Abstract
Hypertension is a major harbinger of cardiovascular morbidity and mortality. It predisposes to higher rates of myocardial infarction, chronic kidney failure, stroke, and heart failure than most other risk factors. By 2025, the prevalence of hypertension is projected to reach 1.5 billion people. The pathophysiology of this disease is multifaceted, as it involves nitric oxide and endothelin dysregulation, reactive oxygen species, vascular smooth muscle proliferation, and vessel wall calcification, among others. With the advent of new biomolecular techniques, various studies have elucidated a gaping hole in the etiology and mechanisms of hypertension. Indeed, epigenetics, DNA methylation, histone modification, and microRNA-mediated translational silencing appear to play crucial roles in altering the molecular phenotype into a hypertensive profile. Here, we critically review the experimentally determined associations between microRNA (miRNA) molecules and hypertension pharmacotherapy. Particular attention is given to the epigenetic mechanisms underlying the physiological responses to antihypertensive drugs like candesartan, and other relevant drugs like clopidogrel, aspirin, and statins among others. Furthermore, how miRNA affects the pharmaco-epigenetics of hypertension is especially highlighted.
Similar content being viewed by others
Introduction
The evolution of hypertension is multifactorial [1,2,3,4]. Most hypertension cases worldwide are due to primary causes, i.e. essential hypertension [5]. A smaller subset of hypertensive individuals around the world suffers from secondarily induced hypertension [6]. The most common causes of secondary hypertension include coarctated aorta, renal parenchymal diseases, renovascular diseases, endocrine disorders, pregnancy-induced hypertension, drug-related hypertension, and sleep apnea [6].
The pathophysiological basis of hypertension is multifactorial [7]. Dysregulation of pressure natriuresis via excessive sympathetic nervous system stimulation, impaired kidney functionality, and improper hormonal activation of salt and water excretion regulators can alter vascular tone and thus predispose to a hypertensive state [8, 9]. Moreover, the vascular endothelium provides homeostasis in the cardiovascular system [10]. This is attained by the incessant release of elements that act to modulate smooth muscle cell contraction, cellular proliferation, the aggregation of platelets, and vascular wall permeability [11]. The dysfunction of this endothelium is due to the imbalance of its regulatory elements [10]. This has a pertinent effect on the development of various diseases, including hypertension. This endothelial dysfunction, in its chronic form, leads to considerable vascular wall remodeling. Moreover, it impacts blood pressure regulation in the context of hypertension [10]. The functionality of this endothelial system has been widely targeted by drug therapies in the context of various disease states [12]. With regards to hypertension, this includes inhibitors of the renin-angiotensin aldosterone system (RAAS), in addition to other therapies like statins and antioxidants [10]. Other complementary approaches like herbal medicine have also been employed in the war against cardiovascular disease in general or hypertension in particular [13,14,15,16,17]. Importantly, these diverse approaches all support the vital role of the endothelial system in hypertension development.
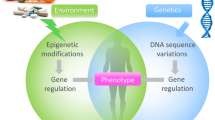
The onset and pathogenesis of hypertension involve several molecular and cellular parameters. Hence, a better understanding of the molecular basis of hypertension is vital for generating new treatment modalities. Many players like reactive oxygen species (ROS), endothelin, and vascular endothelial growth factor (VEGF) are among the key molecules implicated in this pathology [18]. Likewise, vascular smooth muscle cell (VSMC) proliferation and calcification are among the critical cellular aspects that precipitate or exacerbate hypertension [19]. Other players, however, play a protective role in preventing the cardiovascular complications of hypertension and heart failure. These include the cardiac hormone atrial natriuretic peptide (ANP) [18]. It is the interplay between these various adverse and protective mediators that constitutes a framework of hypertension pathophysiology (Fig. 1).
The interplay between the sympathetic nervous system (SNS) and renin-angiotensin aldosterone system (RAAS) in altering cardiovascular hemodynamics. SNS and RAAS activation induce the heart to secrete protective mediators (atrial natriuretic and brain natriuretic peptides) capable of ameliorating a hypertensive state
It is well-established that hypertension also has a solid genetic basis [20]. Generally, hypertension evolves as a combined effect of several factors in the endocrine, renal, and cardiovascular systems. This is especially well-documented in obese people suffering from metabolic syndrome, a phenomenon of extensive genetic interplay [21]. This underpins the importance of genetic input into disease phenotype. More recently, an epigenetic link between molecules like MicroRNA (miRNA) and the evolution of hypertension, both primary and secondary, has been highlighted [22]. In this manuscript, we aim to review the experimentally determined associations between miRNA molecules and hypertension treatment. This is embodied by the physiological responses to antihypertensive drugs due to epigenetic mechanisms, i.e., the pharmaco-epigenetics of hypertension, with a focus on miRNA effects.
Epigenetics and hypertension
DNA methylation
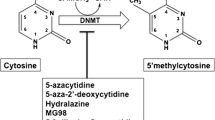
DNA methylation is an example of an epigenetic mark that is responsive to environmental cues and is mitotically stable. It is associated with a range of biological processes, including those involved in the development of hypertension and stroke [23,24,25]. Candidate gene studies in animals and cell lines have demonstrated the role of DNA methylation in the pathogenesis of hypertension, including the HSD11B2 gene [26]. In humans, DNA methylation has been linked to hypertension for this gene as well [27].
Histone modification
Histone modification is known to be one player in the regulation of vascular function in hypertension. That is not surprising given that histones play a crucial role in maintaining chromatin structure and regulating gene expression. One of the five histones found in eukaryotic nuclei, histone 3 (H3), has an N-terminal tail that can be modified by methylation or acetylation of lysine and arginine residues, as well as phosphorylation of serine and threonine residues [28]. Histone acetyltransferases (HAT) add acetyl groups to histones, while histone deacetylases (HDAC) remove them. The effects of these modifications can vary depending on the residue or moiety that is modified.
Non-coding RNAs
Advances in the field of epigenetics have revealed some of the missing pieces in understanding the hereditary puzzle, which can explain why the same genome can lead to different phenotypes without changes to the primary DNA structure. The elusive factor in comprehending the complex and multifactorial nature of hypertension may be the non-coding portion of the human genome [29]. Previously, it was widely believed that all human genes coded for proteins, but it is now known that more than 95% of these genes do not produce proteins. Instead, they are transcribed into non-coding RNA (ncRNAs) molecules, which have vital roles in regulating protein-coding genes [30].
The purpose of this review is to provide an overview of the current understandings for the role of miRNA in the intricate regulatory processes involved in the pathophysiology of hypertension.
MicroRNA and hypertension
MicroRNAs are molecules transcribed from DNA into “non-coding” single-stranded RNA molecules [31] (Fig. 2). These molecules exhibit short, conserved sequences that function as modulators of gene expression [32]. This modulation is achieved by binding the 3’UTR of the mRNA target, thereby regulating its translation [33]. Gene expression regulation is exhibited by mRNA strand de-adenylation, degradation, and inhibition of the ribosomal apparatus assembly, among other mechanisms that remain to be fully elucidated [34].
Transcription of miRNA begins in the nucleus. A miRNA precursor is initially yielded from transcription. It is then transported from the nucleus via the nuclear envelope using the exportin 5 molecule. The resultant miRNA precursor molecule in the cytoplasm is transformed into a double-stranded form via the DICER proteins in an ATP-dependent fashion. Finally, the mature miRNA molecule strand can exercise its degradative, repressive, or even in some cases translation activities
A plethora of miRNA molecules have been linked with the emergence of traditional primary hypertension [35]. They have also been correlated with pulmonary hypertension and pre-eclampsia [36]. In fact, some miRNA molecules may predict the development of hypertension (e.g., miRNA 4516 upregulation and miRNA 145 downregulation) [36]. For instance, diminished levels of miRNA-21 were strongly correlated with ameliorated arterial stiffness in patients with well-controlled essential hypertension [22, 37]. Hence, miRNA-21 may serve as a potential prognostic predictive marker and target for therapy [22, 37]. Moreover, other molecular non-coding RNA agents still need further studies to be assertively dubbed a protective or harmful mediators with regards to a hypertensive phenotype. This is the case with molecules like miRNA-92a-3p, which is proposed to have a positive correlation with systolic and diastolic blood pressure measurements, despite having a negative association with occupational noise exposure, a contributor to hypertensive events [38].
Genetic pre-disposition towards hypertension is becoming increasingly unraveled as more research on the matter prevails. Nearly 2 percent of our entire genome pertains to coding regions that deal with blood pressure regulation. The field of exploring the role of non-coding RNA molecules has thus been extensively revamped. However, several limitations, including verifiable modalities by which efficient molecular standardization in these studies, exist and are being investigated [39, 40]. Nevertheless, further studies delineating the roles of all non-coding RNA molecules may serve as an attractive avenue for potential prevention of the progression of hypertension [41].
