Abstract
Background
Plant-derived phytochemicals such as flavonoids have been explored to be powerful antioxidants that protect against oxidative stress-related diseases. In the present study, Morin, a flavonoid compound was studied for its antioxidant and antidiabetic properties in relation to oxidative stress in insulin resistant models conducted in rat skeletal muscle L6 cell line model.
Methods
Evaluation of antioxidant property of morin was assayed using in vitro methods such as cell viability by MTT assay, estimation of SOD and CAT activity and NO scavenging activity. The anti-oxidative nature of morin on L6 cell line was conducted by the DCF-DA fluorescent activity. Glucose uptake in morin treated L6 myotubes are accessed by 2-NBDG assay in the presence or absence of IRTK and PI3K inhibitors. Further glycogen content estimation due to the morin treatment in L6 myotubes was performed. Antioxidant and insulin signaling pathway gene expression was examined over RT-PCR analysis.
Results
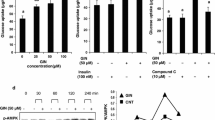
Morin has a negligible cytotoxic effect at doses of 20, 40, 60, 80, and 100 µM concentration according to cell viability assay. Morin revealed that the levels of the antioxidant enzymes SOD and CAT in L6 myotubes had increased. When the cells were subjected to the nitro blue tetrazolium assay, morin lowered reactive oxygen species (ROS) formation at 60 µM concentration displaying 39% ROS generation in oxidative stress condition. Lesser NO activity and a drop in green fluorescence emission in the DCFDA assay, demonstrating its anti-oxidative nature by reducing ROS formation in vitro. Glucose uptake by the L6 myotube cells using 2-NBDG, and with IRTK and PI3K inhibitors (genistein and wortmannin) showed a significant increase in glucose uptake by the cells which shows the up regulated GLUT-4 movement from intracellular pool to the plasma membrane. Morin (60 µM) significantly enhanced the expression of antioxidant genes GPx, GST and GCS as well as insulin signalling genes IRTK, IRS-1, PI3K, GLUT-4, GSK-3β and GS in L6 myotubes treated cells.
Conclusion
Morin has the ability to act as an anti-oxidant by lowering ROS levels and demonstrating insulin mimetic activity by reversing insulin resistance associated with oxidative stress.





Similar content being viewed by others
Abbreviations
- 2-NBDG:
-
2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl) amino)-2-deoxyglucose
- CAT:
-
Catalase
- DCF-DA:
-
Dichlorofluorescein diacetate
- DM:
-
Diabetes mellitus
- DMEM:
-
Dulbecco’s modified eagle’s medium
- DMSO:
-
Dimethyl sulfoxide
- FBS:
-
Fetal bovine serum
- GCS:
-
Glutamyl cysteine synthetase
- GLUT-4:
-
Glucose transporter type 4
- GPx:
-
Glutathione peroxidase
- GS:
-
Glycogen synthase
- GSK-3β:
-
Glycogen synthase kinase 3β
- GST:
-
Glutathione S-transferase
- H2O2 :
-
Hydrogen peroxide
- HEPES:
-
4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid
- IRS:
-
Insulin receptor substrate
- IRTK:
-
Insulin receptor tyrosine kinase
- IRβ:
-
Insulin receptor β
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide
- NBT:
-
Nitro blue tetrazolium
- NCCS:
-
National center for cell science
- PBS:
-
Phosphate buffer saline
- PI3K:
-
Phosphoinositide 3-kinases
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- T2DM:
-
Type 2 diabetic mellitus
References
Son D, Hwang S, Kim M-H et al (2015) Anti-diabetic and hepato-renal protective effects of ziyuglycoside II methyl ester in type 2 diabetic mice. Nutrients 7:5469–5483. https://doi.org/10.3390/nu7075232
George J, Liddle C, Samarasinghe D et al (2002) NASH and insulin resistance: insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology 35:373–379. https://doi.org/10.1053/jhep.2002.30692
Hoehn KL, Hohnen-Behrens C, Cederberg A et al (2008) IRS1-independent defects define major nodes of insulin resistance. Cell Metab 7:421–433. https://doi.org/10.1016/j.cmet.2008.04.005
Issac PK, Guru A, Chandrakumar SS et al (2020) Molecular process of glucose uptake and glycogen storage due to hamamelitannin via insulin signalling cascade in glucose metabolism. Mol Biol Rep 47:6727–6740. https://doi.org/10.1007/s11033-020-05728-5
