen
Measurement and data analysis for engineering and science. Routledge & CRC Press. https://www.routledge.com/Measurement-and-Data-Analysis-for-Engineering-and-Science/Dunn-Davis/p/book/9781138050860. Accessed 29 Mar 2023
Zabell SL, Stigler SM, Aldrich J et al (2008) On student’s 1908 article “the probable error of a mean” [with Comments, Rejoinder]. J Am Stat Assoc 103:1–20
Brandenburg R (2017) Dielectric barrier discharges: progress on plasma sources and on the understanding of regimes and single filaments. Plasma Sources Sci Technol 26:053001. https://doi.org/10.1088/1361-6595/aa6426
Kogelschatz U (2003) Dielectric-barrier discharges: their history, discharge physics, and industrial applications. Plasma Chem Plasma Process 23:1–46. https://doi.org/10.1023/A:1022470901385
Sergeev OA, Shashkov AG, Umanskii AS (1982) Thermophysical properties of quartz glass. J Eng Phys 43:1375–1383. https://doi.org/10.1007/BF00824797
Peeters FJJ, van de Sanden MCM (2014) The influence of partial surface discharging on the electrical characterization of DBDs. Plasma Sources Sci Technol 24:015016. https://doi.org/10.1088/0963-0252/24/1/015016
Pipa AV, Brandenburg R (2019) The equivalent circuit approach for the electrical diagnostics of dielectric barrier discharges: the classical theory and recent developments. Atoms 7:14. https://doi.org/10.3390/atoms7010014
Stuart MR (1955) Dielectric constant of quartz as a function of frequency and temperature. J Appl Phys 26:1399–1404. https://doi.org/10.1063/1.1721922
Saeed A, Adewuyi SO, Ahmed HAM et al (2022) Electrical and dielectric properties of the natural calcite and quartz. Silicon 14:5265–5276. https://doi.org/10.1007/s12633-021-01318-7
Fang Z, Ji S, Pan J et al (2012) Electrical model and experimental analysis of the atmospheric-pressure homogeneous dielectric barrier discharge in He. IEEE Trans Plasma Sci 40:883–891. https://doi.org/10.1109/TPS.2011.2180544
Pal UN, Sharma AK, Soni JS et al (2009) Electrical modelling approach for discharge analysis of a coaxial DBD tube filled with argon. J Phys D: Appl Phys 42:045213. https://doi.org/10.1088/0022-3727/42/4/045213
Yao S, Nakayama A, Suzuki E (2001) Methane conversion using a high-frequency pulsed plasma: discharge features. AIChE J 47:419–426. https://doi.org/10.1002/aic.690470218
Saville DA (1997) Electrohydrodynamics: the Taylor-Melcher leaky dielectric model. Annu Rev Fluid Mech 29:27–64. https://doi.org/10.1146/annurev.fluid.29.1.27
Bastin O, Thulliez M, Serra T et al (2023) Electrical equivalent model of a long dielectric barrier discharge plasma jet for endoscopy. J Phys D Appl Phys 56:125201. https://doi.org/10.1088/1361-6463/acb603
Fridman A (2008) Plasma chemistry. Cambridge University Press, Cambridge
Luo H, Liang Z, Lv B et al (2007) Observation of the transition from a Townsend discharge to a glow discharge in helium at atmospheric pressure. Appl Phys Lett 91:221504. https://doi.org/10.1063/1.2819073
Davydov YI (2006) On the first townsend coefficient at high electric field. IEEE Trans Nucl Sci 53:2931–2935. https://doi.org/10.1109/TNS.2006.881543
Hagelaar GJM, Pitchford LC (2005) Solving the Boltzmann equation to obtain electron transport coefficients and rate coefficients for fluid models. Plasma Sources Sci Technol 14:722–733. https://doi.org/10.1088/0963-0252/14/4/011
Gherardi N, Gouda G, Gat E et al (2000) Transition from glow silent discharge to micro-discharges in nitrogen gas. Plasma Sources Sci Technol 9:340. https://doi.org/10.1088/0963-0252/9/3/312
Rousso AC, Goldberg BM, Chen TY et al (2020) Time and space resolved diagnostics for plasma thermal-chemical instability of fuel oxidation in nanosecond plasma discharges. Plasma Sources Sci Technol 29:105012. https://doi.org/10.1088/1361-6595/abb7be
Zhong H, Shneider MN, Mokrov MS, Ju Y (2019) Thermal-chemical instability of weakly ionized plasma in a reactive flow. J Phys D: Appl Phys 52:484001. https://doi.org/10.1088/1361-6463/ab3d69
Olm C, Varga T, Valkó É et al (2016) Development of an ethanol combustion mechanism based on a hierarchical optimization approach. Int J Chem Kinet 48:423–441. https://doi.org/10.1002/kin.20998
Baulch DL, Cobos CJ, Cox RA et al (1992) Evaluated kinetic data for combustion modelling. J Phys Chem Ref Data 21:411–734. https://doi.org/10.1063/1.555908
Pearce BKD, Ayers PW, Pudritz RE (2019) A consistent reduced network for HCN chemistry in early earth and titan atmospheres: quantum calculations of reaction rate coefficients. J Phys Chem A 123:1861–1873. https://doi.org/10.1021/acs.jpca.8b11323
Funding
This work was supported by the U.S. Department of Energy by National Energy Technology Laboratory under Award Number DE-FE0031862. F.V. and D.G. also acknowledge ND – UC|Chile Luksic Scholars Joint Research Award.
Author information
Authors and Affiliations
Contributions
IA and DG designed and analyzed the plasma experiments. JY and JS assisted in acquiring experimental data. IA and FV designed and interpreted the circuit analyses. GR and JH designed and analyzed the methane conversion experiments. IA, GR, and DG wrote the main manuscript text. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare there is no conflict of interest related to this work.
Ethical Approval
This declaration is not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Akintola, I., Rivera-Castro, G., Yang, J. et al. Temperature Inhibition of Plasma-Driven Methane Conversion in DBD Systems. Plasma Chem Plasma Process 43, 1999–2016 (2023). https://doi.org/10.1007/s11090-023-10388-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-023-10388-x