MicroRNAs and essential hypertension
The evolution of hypertension is underpinned by inflammatory processes [42]. This includes vascular inflammation, endothelial dysfunction, reactive oxygen species (ROS) production secondary to a plethora of dynamic factors [43,44,45,46,47,48,49,50]. Moreover, as will be discussed in this paper, miRNA is linked to upholding and maintaining these inflammatory processes [51]. An example of this is miRNA-122 which perpetuates cardiovascular fibrosis mechanisms by downregulating several RAAS molecules such as ACE2, or agents from other systems that mediate hypertension when altered, such as apelin [52]. Similarly, other miRNAs such as miR-155, miR-212, miR-21, miR-19a/b and miR-20b mediate inflammatory processes that portend hypertension [53]. These miRNA molecules are active agents in the development of hypertension because of their involvement in various fibrotic vascular processes.
The Renin-Angiotensin-Aldosterone System (RAAS) is an important neurohormonal modulator of blood pressure and volume status [54]. Angiotensin II and aldosterone are pertinent mediators [55]. Angiotensin II helps in facilitating arterial vasoconstriction, especially in the context of blood loss. The autoregulatory RAAS helps maintain sodium balance over various fluid intake states with minor blood pressure alterations [54]. A less studied pathway is that of apelin and its associated proteins [52]. Indeed, it is intimately interlinked with the RAAS pathway so much so that ACE2 modulates its levels [56]. This apelin acts as an endothelial vasodilator and functions through the eNOS pathway, with a documented interplay with the MAPK pathway [57]. The following section portrays how miRNA molecules are physiologically related to RAAS and hypertension evolution or amelioration.
MiRNA-181a
An excessively active sympathetic nervous system generally predisposes to a hypertensive state [34]. This is due to the upregulation of the RAAS system [58] (Fig. 3). Renal hyperactivation by the sympathetic nervous system is known to predispose to excessive renin secretion. This process is potentially mediated by miRNA [58]. For instance, relatively lower miRNA-181a, which is a negative regulator of Ren1 mRNA, leads to a hypertensive state in BPH/2 J mice [58]. Bilateral Renal denervation was shown to amplify miRNA-181a levels and its transcription factor Tcf7l2 such that hypertension in BPH/2 J mice was reversed [34]. Hence, renal sympathetic nerves contribute to the downregulation of miR-181a, a derivative to RAAS overactivity [59]. These findings support the inverse relationship between miRNA-181a and hypertension development in the context of an activated sympathetic nervous system.
MiRNA-132 and miRNA-212
In cases of in-vivo angiotensin II-induced hypertension, miRNA molecules such as miRNAs 132 and 212 were found to be elevated in the heart, kidney, aortic wall tissues of rats [60]. The activation of the Gαq-coupled endothelin receptor was found to elevate the levels of these two miRNA molecules. Contextually, a decrease in these two molecules was noted after administration of Angiotensin II type 1 receptor (AT1-R) blockers, as opposed to treatment with beta-blockers [60]. Subsequently, one can deduce a correlation between these two miRNA molecules and a hypertensive status in response to angiotensin receptor blockers. Moreover, miRNA-132 has additional implications in the realm of cardiovascular morbidity [60]. MiRNA-132 might also be involved in the metabolic and CVD implications of frank obesity [61]. Studies with large sample sizes are needed to correlate miRNA-132 levels in subcutaneous tissue and hypertension in these individuals [61]. These findings all underscore a need for further research to elucidate underpinned relations between these miRNAs and hypertensive sequelae.
MiRNA-483-3p
Angiotensin II-induced activation of AT1-R in VSMCs elicits a miRNA expression signature. For instance, AT-II regulates miRNA 483-3p amongst other molecules [62]. Multiple proponents of the RAAS are targeted by miRNA-483-p, including angiotensinogen and angiotensin-converting enzyme 1 (ACE-1), in VSMCs [62]. Binding sites of this miRNA include RAAS genes AGTR2, ACE-1 and 2, and AGT. Thereby, RAAS homeostasis coordination is achieved. Moreover, miR-483 expression is decreased in the sera of patients with idiopathic pulmonary hypertension, peculiarly in more severe cases [63]. Overexpression of miRNA-483 in pulmonary arterial endothelial cells inhibits the genes related to pulmonary arterial hypertension [63]. Additional findings indicate that miR-483-3p has a pertinent protective impact on endothelial cell functionality during hypertension onset [64]. MiRNA-483 may be a future therapeutic target with regards to CVD sequelae within the context of hypertension [64].
MiRNA-155
In the context of vascular remodeling diseases, miRNA-155 has been shown to be a promising target in ameliorating RAAS-mediated VSMC proliferation [65]. This is due to AT1-R being identified as an important miRNA-155 target in the context of mouse VSMCs [65]. This finding reveals the role of miRNA-155 in mediating anti-proliferative effects.
MicroRNAs and pre-eclampsia
Pre-eclampsia is a disorder of pregnancy that consists of hypertension and proteinuria which occur after the 20th week of the gestational period [66]. It causes significant burden in terms of maternal and neonatal morbidity and mortality [67, 68]. Dysregulation of miRNA molecules have been posited to play a role in the development of pre-eclampsia (Fig. 4) [69]. Some of the miRNA molecules involved in the pathogenesis of pre-eclampsia shall be discussed below.
Depiction of one aspect of pre-eclampsia: trophoblast migration dysfunction. This dysfunction is elicited by microRNAs such as miRNA-210. Shallow trophoblast invasion is seen, resulting in unconverted narrow spiral arteries. This leads to fetal hypoxia virtue of endothelial injury. Sequelae of this hypoxia includes maternal hypertension, edema, and proteinuria
MiRNA-210
MiRNA-210 is one of the most studied miRNA molecules. Its role in the pathophysiology of pre-eclampsia has been explored [70]. MiRNA-210 was seen to be elevated in women with pre-eclampsia [71]. It has been isolated in the placenta and blood of patients with pre-eclampsia [71]. Its effect on trophoblast function (Fig. 4), mitochondrial function, and iron metabolism has been explored [69]. Generally, miRNA-210 expression is increased in hypoxic states. In the context of pre-eclampsia, miRNA-210 plays a role in the incessant inflammation seen. [69]. This is achieved by compromising mitochondrial function, thus stabilizing hypoxia-inducible factor -alpha (HIF-alpha) due to development of reactive oxygen species (ROSs). The expression of miRNA-210 is upregulated due to TLR-3 activation, which also stabilizes HIF-alpha, amongst a cascade of other molecular effects [69]. These findings point to miRNA-210 being deeply involved in pre-eclampsia development.
MiRNA-155
Endothelial nitric oxide synthase (eNOS), a major element in endothelial cell permeability, is regulated by miRNA-155 [72]. This has an implication in endothelial cell function and integrity in the context of pre-eclampsia [72]. In addition, vasodilation homeostasis in human umbilical vein endothelium was seen to be regulated by miRNA-155 [69]. The inhibition of this miRNA-155 molecule was observed to aid in the improvement of endothelial dysfunctional activity [69]. Hence, the role of miRNA-155 potentially involves the development of pre-eclampsia. Further studies are required to mark the pattern of miRNA-155 regulation in the context of pre-eclampsia.
MiRNA-124
The increased expression of miRNA-124-3p inhibits the pro-apoptotic effect of Ang-II, in addition to its role in ROS production in Human umbilical vein endothelial cells (HUVECs) [73]. This is accomplished by targeting EGR1 [73]. This finding reveals a pertinent role of miRNA-144-3p in mediating hypertensive outcomes.
MicroRNAs and pulmonary arterial hypertension
Pulmonary arterial hypertension is a variant of hypertension that has significant burdens. It has unfavorable prognostic morbidity and mortality indicators [74]. Its pathogenesis arises via several mechanisms [75]. On a molecular basis, this entails the proliferation of pulmonary endothelial cells and smooth muscle cells, in addition to the latter’s migration and activation [76]. Many miRNA molecules have been implicated in the pathophysiology of this disease [77]. In fact, pulmonary arterial hypertension shares similar inappropriately activated pathways with cancers [78]. These activated pathways lead to the excessive proliferation and survival pulmonary arterial smooth muscle cells (PASMCs) within the pulmonary arterial wall and resultant lumen restriction [78]. The roles of miRNA-204 and miRNA-206 in PASMCs proliferation has been implicated [78]. Others such as miRNA-145, miRNA-21 and the miR17/92 cluster have been linked with the altered BMPR2 pathway [78]. Some of these miRNA molecules, and others, shall be discussed below (Fig. 5).