Bonnefont-Rousselot D, Bastard JP, Jaudon MC, Delattre J (2000) Consequences of the diabetic status on the oxidant/antioxidant balance. Diabetes Metab 26:163–176
West IC (2000) Radicals and oxidative stress in diabetes. Diabet Med 17:171–180. https://doi.org/10.1046/j.1464-5491.2000.00259.x
Inouye M, Mio T, Sumino K (1999) Link between glycation and lipoxidation in red blood cells in diabetes. Clin Chim Acta 285:35–44. https://doi.org/10.1016/S0009-8981(99)00065-0
Schlernitzauer A, Oiry C, Hamad R et al (2013) Chicoric acid is an antioxidant molecule that stimulates AMP kinase pathway in L6 myotubes and extends lifespan in Caenorhabditis elegans. PLoS ONE 8:1–11. https://doi.org/10.1371/journal.pone.0078788
Rudich A, Tirosh A, Potashnik R et al (1998) Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes 47:1562–1569. https://doi.org/10.2337/diabetes.47.10.1562
Zhang X, Wang L, Lu H et al (2020) Preservation of hydrogen peroxide-induced oxidative damage in HepG-2 cells by rice protein hydrolysates pretreated with electron beams. Sci Rep 10:1–11. https://doi.org/10.1038/s41598-020-64814-7
Kaneto H, Katakami N, Matsuhisa M, Matsuoka TA (2010) Role of reactive oxygen species in the progression of type 2 diabetes and atherosclerosis. Mediators Inflamm 2010:1–11. https://doi.org/10.1155/2010/453892
Metodiewa D, Kochman A, Karolczak S (1997) Evidence for antiradical and antioxidant properties of four biologically active N, N-diethylamioethyl ethers of flavanone oximes: a comparison with natural polyphenolic flavonoid (rutin) action. Biochem Mol Biol Int 41:1067–1075. https://doi.org/10.1080/15216549700202141
Walker EH, Pacold ME, Perisic O et al (2000) Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell 6:909–919. https://doi.org/10.1016/S1097-2765(05)00089-4
Hayashi T, Sawa K, Kawasaki M et al (1988) Inhibition of cow’s milk xanthine oxidase by flavonoids. J Nat Prod 51:345–348. https://doi.org/10.1021/np50056a030
Basile A, Sorbo S, Giordano S et al (2000) Antibacterial and allelopathic activity of extract from Castanea sativa leaves. Fitoterapia 71:110–116. https://doi.org/10.1016/S0367-326X(00)00185-4
Caillet S, Yu H, Lessard S et al (2007) Fenton reaction applied for screening natural antioxidants. Food Chem 100:542–552. https://doi.org/10.1016/j.foodchem.2005.10.009
Gálvez J, Coelho G, Crespo ME et al (2001) Intestinal anti-inflammatory activity of morin on chronic experimental colitis in the rat. Aliment Pharmacol Ther 15:2027–2039. https://doi.org/10.1046/j.1365-2036.2001.01133.x
Manna SK, Aggarwal RS, Sethi G et al (2007) Morin (3,5,7,2′,4′-pentahydroxyflavone) abolishes nuclear factor-κB activation induced by various carcinogens and inflammatory stimuli, leading to suppression of nuclear factor-κB—regulated gene expression and up-regulation of apoptosis. Clin Cancer Res 13:2290–2297. https://doi.org/10.1158/1078-0432.CCR-06-2394
Choi CW, Kim SC, Hwang SS et al (2002) Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci 163:1161–1168. https://doi.org/10.1016/S0168-9452(02)00332-1
Malešev D, Kuntić V (2007) Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J Serbian Chem Soc 72:921–939. https://doi.org/10.2298/JSC0710921M
Wang N, Zhang J, Qin M et al (2018) Amelioration of streptozotocin—induced pancreatic β cell damage by morin: involvement of the AMPK—FOXO3—catalase signaling pathway. Int J Mol Med. https://doi.org/10.3892/ijmm.2017.3357
Kitagawa S, Sakamoto H, Tano H (2004) Inhibitory effects of flavonoids on free radical-induced hemolysis and their oxidative effects on hemoglobin. Chem Pharm Bull 52:999–1001. https://doi.org/10.1248/cpb.52.999
Hodek P, Trefil P, Stiborová M (2002) Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem Biol Interact 139:1–21. https://doi.org/10.1016/S0009-2797(01)00285-X
Rai M, Jogee PS, Agarkar G, Dos SCA (2016) Anticancer activities of Withania somnifera: current research, formulations, and future perspectives. Pharm Biol 54:189–197. https://doi.org/10.3109/13880209.2015.1027778
Guru A, Lite C, Freddy AJ et al (2021) Intracellular ROS scavenging and antioxidant regulation of WL15 from cysteine and glycine-rich protein 2 demonstrated in zebrafish in vivo model. Dev Comp Immunol 114:103863. https://doi.org/10.1016/j.dci.2020.103863
Esfandiari N, Sharma RK, Saleh RA et al (2003) Utility of the nitroblue tetrazolium reduction test for assessment of reactive oxygen species production by seminal leukocytes and spermatozoa. J Androl 24:862–870. https://doi.org/10.1002/j.1939-4640.2003.tb03137.x
Kim KJ, Lee BY (2012) Fucoidan from the sporophyll of Undaria pinnatifida suppresses adipocyte differentiation by inhibition of inflammation-related cytokines in 3T3-L1 cells. Nutr Res 32:439–447. https://doi.org/10.1016/j.nutres.2012.04.003
Sarkar P, Stefi RV, Pasupuleti M et al (2020) Antioxidant molecular mechanism of adenosyl homocysteinase from cyanobacteria and its wound healing process in fibroblast cells. Mol Biol Rep 47:1821–1834. https://doi.org/10.1007/s11033-020-05276-y
Misra HPFI (1972) The role of superoxide anion in the epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Kanaujia A, Duggar R, Pannakal ST et al (2010) Insulinomimetic activity of two new gallotannins from the fruits of Capparis moonii. Bioorg Med Chem 18:3940–3945. https://doi.org/10.1016/j.bmc.2010.04.032
Goth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim acta 196:143–151. https://doi.org/10.1016/0009-8981(91)90067-M
Gomes A, Fernandes E, Lima JLFC (2005) Fluorescence probes used for detection of reactive oxygen species. J Biochem Biophys Methods 65:45–80. https://doi.org/10.1016/j.jbbm.2005.10.003
Sannasimuthu A, Kumaresan V, Anilkumar S et al (2019) Design and characterization of a novel Arthrospira platensis glutathione oxido-reductase-derived antioxidant peptide GM15 and its potent anti-cancer activity via caspase-9 mediated apoptosis in oral cancer cells. Free Radic Biol Med 135:198–209. https://doi.org/10.1016/j.freeradbiomed.2019.03.006
Bernini R, Barontini M, Cis V et al (2018) Synthesis and evaluation of the antioxidant activity of lipophilic phenethyl trifluoroacetate esters by in vitro ABTS, DPPH and in cell-culture DCF assays. Molecules 23:1–14. https://doi.org/10.3390/molecules23010208
Lite C, Ahmed SSSJ, Santosh W, Seetharaman B (2019) Prenatal exposure to bisphenol-A altered miRNA-224 and protein expression of aromatase in ovarian granulosa cells concomitant with elevated serum estradiol levels in F 1 adult offspring. J Biochem Mol Toxicol. https://doi.org/10.1002/jbt.22317
Blodgett AB, Kothinti RK, Kamyshko I et al (2011) A fluorescence method for measurement of glucose transport in kidney cells. Diabetes Technol Ther 13:743–751. https://doi.org/10.1089/dia.2011.0041
Huang R, Fang M, Dillon GH (1999) The tyrosine kinase inhibitor genistein directly inhibits GABA A receptors. Mol Brain Res 67:177–183
Anand S, Arasakumari M, Prabu P, Amalraj AJ (2016) Anti-diabetic and aldose reductase inhibitory potential of Psidium guajava by in vitro analysis. Int J Pharm Pharm Sci 8:271–276
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159. https://doi.org/10.1016/0003-2697(87)90021-2
Bhatt P, Kumaresan V, Palanisamy R et al (2014) Immunological role of C4 CC chemokine-1 from snakehead murrel Channa striatus. Mol Immunol 57:292–301. https://doi.org/10.1016/j.molimm.2013.10.012
Kumaresan V, Bhatt P, Ganesh MR et al (2015) A novel antimicrobial peptide derived from fish goose type lysozyme disrupts the membrane of Salmonella enterica. Mol Immunol 68:421–433. https://doi.org/10.1016/j.molimm.2015.10.001
Arockiaraj J, Gnanam AJ, Muthukrishnan D et al (2013) Macrobrachium rosenbergii cathepsin L: molecular characterization and gene expression in response to viral and bacterial infections. Microbiol Res 168:569–579. https://doi.org/10.1016/j.micres.2013.04.007
Dhanya R, Arun KB, Syama HP et al (2014) Rutin and quercetin enhance glucose uptake in L6 myotubes under oxidative stress induced by tertiary butyl hydrogen peroxide. Food Chem 158:546–554. https://doi.org/10.1016/j.foodchem.2014.02.151