Vascular homeostasis is dictated by the phenotype and integrity of various vascular wall elements. This includes SMC, EC, and myofibroblasts. Dysfunction and hyper-proliferation of these elements disrupts the mentioned homeostasis, leading to the manifestation of Pulmonary hypertension. Various miRNA molecules have been implicated in this disruption. These include miRNA in endothelial cells (miR-17-5p, 20a, 27, 424, 503), miRNA in SMC (miR-17-5-p, 20a, 27, 124, 138, 145, 190, 204, 206, 210), and miRNA in fibroblasts (miR-124, 150)
MiRNA-21
MiRNA-21 is thought to play a potential role in hypoxia-induced PASMC proliferation [22]. Indeed, upregulation of this molecule has been found to be facilitated by BMPR2 expression, whereby the miRNA-21 molecules also inhibit BMPR2 expression in a negative feedback loop. As such, it serves as a protective marker especially that its loss leads to Rho-Kinase activity upregulation, furthering pulmonary hypertension progression. [76]. This is another example whereby a miRNA molecule may alter the balance of hypertension development, here in the context of pulmonary hypertension. However, more research is needed to better elucidate the role of miRNA-21 in the context of resistant hypertension [79].
MiRNA-124
MiRNA-124 is downregulated in hypoxic conditions, and when overexpressed it can inhibit PASMC proliferation [76]. This may be capitalized on in future potential treatment modalities, particularly because miRNA-124 inhibits the NFAT activity as well as the subsequent transcription of interleukin-2, a key culprit in hypertension [76]. Furthermore, reduced levels of miRNA-124 has been reported in fibroblasts of patients suffering from pulmonary arterial hypertension [76]. This has an implication for its role in the migration and hyperproliferation of the respective fibroblasts [80], and thus in the pathogenesis of pulmonary hypertension. It appears that one of the underlying mechanisms of action employed by miRNA 124 is suppressing MCP-1 and PTBP-1, which in turn regulate a series of signaling pathways, some of which involve fibroblast proliferation [76]. Thus, this miRNA molecule may serve as a protective factor in the context of pulmonary hypertension [81].
MiRNA-210
As discussed previously, miRNA-210 is upregulated in the context of hypoxia in several cells including PASMCs [76], and apparently this increase is facilitated by the HIF-1 pathway. This upregulation leads to the suppression of E2F3, thus increasing the resistance to apoptosis. Eventually, this promotes PASMC hyperplasia, [76], clearly making it a culprit in exacerbating pulmonary hypertension.
MiRNA-150
MiRNA-150 levels have been seen to be diminished in patients with pulmonary hypertension. Moreover, they have been correlated with a rather poor survival [82]. This was also observed in the plasma and pulmonary cells of rats treated with hypoxia [82]. MiRNA-150 upregulation relieves this and suppresses hypoxia-driven collagen fibrous formation, and the expression of markers such as α-SMA, TGF-β1, and collagen I in pulmonary arterial smooth muscle cells and lung tissues [82]. In addition, miRNA-150 upregulation represses the excessive proliferation of these PASMCs driven by hypoxia via the AKT/mTOR signaling pathway. Moreover, this upregulation inhibits proliferation and resistance to apoptosis in relevant endothelial cells [82]. These findings indicate that miRNA-150 is a promising potential pulmonary hypertension ameliorator. Moreover, this molecule acts as an independent prognostic survival indicator [83]. In addition to its previously mentioned proliferation attenuating effects, miRNA-150 alters phospholipid signaling, with PTPMT1 mostly affected [83]. PTPMT1 reduces inflammatory activity, apoptosis and improves mitochondrial function in pulmonary endothelial cells and progenitors in the context of pulmonary hypertension [83]. These effects are mediated by diminished expression of pro-fibrotic, pro-apoptotic, and pro-inflammatory genes. These genes include c-MYB, NOTCH3, transforming growth factor β (TGF-β), and Col1a1. Mi-RNA1-150 thus serves as a major effector in pulmonary hypertension [83].
MiRNA-140-5p
In the context of pulmonary hypertension, reduced levels of miRNA-140-5p molecules were observed [84]. The therapeutic implications of replacing this miRNA were shown. MiRNA-140-5p targets the E3 ubiquitin ligase Smurf1. The regulation of the latter is a key in the context of pulmonary hypertension [84]. Smurf1 itself targets the BMPR2 molecule, the inactivation of which is the main key implicated in causing of pulmonary hypertension. The restoration BMPR2 signaling, by exogenous delivery of this miRNA molecule, and thus targeting Smurf1, is a promising therapeutic goal of this condition [85].
MicroRNAs and responses to antihypertensive drugs
Various antihypertensives have been used over the past several decades to ameliorate hypertensive outcomes [86]. These include ACE-inhibitors, angiotensin receptor blockers (ARBs), aldosterone antagonists, in addition to alpha and beta-blockers, and calcium channel blockers [87]. Recently, miRNA molecules have thus been found to affect the pharmaco-epigenetic basis of antihypertensives [88]. This has vast implications on the response and treatment-resistance in individuals on antihypertensives [88]. This is pertinent given that half of the patients on anti-hypertensives do not have controlled hypertension [89]. The following section shall address the pharmaco-epigenetic relationship between miRNA molecules and the currently available antihypertensive treatment modalities (Table 1).
Overview of candesartan in hypertension
Candesartan is an ARB and one of the most effective treatments of hypertension [37]. As the name implied, one main mechanism by which it mediates its action is via inhibiting the action of angiotensin II, a main effector of the RAAS. This ameliorates the burden of excessive RAAS activation [90]. It is particularly effective in individuals who have unfavorable side effect outcome (e.g., excessive dry cough) when exposed to ACE-inhibitor [91]. The absence of this widely noted side effect with ARBs is due to the lack of inhibition of substance P and bradykinin degradation [91]. Findings related to epigenetic involvement within candesartan response shall be discussed below.
Pharmaco-epigenetics of candesartan
Candesartan has been confirmed to ameliorate hypertensive sequelae via several mechanisms [92]. One of the mechanisms is by preventing angiotensin II–induced vascular smooth muscle cell proliferation [37]. This effect is mediated by alterations of several miRNAs [37]. Notably, miRNA-301b is shown to be a main target of candesartan [37]. It prevents the decrease of miRNA-301b and thus mediates an anti-proliferative action. Furthermore, studies show that inhibition of this miRNA specifically seems to minimize the physiologic effects of candesartan [37]. Thus, the inhibition of SMC proliferation is lifted [37]. MiRNA-301b prohibits SMC proliferation via targeting the 3’UTR of STAT3, preventing its expression and thus role in the cell cycle G1/S transition [37]. These mechanisms delineate the role of candesartan and its respective miRNA counterpart in ameliorating vascular wall alterations in hypertension.
Pharmaco-epigenetics of beta-blockers
Beta-blockers alleviate hypertension by reducing renin release [93]. This is facilitated by antagonizing beta-1 receptors on juxtaglomerular renal cells [94]. In addition, beta-blockers reduce heart rate by antagonizing beta-1 receptors on cardiac cells [95]. Patients that exhibit RAAS-mediated increase in blood pressure benefit the most from beta-blocker treatment. This response to beta-blocker treatment has been shown to be manipulated by plasma miRNA [96]. This reveals a pertinent need to investigate the relationship between miRNA molecules and beta-blocker treatment.
MiRNA 19a was shown to be a possible biomarker of beta-blocker effectiveness in the setting of patient’s selective response to antihypertensive [94]. This is mediated by several points of regulation in the beta-adrenergic signaling pathway: ADRB1 (the beta-blocker receptor protein target), beta-adrenergic kinase, adenylyl cyclase, and others [94]. In fact, beta-blocker treatment may induce miRNA 19a expression which subsequently downregulates ADRB1 expression [94]. Moreover, molecules such as miRNA-101 and miRNA let-7e have been posited to modulate antihypertensive beta-blocker response [94]. More research is needed to elucidate the exact mechanistic response of these miRNAs to beta-blockers.
MicroRNA: dictating interpersonal beta-blocker effectiveness
Different beta-blocker classes elicit various responses [97]. Atenolol and Nebivolol have similar effects on blood pressure and heart rate. However, they differ in their effects on left ventricular integrity in rodent-models of hypertension. This is through differing effects on miRNA molecules [98]. Nebivolol was shown to better ameliorate left ventricular systolic function decline. In addition, it decreases the burden of ventricular fibrosis and remodeling [98]. This is achieved by preventing the decrease in levels of miRNA-27a and miRNA-29a (which target Sp1) in rodent receiving high-salt diet, in addition to miRNA-133a (which targets Cdc42) [98]. These findings portray the various effects of miRNA molecules in affecting therapeutic drug response in addition to differential drug effects even within the same class of beta-blockers.
MicroRNA in the context of dyslipidemia and hypertension
Dyslipidemia involves an unfavorable lipid profile [99]. This entails high plasma low-density lipoprotein cholesterol (LDL-C), triglycerides (TGs), total cholesterol (TC), and reduced high density lipoprotein cholesterol (HDL-C) [100]. Atherosclerosis is a long-term sequela of dyslipidemia [101, 102]. This phenomenon eventually disrupts blood flow in affected blood vessels [103]. This disruption induces a pro-inflammatory state [104]. Peculiarly, it represses protective factors such as eNOS [103]. This process has been shown to be regulated by miRNA involvement [103]. The development of atheromatous plaques leads to potentially lethal consequences such as myocardial infarction (MI) [105]. This appears to be influenced by miRNA molecules, such as miRNA-10a, -126, -145a/b, -185, -210, and -326 [103]. These molecules have been seen to be elevated inside these arterial plaques in comparison to arteries with no plaque [106]. Meanwhile, certain miRNA molecules appear to be protective [103]. These findings support the hypothesis that epigenetic mechanisms involving miRNA regulate not only hypertensive mechanisms in bringing about unfavorable cardiovascular outcomes, but also dyslipidemia-related sequelae.
Statin overview in context of microRNA
Statins are 3-hydroxy-3methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors [107]. They are a reliable choice in ameliorating unfavorable lipid profiles [108]. They hinder the progression of the lipid synthesis pathway. This is facilitated by preventing the formation of the mevalonate (MVA) intermediate via inhibiting the HMG-CoA reductase rate-limiting enzymatic step [107]. This decreases de novo cholesterol synthesis and increases the feedback expression of low-density lipoprotein receptors (LDL-R) in tissues like the liver. Thus, statins decrease the amount of circulating cholesterol in the blood [109].
It has been demonstrated that statins’ pleiotropic and adverse effects are mediated by miRNA molecules [110]. The pleiotropic effects include anti-inflammatory, antioxidant, anti-thrombotic, and anti-proliferative consequences on the blood vessel wall. This is pertinent to hypertension as it reduces the vascular remodeling profile [103]. The involvement of miRNA processes, however, in these latter pleiotropic effects of statins remains to be further explored in future research.
Pharmaco-epigenetics of statins
The response to statin therapy seems to be influenced by selective miRNA markers [110]. These markers regulate drug transporter and nuclear CYP450 receptors [111]. This is achieved by modulating CYP3A enzyme functionality and expression. Culprits of this regulation include miRNA-27b which affects CYP3A4 gene expression [103]. In fact, inter-individual discrepancies in statin (e.g., atorvastatin) metabolism is due to varying expression levels of miRNA-27b and miRNA-206 [103]. Moreover, miRNA-142 was affirmed to be a major variable in determining whether expression of CYP3A4 and CYP3A5 occurred. This is due to miRNA-142’s transcriptional silencing. MiRNA-133a has also been shown to play a role in mediating statin effects such as lovastatin in the context of endothelial cell function [103]. These findings reveal the pertinence of miRNA molecules in mediating statin effects and dictating its metabolism and therapeutic response.
Aspirin and hypertension
Aspirin, the non-selective cyclooxygenase (COX) inhibitor, has been widely used for its effects as an antiplatelet drug at low doses (< 300 mg) [112]. It prevents platelet aggregation via preferential irreversible blockade of COX-1 inside platelets [113]. This prompts the need for the generation of new platelets to produce thromboxane A2 [114]. However, its usage as a potential management option in hypertensive patients has been discussed in the literature in recent years [115]. The relevant studies have been limited though, with discrepancies in factors like concomitant drug utilization and dosage of aspirin [114]. Aspirin’s benefits as a possible adjunct therapeutic agent in the context of hypertension also differ in outcomes with respect to males and females [114]. Future research is needed to elucidate aspirin’s feasibility as an option in modulating arterial vascular tone, amongst other blood pressure effectors [10].
Pharmaco-epigenetics of aspirin
The physiologic response to aspirin is regulated by certain miRNA molecules [116]. miRNA serve as markers for aspirin resistance, a condition that plagues one fourth of CVD patients [88]. MiRNA-135a-5p and miRNA-204-5p are implicated in this resistance. There are inter-individual differences in relative expression dictating varying response to treatment. These 2 miRNA molecules have been claimed to affect the expression of genes such as THBS1, CORO1C, CDC42, MAPRE2 [88]. Moreover, when indomethacin, a mimicker of aspirin in terms of effects, was used to monitor platelet reactivity, miRNA-19b-1-5p expression was decreased [116]. This serves as an instance of insensitive drug response. This qualifies miRNA to be a potential biomarker of aspirin resistance [88].
In addition, aspirin appears to prevent the abnormal proliferation and calcification of VSMCs [117], a marker of atherosclerotic disease. Contextually, this anti-proliferative and anti-inflammatory effect of aspirin in VSMCs is mediated, at least partly, by miRNA-145 [118]. When atherosclerotic plaques from patients treated with aspirin were compared with those from untreated patients, it was found that there is a significantly higher level of miR-145 and lower CD40 levels in plaques from the treated patients [118]. Moreover, aspirin’s role as an antiplatelet therapeutic agent also seems to be linked to miRNA regulation. For instance, aspirin robustly decreases the expression of miRNA-126 and miRNA-223 [119, 120]. These findings reveal the interlinkage between miRNA and responsiveness to aspirin.
Aspirin in pre-eclampsia: focus on microRNA
Endothelial cell dysfunction is an infamous contributor to the pathogenesis of the inflammatory gestational disease, pre-eclampsia. This is due to reduced eNOS/NO vasodilatory activity when the endothelium is compromised [121]. Aspirin has been shown to have a preventive effect with regards to pre-eclampsia [122]. Indeed, evidence shows that aspirin could abrogate TNF-α induced endothelial dysfunction by suppressing miR-155 [121]. This is another example whereby a hypertensive state is influenced by aspirin via modulating miRNA effects.
Overview of clopidogrel in hypertension
Clopidogrel is an antiplatelet drug that serves to inhibit the activation and aggregation of platelets [90]. This is facilitated via the irreversible binding of active metabolites to the platelet P2Y12 variant of ADP receptors [90]. As is the case with aspirin, clopidogrel may have some utility in subsets of hypertensive patients [123]. Indeed, clopidogrel elicits a preventive effect on angiotensin II-induced inflammation and fibrosis of the heart [90].
Acute rises of blood pressure have been known to platelet activation, a phenomenon that clopidogrel inhibits [90]. It is also now recognized that hypertension is associated with inflammatory processes in vessel wall dynamics [124]. This is in part mediated by incessant platelet activation, particularly in patients with microalbuminuria and vascular lesions [125]. In addition, another major platelet inflammatory mechanism known as the platelet-leukocyte conjugation is a culprit in this process [126]. The nature of platelet involvement in vessel wall dynamics reveals a potential future use of clopidogrel in hypertension. However, future research is needed to elucidate the major potential beneficiaries of clopidogrel in modulating inflammation in hypertension and preventing adverse critical outcomes like MI.
Pharmaco-epigenetics of clopidogrel
Clopidogrel, as an antiplatelet, is a non-active prodrug. It is primarily absorbed in the intestine via ABCB1 transporters. It is then activated to a metabolite by CYP450 enzymes CYP2A4, CYP3A5, CYP2B6, CYP2C19, CYP2C9, and CYP1A2 [90, 127]. Irreversible ADP-receptor binding by way of this active metabolite thus prevents the activation of platelets [128]. However, conventional dosing of clopidogrel has shown, in a considerable percentage of recipients, to elicit a subpar antiplatelet response [90]. Pharmacogenomic bases of interpersonal differences in responses to clopidogrel by way of single nucleotide polymorphisms (SNPs) in certain CYP enzymes were not shown to account for the whole picture [90, 127]. On the other hand, miRNA molecule polymorphisms were shown to play a central part in these interpersonal differences in responses [90]. P2RY12 and CYP2B6 are targets of miRNA-605 [90]. Polymorphisms that give rise to miRNA-605 A/G instead of the G allelic variant prevent the maturation of the miRNA molecules. This prevents the aversion of unfavorable coronary syndrome outcomes in patients on clopidogrel [90]. In fact, this polymorphism may serve as a future biomarker in the context of predicting future events in patients on clopidogrel for the long-term [90]. These findings demonstrate the pertinent effect of miRNA molecules in altering clopidogrel response.
Other therapeutic strategies
With the advent of advanced molecular technologies, miRNA involvement as pathogenesis mediators in many diseases has become more apparent. However, as delineated in this paper, some also serve as protective factors in hypertension. Thus, therapeutic modalities may make use of miRNA manipulation to maintain a balance at the cellular level with regards to gene regulation. In this section, potential miRNA therapies shall be discussed.