Praveen Kumar I, Arun J, Sri Snehaa C, Sujatha S (2018) Tannins of Jatropha gossypifolia exert anti-hyperlipidemic effect. Eur Biomed Pharm Sci 5:607–614
Issac PK, Ishan M, Sujatha S, Alwin D (2017) Antihyperglycemic and antihyperlipidemic activity of Jatropha gossypifolia methanolic extract in streptozotocin-nicotinamide induced diabetic rats. Asian J Pharm Clin Res 10:326–330
Pandey KB, Rizvi SI (2011) Biomarkers of oxidative stress in red blood cells. Biomed Pap 155:131–136. https://doi.org/10.5507/bp.2011.027
Qureshi AA, Khan DA, Mahjabeen W et al (2012) Suppression of nitric oxide production and cardiovascular risk factors in healthy seniors and hypercholesterolemic subjects by a combination of polyphenols and vitamins. J Clin Exp Cardiolog 01:1–30. https://doi.org/10.4172/2155-9880.s5-008
Kumar P, Ajay I, Sri G et al (2020) Molecular process of glucose uptake and glycogen storage due to hamamelitannin via insulin signalling cascade in glucose metabolism. Mol Biol Rep. https://doi.org/10.1007/s11033-020-05728-5
Issac PK, Lite C, Guru A et al (2021) Tryptophan-tagged peptide from serine threonine-protein kinase of Channa striatus improves antioxidant defence in L6 myotubes and attenuates caspase 3–dependent apoptotic response in zebrafish larvae. Fish Physiol Biochem 47:293–311. https://doi.org/10.1007/s10695-020-00912-7
Sujatha S, Anand S, Sangeetha KN et al (2010) Biological evaluation of (3β)-STIGMAST-5-EN-3-OL as potent anti-diabetic agent in regulating glucose transport using in vitro model. Int J Diabetes Mellit 2:101–109. https://doi.org/10.1016/j.ijdm.2009.12.013
Gumieniczek A, Hopkała H, Wójtowicz Z, Nieradko M (2001) Differences in antioxidant status in skeletal muscle tissue in experimental diabetes. Clin Chim Acta 314:39–45. https://doi.org/10.1016/S0009-8981(01)00680-5
Nagalakshmi K, Rajaguru PY, Issac PK, Sujatha S (2017) Fabrication, characterization, in vitro drug release and glucose uptake activity of 14-deoxy, 11, 12-didehydroandrographolide loaded polycaprolactone nanoparticles. Asian J Pharm Sci 12:353–362. https://doi.org/10.1016/j.ajps.2017.02.003
Bharadwaja S, Issac PK, Cleta J et al (2021) An in vitro mechanistic approach towards understanding the distinct pathways regulating insulin resistance and adipogenesis by apocynin. J Biosci 46:1–16. https://doi.org/10.1007/s12038-020-00134-2
Ishiki M, Klip A (2005) Minireview: recent developments in the regulation of glucose transporter-4 traffic: new signals, locations, and partners. Endocrinology 146:5071–5078. https://doi.org/10.1210/en.2005-0850
Guru A, Issac PK, Saraswathi N et al (2021) Deteriorating insulin resistance due to WL15 peptide from cysteine and glycine-rich protein 2 in high glucose induced rat skeletal muscle L6 cells. Cell Biol Int. https://doi.org/10.1002/cbin.11608
Koivisto UM, Martinez-Valdez H, Bilan PJ et al (1991) Differential regulation of the GLUT-1 and GLUT-4 glucose transport systems by glucose and insulin in L6 muscle cells in culture. J Biol Chem 266:2615–2621
Ciaraldi TP, Huber-Knudsen K, Hickman M, Olefsky JM (1995) Regulation of glucose transport in cultured muscle cells by novel hypoglycemic agents. Metabolism 44:976–981
Parry HA, Rivera ME, Vaughan RA, Sunderland KL (2020) Uncarboxylated osteocalcin decreases insulin-stimulated glucose uptake without affecting insulin signaling and regulators of mitochondrial biogenesis in myotubes. J Physiol Biochem. https://doi.org/10.1007/s13105-020-00732-6
Schrauwen P, Hesselink MKC (2004) Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes 53:1412–1417. https://doi.org/10.2337/diabetes.53.6.1412
Chen HL, Lan XZ, Wu YY et al (2017) The antioxidant activity and nitric oxide production of extracts obtained from the leaves of Chenopodium quinoa Willd. Biomed 7:24–28. https://doi.org/10.1051/bmdcn/2017070424
Itharat A, Sayompark S, Hansakul P, Dechayont B (2016) In vitro antioxidant activities of extracts of bauhinia strychnifolia stems and leaves: comparison with activities in green tea extracts. Med Aromat Plants 05:1–7. https://doi.org/10.4172/2167-0412.1000243