Chemically manipulated oligonucleotide molecules termed ‘antagomirs’ have been observed to target miRNA molecules and silence them effectively. The mode of delivery of these molecules remains an important obstacle to overcome in the future [129]. Nevertheless, this can herald several therapeutic outcomes. For example, utilizing intravenously given antagomirs conjugated to cholesterol is a possible mechanism [129]. Antagomirs against miRNA-21 were shown to block cardiac, renal, and pulmonary fibrosis [129]. Moreover, it was shown that targeting both miRNA-425 and miRNA-155 with anti-miRNAs is effective in modulating ANP levels, hence ameliorating hypertension [130]. Examples of targeting miRNA in alleviating pulmonary hypertension have been delineated [131]. This includes inhibiting miRNA-17 via an antagomir, which leads to the upregulation of the BMPR-2 pathway, thus ameliorating hypertension [132]. Moreover, findings have shown that restoring certain miRNA molecules in hepatic stellate cells may potentially alleviate intrahepatic portal hypertension [133]. Other molecules such as miRNA sponges, and locked-nucleic-acid oligonucleotides have also been studied in the context of diseases not isolated to the cardiovascular domain [129]. As a better understanding is attained with regards to miRNA chemical safety, stability, and delivery as a therapeutic modality, better outcomes can be reached [131].
Other considerations
MiRNA molecules are involved in the pathogenesis of many diseases, including cancer. They are interlinked with cancer biological processes such as angiogenesis, apoptosis, proliferation, and invasion/metastasis [134]. In fact, they have been implicated with resistance that some cancer therapeutic regimens have [135, 136]. Cancer cells exhibit certain phenotypic profiles, through miRNA signatures; this can help overcome diagnostic and therapeutic dilemmas [137]. As a matter of fact, miRNA-based therapies have been studied in the context of breast cancer cases [138] (Fig. 6). These phenotypic markers are also exhibited in the context of spinal muscular atrophy through miRNA signatures as well [139]. This can also exhibit a form of biomarker tracking, which can help surmount therapeutic challenges. These encompass a few of the examples whereby a form of epigenetic signaling can make all the difference. Future genomic and epigenetic studies are required in each respective medical field to better help elucidate gaps in diagnostics and therapeutics.
MiRNA molecules may aid in shifting the balance of cells towards an oncogenic phenotype. Others have a tumor suppressive function. Therapies focused on manipulating these individualized miRNA molecules’ respective function are on the rise. This includes antagomirs that can silence certain oncogenic miRNA. Moreover, synthetic miRNA can be utilized to replace miRNA with loss of tumor suppressor function. These can help prevent the propagation of signaling pathways that favor tumorigenesis
Conclusion
Pharmaco-epigenetics is a promising field in the context of personalized health optimization. It is one of the major effectors in predisposing to hypertension. Here, we delineated various means by which epigenetics plays its part, with a primary focus on how miRNA molecules could exacerbate, or ameliorate, hypertension. Moreover, antihypertensive drug response and resistance was seen to be drastically affected by miRNA. Further research is required to better elucidate the miRNA involvement in dictating antihypertensive outcomes. Moreover, additional studies in the field of epigenetics are needed to develop personalized antihypertensive pharmacologic treatment. Given the well-established therapies that already being utilized for hypertension, recognizing the pharmaco-epigenetic implications of miRNA molecules affecting the response to medications is vital for providing maximal treatment efficacy. Finally, given the loose associations between certain discussed treatment modalities (aspirin, clopidogrel, and statins) and hypertension, miRNA molecules may serve as a bridging gap in ultimately realizing the epigenetic understudied links of cardiovascular disease pathophysiology.
Data availability
No datasets were generated or analysed during the current study.
References
Al Attar AA, Fahed GI, Hoballah MM, Pedersen S, El-Yazbi AF, Nasser SA, Bitto A, Orekhov AN, Eid AH (2022) Mechanisms underlying the effects of caloric restriction on hypertension. Biochem Pharmacol 200:115035. https://doi.org/10.1016/j.bcp.2022.115035
Wehbe Z, Nasser SA, El-Yazbi A, Nasreddine S, Eid AH (2020) Estrogen and bisphenol A in hypertension. Curr Hypertens Rep 22:23. https://doi.org/10.1007/s11906-020-1022-z
Fardoun M, Dehaini H, Shaito A, Mesmar J, El-Yazbi A, Badran A, Beydoun E, Eid AH (2020) The hypertensive potential of estrogen: an untold story. Vascul Pharmacol 124:106600. https://doi.org/10.1016/j.vph.2019.106600
Dehaini H, Fardoun M, Abou-Saleh H, El-Yazbi A, Eid AA, Eid AH (2018) Estrogen in vascular smooth muscle cells: a friend or a foe? Vascul Pharmacol 111:15–21. https://doi.org/10.1016/j.vph.2018.09.001
Litwin M, Kułaga Z (2021) Obesity, metabolic syndrome, and primary hypertension. Pediatr Nephrol 36:825–837. https://doi.org/10.1007/s00467-020-04579-3
Charles L, Triscott J, Dobbs B (2017) Secondary hypertension: discovering the underlying cause. Am Fam Physician 96:453–461
Fuchs FD, Whelton PK (2020) High blood pressure and cardiovascular disease. Hypertension 75:285–292. https://doi.org/10.1161/hypertensionaha.119.14240
Garfinkle MA (2017) Salt and essential hypertension: pathophysiology and implications for treatment. J Am Soc Hypertens 11:385–391. https://doi.org/10.1016/j.jash.2017.04.006
Ivy JR, Bailey MA (2014) Pressure natriuresis and the renal control of arterial blood pressure. J Physiol 592:3955–3967. https://doi.org/10.1113/jphysiol.2014.271676
Dzeshka MS, Shantsila A, Lip GY (2016) Effects of aspirin on endothelial function and hypertension. Curr Hypertens Rep 18:83. https://doi.org/10.1007/s11906-016-0688-8
Cyr AR, Huckaby LV, Shiva SS, Zuckerbraun BS (2020) Nitric oxide and endothelial dysfunction. Crit Care Clin 36:307–321. https://doi.org/10.1016/j.ccc.2019.12.009
Rohlenova K, Veys K, Miranda-Santos I, De Bock K, Carmeliet P (2018) Endothelial cell metabolism in health and disease. Trends Cell Biol 28:224–236. https://doi.org/10.1016/j.tcb.2017.10.010
Samaha AA, Fawaz M, Salami A, Baydoun S, Eid AH (2019) Antihypertensive indigenous lebanese plants: ethnopharmacology and a clinical trial. Biomolecules. https://doi.org/10.3390/biom9070292
Anwar MA, Al Disi SS, Eid AH (2016) Anti-hypertensive herbs and their mechanisms of action: part II. Front Pharmacol 7:50. https://doi.org/10.3389/fphar.2016.00050
Al Disi SS, Anwar MA, Eid AH (2015) Anti-hypertensive herbs and their mechanisms of action: part I. Front Pharmacol 6:323. https://doi.org/10.3389/fphar.2015.00323
Shouk R, Abdou A, Shetty K, Sarkar D, Eid AH (2014) Mechanisms underlying the antihypertensive effects of garlic bioactives. Nutr Res 34:106–115. https://doi.org/10.1016/j.nutres.2013.12.005
Maaliki D, Shaito AA, Pintus G, El-Yazbi A, Eid AH (2019) Flavonoids in hypertension: a brief review of the underlying mechanisms. Curr Opin Pharmacol 45:57–65. https://doi.org/10.1016/j.coph.2019.04.014
Konukoglu D, Uzun H (2017) Endothelial dysfunction and hypertension. Adv Exp Med Biol 956:511–540. https://doi.org/10.1007/5584_2016_90
Jaminon A, Reesink K, Kroon A, Schurgers L (2019) The role of vascular smooth muscle cells in arterial remodeling: focus on calcification-related processes. Int J Mol Sci. https://doi.org/10.3390/ijms20225694
Arnett DK, Claas SA (2018) Omics of blood pressure and hypertension. Circ Res 122:1409–1419. https://doi.org/10.1161/circresaha.118.311342
Improta Caria AC, Nonaka CKV, Pereira CS, Soares MBP, Macambira SG, Souza BSF (2018) Exercise training-induced changes in microRNAs: beneficial regulatory effects in hypertension, type 2 diabetes, and obesity. Int J Mol Sci. https://doi.org/10.3390/ijms19113608
Li X, Wei Y, Wang Z (2018) microRNA-21 and hypertension. Hypertens Res 41:649–661. https://doi.org/10.1038/s41440-018-0071-z
Alexeeff SE, Baccarelli AA, Halonen J, Coull BA, Wright RO, Tarantini L, Bollati V, Sparrow D, Vokonas P, Schwartz J (2013) Association between blood pressure and DNA methylation of retrotransposons and pro-inflammatory genes. Int J Epidemiol 42:270–280. https://doi.org/10.1093/ije/dys220
Wei LK, Sutherland H, Au A, Camilleri E, Haupt LM, Gan SH, Griffiths LR (2015) A potential epigenetic marker mediating serum folate and vitamin B12 levels contributes to the risk of ischemic stroke. Biomed Res Int 2015:167976. https://doi.org/10.1155/2015/167976
Smolarek I, Wyszko E, Barciszewska AM, Nowak S, Gawronska I, Jablecka A, Barciszewska MZ (2010) Global DNA methylation changes in blood of patients with essential hypertension. Med Sci Monit 16:149–155
Riviere G, Lienhard D, Andrieu T, Vieau D, Frey BM, Frey FJ (2011) Epigenetic regulation of somatic angiotensin-converting enzyme by DNA methylation and histone acetylation. Epigenetics 6:478–489. https://doi.org/10.4161/epi.6.4.14961
Friso S, Pizzolo F, Choi SW, Guarini P, Castagna A, Ravagnani V, Carletto A, Pattini P, Corrocher R, Olivieri O (2008) Epigenetic control of 11 beta-hydroxysteroid dehydrogenase 2 gene promoter is related to human hypertension. Atherosclerosis 199:323–327. https://doi.org/10.1016/j.atherosclerosis.2007.11.029
Millis RM (2011) Epigenetics and hypertension. Curr Hypertens Rep 13:21–28. https://doi.org/10.1007/s11906-010-0173-8
Raftopoulos L, Katsi V, Makris T, Tousoulis D, Stefanadis C, Kallikazaros I (2015) Epigenetics, the missing link in hypertension. Life Sci 129:22–26. https://doi.org/10.1016/j.lfs.2014.08.003
Consortium EP (2012) An integrated encyclopedia of DNA elements in the human genome. Nature 489:57–74. https://doi.org/10.1038/nature11247
Hill M, Tran N (2021) miRNA interplay: mechanisms and consequences in cancer. Dis Model Mech. https://doi.org/10.1242/dmm.047662
Socco S, Bovee RC, Palczewski MB, Hickok JR, Thomas DD (2017) Epigenetics: the third pillar of nitric oxide signaling. Pharmacol Res 121:52–58. https://doi.org/10.1016/j.phrs.2017.04.011
Correia de Sousa M, Gjorgjieva M, Dolicka D, Sobolewski C, Foti M (2019) Deciphering miRNAs’ action through miRNA editing. Int J Mol Sci. https://doi.org/10.3390/ijms20246249
Biancardi VC, Sharma NM (2020) Connecting sympathetic and renin-angiotensin system overdrive in neurogenic hypertension through miRNA-181a. Hypertens Res 43:1309–1310. https://doi.org/10.1038/s41440-020-0492-3
Duan L, Xiong XJ, Wang J (2014) MicroRNA and hypertension. Zhongguo Zhong Yao Za Zhi 39:397–401
Ozkan G, Ulusoy S, Geyik E, Erdem Y (2019) Down-regulation of miRNA 145 and up-regulation of miRNA 4516 may be associated with primary hypertension. J Clin Hypertens (Greenwich) 21:1724–1731. https://doi.org/10.1111/jch.13704
Zhang L, Yang F, Yan Q (2020) Candesartan ameliorates vascular smooth muscle cell proliferation via regulating miR-301b/STAT3 axis. Hum Cell 33:528–536. https://doi.org/10.1007/s13577-020-00333-x
Li WZ, Zhang HZ, Chen ZM, Tao YQ, Huang XZ, Chen WH, Wang DM (2024) MiRNA-92a-3p mediated the association between occupational noise exposure and blood pressure among Chinese adults. Sci Total Environ 907:168148. https://doi.org/10.1016/j.scitotenv.2023.168148
Marques FZ, Charchar FJ (2015) microRNAs in essential hypertension and blood pressure regulation. Adv Exp Med Biol 888:215–235. https://doi.org/10.1007/978-3-319-22671-2_11
Marques FZ, Booth SA, Charchar FJ (2015) The emerging role of non-coding RNA in essential hypertension and blood pressure regulation. J Hum Hypertens 29:459–467. https://doi.org/10.1038/jhh.2014.99
Kim JD, Lee A, Choi J, Park Y, Kang H, Chang W, Lee MS, Kim J (2015) Epigenetic modulation as a therapeutic approach for pulmonary arterial hypertension. Exp Mol Med 47:e175. https://doi.org/10.1038/emm.2015.45
Wojtasinska A, Frak W, Lisinska W, Sapeda N, Mlynarska E, Rysz J, Franczyk B (2023) Novel insights into the molecular mechanisms of atherosclerosis. Int J Mol Sci 24:13434. https://doi.org/10.3390/ijms241713434
Aboukhater D, Morad B, Nasrallah N, Nasser SA, Sahebkar A, Kobeissy F, Boudaka A, Eid AH (2023) Inflammation and hypertension: underlying mechanisms and emerging understandings. J Cell Physiol 238:1148–1159. https://doi.org/10.1002/jcp.31019
Aramouni K, Assaf R, Shaito A, Fardoun M, Al-Asmakh M, Sahebkar A, Eid AH (2023) Biochemical and cellular basis of oxidative stress: Implications for disease onset. J Cell Physiol 238:1951–1963. https://doi.org/10.1002/jcp.31071
Aramouni K, Assaf RK, Azar M, Jabbour K, Shaito A, Sahebkar A, Eid AA, Rizzo M, Eid AH (2023) Infection with Helicobacter pylori may predispose to atherosclerosis: role of inflammation and thickening of intima-media of carotid arteries. Front Pharmacol 14:1285754. https://doi.org/10.3389/fphar.2023.1285754
Eid AH, Parenti A (2021) Vascular inflammation: players and modulators. Curr Pharm Des 27:2097–2098. https://doi.org/10.2174/138161282718210531101018
Fardoun M, Al-Shehabi T, El-Yazbi A, Issa K, Zouein F, Maaliki D, Iratni R, Eid AH (2017) Ziziphus nummularia inhibits inflammation-induced atherogenic phenotype of human aortic smooth muscle cells. Oxid Med Cell Longev 2017:4134093. https://doi.org/10.1155/2017/4134093
Fardoun MM, Maaliki D, Halabi N, Iratni R, Bitto A, Baydoun E, Eid AH (2020) Flavonoids in adipose tissue inflammation and atherosclerosis: one arrow, two targets. Clin Sci (Lond) 134:1403–1432. https://doi.org/10.1042/CS20200356
Nasser SA, Afify EA, Kobeissy F, Hamam B, Eid AH, El-Mas MM (2021) Inflammatory basis of atherosclerosis: modulation by sex hormones. Curr Pharm Des 27:2099–2111. https://doi.org/10.2174/1381612827666210122142811
Shaito A, Aramouni K, Assaf R, Parenti A, Orekhov A, Yazbi AE, Pintus G, Eid AH (2022) Oxidative stress-induced endothelial dysfunction in cardiovascular diseases. Front Biosci (Landmark Ed) 27:105. https://doi.org/10.31083/j.fbl2703105
Wang G, Luo YL, Gao XJ, Liang Y, Yang FF, Wu JB, Fang D, Luo M (2023) MicroRNA regulation of phenotypic transformations in vascular smooth muscle: relevance to vascular remodeling. Cell Mol Life Sci 80:144. https://doi.org/10.1007/s00018-023-04793-w
Ali F, Shen AL, Islam W, Saleem MZ, Muthu R, Xie QR, Wu MZ, Cheng Y, Chu JF, Lin W, Peng J (2022) Role of MicroRNAs and their corresponding ACE2/Apelin signaling pathways in hypertension. Microb Pathog 162:105361. https://doi.org/10.1016/j.micpath.2021.105361
Adamcova M, Kawano I, Simko F (2021) The impact of microRNAs in renin-angiotensin-system-induced cardiac remodelling. Int J Mol Sci. https://doi.org/10.3390/ijms22094762
Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH (2015) Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res 116:960–975. https://doi.org/10.1161/CIRCRESAHA.116.303587
Patel S, Rauf A, Khan H, Abu-Izneid T (2017) Renin-angiotensin-aldosterone (RAAS): the ubiquitous system for homeostasis and pathologies. Biomed Pharmacother 94:317–325. https://doi.org/10.1016/j.biopha.2017.07.091
Mohammadi M, Mohamadi M, Moradi A, Ramawad HA, Gharin P, Azizi Y, Yousefifard M (2022) Apelin as a candidate for hypertension management; a review and meta on animal studies. Archiv Acad Emerg Med 10:1–13. https://doi.org/10.22037/aaem.v10i1.1704
He Q, Wang Y, Yang H, Wang J, Zhang J, Liu D (2021) Apelin-36 protects against lipopolysaccharide-induced acute lung injury by inhibiting the ASK1/MAPK signaling pathway. Mol Med Rep. https://doi.org/10.3892/mmr.2020.11644
Jackson KL, Marques FZ, Watson AM, Palma-Rigo K, Nguyen-Huu TP, Morris BJ, Charchar FJ, Davern PJ, Head GA (2013) A novel interaction between sympathetic overactivity and aberrant regulation of renin by miR-181a in BPH/2J genetically hypertensive mice. Hypertension 62:775–781. https://doi.org/10.1161/HYPERTENSIONAHA.113.01701
Jackson KL, Gueguen C, Lim K, Eikelis N, Stevenson ER, Charchar FJ, Lambert GW, Burke SL, Paterson MR, Marques FZ, Head GA (2020) Neural suppression of miRNA-181a in the kidney elevates renin expression and exacerbates hypertension in Schlager mice. Hypertens Res 43:1152–1164. https://doi.org/10.1038/s41440-020-0453-x
Eskildsen TV, Jeppesen PL, Schneider M, Nossent AY, Sandberg MB, Hansen PB, Jensen CH, Hansen ML, Marcussen N, Rasmussen LM, Bie P, Andersen DC, Sheikh SP (2013) Angiotensin II regulates microRNA-132/-212 in hypertensive rats and humans. Int J Mol Sci 14:11190–11207. https://doi.org/10.3390/ijms140611190
Eikelis N, Dixon JB, Lambert EA, Hanin G, Tzur Y, Greenberg DS, Soreq H, Marques FZ, Fahey MT, Head GA, Schlaich MP, Lambert GW (2021) MicroRNA-132 may be associated with blood pressure and liver steatosis-preliminary observations in obese individuals. J Hum Hypertens. https://doi.org/10.1038/s41371-021-00597-2
Kemp JR, Unal H, Desnoyer R, Yue H, Bhatnagar A, Karnik SS (2014) Angiotensin II-regulated microRNA 483–3p directly targets multiple components of the renin-angiotensin system. J Mol Cell Cardiol 75:25–39. https://doi.org/10.1016/j.yjmcc.2014.06.008
Zhang J, He Y, Yan X, Chen S, He M, Lei Y, Zhang J, Gongol B, Gu M, Miao Y, Bai L, Cui X, Wang X, Zhang Y, Fan F, Li Z, Shen Y, Chou CH, Huang HD, Malhotra A, Rabinovitch M, Jing ZC, Shyy JY (2020) MicroRNA-483 amelioration of experimental pulmonary hypertension. EMBO Mol Med 12:e11303. https://doi.org/10.15252/emmm.201911303
Shang F, Guo X, Chen Y, Wang C, Gao J, Wen E, Lai B, Bai L (2022) Endothelial microRNA-483-3p is hypertension-protective. Oxid Med Cell Longev 2022:3698219. https://doi.org/10.1155/2022/3698219
Yang LX, Liu G, Zhu GF, Liu H, Guo RW, Qi F, Zou JH (2014) MicroRNA-155 inhibits angiotensin II-induced vascular smooth muscle cell proliferation. J Renin Angiotensin Aldosterone Syst 15:109–116. https://doi.org/10.1177/1470320313503693
Burton GJ, Redman CW, Roberts JM, Moffett A (2019) Pre-eclampsia: pathophysiology and clinical implications. BMJ 366:l2381. https://doi.org/10.1136/bmj.l2381
Wedn AM, El-Bassossy HM, Eid AH, El-Mas MM (2021) Modulation of preeclampsia by the cholinergic anti-inflammatory pathway: therapeutic perspectives. Biochem Pharmacol 192:114703. https://doi.org/10.1016/j.bcp.2021.114703
Maaliki D, Issa K, Al Shehabi T, El-Yazbi A, Eid AH (2019) The role of alpha2-adrenergic receptors in hypertensive preeclampsia: a hypothesis. Microcirculation 26:e12511. https://doi.org/10.1111/micc.12511
Lv Y, Lu C, Ji X, Miao Z, Long W, Ding H, Lv M (2019) Roles of microRNAs in preeclampsia. J Cell Physiol 234:1052–1061. https://doi.org/10.1002/jcp.27291
Koushki M, Amiri Dash Atan N, Omidi-Ardali H, Rezaei Tavirani M (2018) Assessment of correlation between miR-210 expression and pre-eclampsia risk: a meta-analysis. Rep Biochem Mol Biol 7:94–101
Biro O, Alasztics B, Molvarec A, Joo J, Nagy B, Rigo J Jr (2017) Various levels of circulating exosomal total-miRNA and miR-210 hypoxamiR in different forms of pregnancy hypertension. Pregnancy Hypertens 10:207–212. https://doi.org/10.1016/j.preghy.2017.09.002
Kim JH, Kim JY, Park M, Kim S, Kim T, Kim J, Choi S, Park W, Hwang JY, Choe J, Ha KS, Won MH, Ryoo S, Kwon YG, Kim YM (2020) NF-κB-dependent miR-31/155 biogenesis is essential for TNF-α-induced impairment of endothelial progenitor cell function. Exp Mol Med 52:1298–1309. https://doi.org/10.1038/s12276-020-0478-x
Lv L, Shen J, Xu J, Wu X, Zeng C, Lin L, Mao W, Wei T (2021) MiR-124-3p reduces angiotensin II-dependent hypertension by down-regulating EGR1. J Hum Hypertens 35:696–708. https://doi.org/10.1038/s41371-020-0381-x
Vonk Noordegraaf A, Groeneveldt JA, Bogaard HJ (2016) Pulmonary hypertension. Eur Respir Rev 25:4–11. https://doi.org/10.1183/16000617.0096-2015
Mandras SA, Mehta HS, Vaidya A (2020) Pulmonary hypertension: a brief guide for clinicians. Mayo Clin Proc 95:1978–1988. https://doi.org/10.1016/j.mayocp.2020.04.039
Zhou G, Chen T, Raj JU (2015) MicroRNAs in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 52:139–151. https://doi.org/10.1165/rcmb.2014-0166TR
Boucherat O, Potus F, Bonnet S (2015) microRNA and pulmonary hypertension. Adv Exp Med Biol 888:237–252. https://doi.org/10.1007/978-3-319-22671-2_12
Meloche J, Paulin R, Provencher S, Bonnet S (2015) Therapeutic potential of microRNA modulation in pulmonary arterial hypertension. Curr Vasc Pharmacol 13:331–340. https://doi.org/10.2174/15701611113119990010
Kara SP, Ozkan G, Yılmaz A, Bayrakçı N, Güzel S, Geyik E (2021) MicroRNA 21 and microRNA 155 levels in resistant hypertension, and their relationships with aldosterone. Ren Fail 43:676–683. https://doi.org/10.1080/0886022x.2021.1915800
Zhang H, Laux A, Stenmark KR, Hu CJ (2021) Mechanisms contributing to the dysregulation of miRNA-124 in pulmonary hypertension. Int J Mol Sci. https://doi.org/10.3390/ijms22083852
Zhang H, Wang D, Li M, Plecitá-Hlavatá L, D’Alessandro A, Tauber J, Riddle S, Kumar S, Flockton A, McKeon BA, Frid MG, Reisz JA, Caruso P, El Kasmi KC, Ježek P, Morrell NW, Hu CJ, Stenmark KR (2017) Metabolic and proliferative state of vascular adventitial fibroblasts in pulmonary hypertension is regulated through a microRNA-124/PTBP1 (polypyrimidine tract binding protein 1)/pyruvate kinase muscle axis. Circulation 136:2468–2485. https://doi.org/10.1161/circulationaha.117.028069
Li Y, Ren W, Wang X, Yu X, Cui L, Li X, Zhang X, Shi B (2019) MicroRNA-150 relieves vascular remodeling and fibrosis in hypoxia-induced pulmonary hypertension. Biomed Pharmacother 109:1740–1749. https://doi.org/10.1016/j.biopha.2018.11.058
Russomanno G, Jo KB, Abdul-Salam VB, Morgan C, Endruschat J, Schaeper U, Osman AH, Alzaydi MM, Wilkins MR, Wojciak-Stothard B (2021) miR-150-PTPMT1-cardiolipin signaling in pulmonary arterial hypertension. Mol Ther Nucleic Acids 23:142–153. https://doi.org/10.1016/j.omtn.2020.10.042
Rothman AM, Rowlands DJ, Lawrie A (2016) miRNA-140-5p: new avenue for pulmonary arterial hypertension drug development? Epigenomics 8:1311–1313. https://doi.org/10.2217/epi-2016-0089
Orriols M, Gomez-Puerto MC, Ten Dijke P (2017) BMP type II receptor as a therapeutic target in pulmonary arterial hypertension. Cell Mol Life Sci 74:2979–2995. https://doi.org/10.1007/s00018-017-2510-4