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247. https://doi.org/10.1038/35041687
Lee MH, Cha HJ, Choi EO et al (2017) Antioxidant and cytoprotective effects of morin against hydrogen peroxide-induced oxidative stress are associated with the induction of Nrf-2-mediated HO-1 expression in V79–4 Chinese hamster lung fibroblasts. Int J Mol Med 39:672–680. https://doi.org/10.3892/ijmm.2017.2871
Bachewal P, Gundu C, Yerra VG et al (2018) Morin exerts neuroprotection via attenuation of ROS induced oxidative damage and neuroinflammation in experimental diabetic neuropathy. BioFactors 44:109–122. https://doi.org/10.1002/biof.1397
Zou C, Wang Y, Shen Z (2005) 2-NBDG as a fluorescent indicator for direct glucose uptake measurement. J Biochem Biophys Methods 64:207–215. https://doi.org/10.1016/j.jbbm.2005.08.001
Muthusamy VS, Anand S, Sangeetha KN et al (2008) Tannins present in Cichorium intybus enhance glucose uptake and inhibit adipogenesis in 3T3-L1 adipocytes through PTP1B inhibition. Chem Biol Interact 174:69–78. https://doi.org/10.1016/j.cbi.2008.04.016
Anand S, Muthusamy VS, Sujatha S et al (2010) Aloe emodin glycosides stimulates glucose transport and glycogen storage through PI3K dependent mechanism in L6 myotubes and inhibits adipocyte differentiation in 3T3L1 adipocytes. FEBS Lett 584:3170–3178. https://doi.org/10.1016/j.febslet.2010.06.004
Choi CY, An KW, An MI (2008) Molecular characterization and mRNA expression of glutathione peroxidase and glutathione S-transferase during osmotic stress in olive flounder (Paralichthys olivaceus). Comp Biochem Physiol A Mol Integr Physiol 149:330–337. https://doi.org/10.1016/j.cbpa.2008.01.013
El MS, Guérin P, Ménézo Y (1999) Expression of genes encoding antioxidant enzymes in human and mouse oocytes during the final stages of maturation. Mol Hum Reprod 5:720–725. https://doi.org/10.1093/molehr/5.8.720
Laville M, Auboeuf D, Khalfallah Y et al (1996) Acute regulation by insulin of phosphatidylinositol-3-kinase, Rad, Glut 4, and lipoprotein lipase mRNA levels in human muscle. J Clin Invest 98:43–49. https://doi.org/10.1172/JCI118775
Buhl ES, Jessen N, Schmitz O et al (2001) Chronic treatment with 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside increases insulin-stimulated glucose uptake and GLUT4 translocation in rat skeletal muscles in a fiber type-specific manner. Diabetes 50:12–17
Woodgett JR (1990) Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J 9:2431–2438
Magnoni LJ, Vraskou Y, Palstra AP et al (2012) AMP-activated protein kinase plays an important evolutionary conserved role in the regulation of glucose metabolism in fish skeletal muscle cells. PLoS ONE 7:e31219. https://doi.org/10.1371/journal.pone.0031219
Sahni SK, Turpin LC, Brown TL, Sporn LA (1999) Involvement of protein kinase C in Rickettsia rickettsii-induced transcriptional activation of the host endothelial cell. Infect Immun 67:6418–6423. https://doi.org/10.1128/iai.67.12.6418-6423.1999
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project (RSP-2021/20), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
PKI, RK, AG, PR, JA conceived the study. PR provided the Morin. PKI, RK, AG performed the experiments. PR, MVA, NAA, KCC, RH, JA provided the reagents for the study. All authors designed the experiments, analysed the data, wrote the manuscript, read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
All the authors listed in the manuscript have approved the manuscript.
Consent for publication
The data provided in the manuscript is approved by all authors for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Issac, P.K., Karan, R., Guru, A. et al. Insulin signaling pathway assessment by enhancing antioxidant activity due to morin using in vitro rat skeletal muscle L6 myotubes cells. Mol Biol Rep 48, 5857–5872 (2021). https://doi.org/10.1007/s11033-021-06580-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-021-06580-x