Reddy P, Dupree L (2016) Approach to antihypertensive therapy. Am J Ther 23:e451–e473. https://doi.org/10.1097/mjt.0000000000000224
Tsioufis C, Thomopoulos C (2017) Combination drug treatment in hypertension. Pharmacol Res 125:266–271. https://doi.org/10.1016/j.phrs.2017.09.011
Paseban M, Marjaneh RM, Banach M, Riahi MM, Bo S, Sahebkar A (2020) Modulation of microRNAs by aspirin in cardiovascular disease. Trends Cardiovasc Med 30:249–254. https://doi.org/10.1016/j.tcm.2019.08.005
Stoll S, Wang C, Qiu H (2018) DNA methylation and histone modification in hypertension. Int J Mol Sci. https://doi.org/10.3390/ijms19041174
Zhou WL, Mo ZZ, Xiao FY, Dai W, Wang G, Zhou G, Zhang W, Chen BL (2020) microRNA-605 rs2043556 polymorphisms affect clopidogrel therapy through modulation of CYP2B6 and P2RY12 in acute coronary syndrome patients. Platelets 31:897–905. https://doi.org/10.1080/09537104.2019.1696455
Yilmaz I (2019) Angiotensin-converting enzyme inhibitors induce cough. Turk Thorac J 20:36–42. https://doi.org/10.5152/TurkThoracJ.2018.18014
Zhenfeng Z, Huilan S, Junya J, Dong L, Shan L (2011) A systematic review and meta-analysis of candesartan and losartan in the management of essential hypertension. J Renin Angiotensin Aldosterone Syst 12:365–374. https://doi.org/10.1177/1470320310391503
Stokes GS, Weber MA, Thornell IR (1974) Beta-blockers and plasma renin activity in hypertension. Br Med J 1:60–62. https://doi.org/10.1136/bmj.1.5897.60
Solayman MH, Langaee TY, Gong Y, Shahin MH, Turner ST, Chapman AB, Gums JG, Boerwinkle E, Beitelshees AL, El-Hamamsy M, El-Wakeel L, Cooper-DeHoff RM, Badary OA, Johnson JA (2019) Effect of plasma MicroRNA on antihypertensive response to beta blockers in the pharmacogenomic evaluation of antihypertensive responses (PEAR) studies. Eur J Pharm Sci 131:93–98. https://doi.org/10.1016/j.ejps.2019.02.013
Kotecha D, Flather MD, Altman DG, Holmes J, Rosano G, Wikstrand J, Packer M, Coats AJS, Manzano L, Böhm M, van Veldhuisen DJ, Andersson B, Wedel H, von Lueder TG, Rigby AS, Hjalmarson Å, Kjekshus J, Cleland JGF (2017) Heart rate and rhythm and the benefit of beta-blockers in patients with heart failure. J Am Coll Cardiol 69:2885–2896. https://doi.org/10.1016/j.jacc.2017.04.001
Ling S, Nanhwan M, Qian J, Kodakandla M, Castillo AC, Thomas B, Liu H, Ye Y (2013) Modulation of microRNAs in hypertension-induced arterial remodeling through the β1 and β3-adrenoreceptor pathways. J Mol Cell Cardiol 65:127–136. https://doi.org/10.1016/j.yjmcc.2013.10.003
Del Mauro JS, Prince PD, Santander Plantamura Y, Allo MA, Parola L, Fernandez Machulsky N, Morettón MA, Bin EP, González GE, Bertera FM, Carranza A, Berg G, Taira CA, Donato M, Chiappetta DA, Polizio AH, Höcht C (2021) Nebivolol is more effective than atenolol for blood pressure variability attenuation and target organ damage prevention in L-NAME hypertensive rats. Hypertens Res 44:791–802. https://doi.org/10.1038/s41440-021-00630-4
Ye H, Ling S, Castillo AC, Thomas B, Long B, Qian J, Perez-Polo JR, Ye Y, Chen X, Birnbaum Y (2013) Nebivolol induces distinct changes in profibrosis microRNA expression compared with atenolol, in salt-sensitive hypertensive rats. Hypertension 61:1008–1013. https://doi.org/10.1161/HYPERTENSIONAHA.111.00892
Shahwan MJ, Jairoun AA, Farajallah A, Shanabli S (2019) Prevalence of dyslipidemia and factors affecting lipid profile in patients with type 2 diabetes. Diabetes Metab Syndr 13:2387–2392. https://doi.org/10.1016/j.dsx.2019.06.009
Diabetes Canada Clinical Practice Guidelines Expert C, Mancini GBJ, Hegele RA, Leiter LA (2018) Dyslipidemia. Can J Diabetes 42(Suppl 1):S178–S185. https://doi.org/10.1016/j.jcjd.2017.10.019
Kopin L, Lowenstein C (2017) Dyslipidemia. Ann Intern Med 167:Itc81–Itc96. https://doi.org/10.7326/aitc201712050
Al Zein M, Zein O, Diab R, Dimachkie L, Sahebkar A, Al-Asmakh M, Kobeissy F, Eid AH (2023) Intermittent fasting favorably modulates adipokines and potentially attenuates atherosclerosis. Biochem Pharmacol 218:115876. https://doi.org/10.1016/j.bcp.2023.115876
Mohajeri M, Banach M, Atkin SL, Butler AE, Ruscica M, Watts GF, Sahebkar A (2018) MicroRNAs: novel molecular targets and response modulators of statin therapy. Trends Pharmacol Sci 39:967–981. https://doi.org/10.1016/j.tips.2018.09.005
Wolf D, Ley K (2019) Immunity and inflammation in atherosclerosis. Circ Res 124:315–327. https://doi.org/10.1161/circresaha.118.313591
Li F, Li D, Yu J, Jia Y, Liu Y, Liu Y, Wu Q, Liao X, Zeng Z, Wan Z, Zeng R (2021) Silent myocardial infarction and long-term risk of frailty: the atherosclerosis risk in communities study. Clin Interv Aging 16:1139–1149. https://doi.org/10.2147/cia.S315837
Chen WJ, Yin K, Zhao GJ, Fu YC, Tang CK (2012) The magic and mystery of microRNA-27 in atherosclerosis. Atherosclerosis 222:314–323. https://doi.org/10.1016/j.atherosclerosis.2012.01.020
Almeida SO, Budoff M (2019) Effect of statins on atherosclerotic plaque. Trends Cardiovasc Med 29:451–455. https://doi.org/10.1016/j.tcm.2019.01.001
Rissetti G, Zeni D, Ongaratti BR, Pereira-Lima JFS, Rech C, da Costa OM (2021) Lipid profile and response to statin therapy in patients with hypopituitarism. Arch Endocrinol Metab 64:673–678. https://doi.org/10.20945/2359-3997000000292
Sirtori CR (2014) The pharmacology of statins. Pharmacol Res 88:3–11. https://doi.org/10.1016/j.phrs.2014.03.002
Allen SC, Mamotte CDS (2017) Pleiotropic and adverse effects of statins-do epigenetics play a role? J Pharmacol Exp Ther 362:319–326. https://doi.org/10.1124/jpet.117.242081
Hirota T, Fujita Y, Ieiri I (2020) An updated review of pharmacokinetic drug interactions and pharmacogenetics of statins. Expert Opin Drug Metab Toxicol 16:809–822. https://doi.org/10.1080/17425255.2020.1801634
Santos-Gallego CG, Badimon J (2021) Overview of aspirin and platelet biology. Am J Cardiol 144(Suppl 1):S2-s9. https://doi.org/10.1016/j.amjcard.2020.12.018
Ornelas A, Zacharias-Millward N, Menter DG, Davis JS, Lichtenberger L, Hawke D, Hawk E, Vilar E, Bhattacharya P, Millward S (2017) Beyond COX-1: the effects of aspirin on platelet biology and potential mechanisms of chemoprevention. Cancer Metastasis Rev 36:289–303. https://doi.org/10.1007/s10555-017-9675-z
Soodi D, VanWormer JJ, Rezkalla SH (2020) Aspirin in primary prevention of cardiovascular events. Clin Med Res 18:89–94. https://doi.org/10.3121/cmr.2020.1548
Costa AC, Reina-Couto M, Albino-Teixeira A, Sousa T (2017) Aspirin and blood pressure: effects when used alone or in combination with antihypertensive drugs. Rev Port Cardiol 36:551–567. https://doi.org/10.1016/j.repc.2017.05.008
Kok MG, Mandolini C, Moerland PD, de Ronde MW, Sondermeijer BM, Halliani A, Nieuwland R, Cipollone F, Creemers EE, Meijers JC, Pinto-Sietsma SJ (2016) Low miR-19b-1-5p expression in isolated platelets after aspirin use is related to aspirin insensitivity. Int J Cardiol 203:262–263. https://doi.org/10.1016/j.ijcard.2015.10.098
Shen Q, Chen Q, Liu Y, Xue X, Shen X, He Q, Wang G, Han F (2022) Aspirin relieves the calcification of aortic smooth muscle cells by enhancing the heat shock response. Pharm Biol 60:17–24. https://doi.org/10.1080/13880209.2021.2007268
Guo X, Yu L, Chen M, Wu T, Peng X, Guo R, Zhang B (2016) miR-145 mediated the role of aspirin in resisting VSMCs proliferation and anti-inflammation through CD40. J Transl Med 14:211. https://doi.org/10.1186/s12967-016-0961-2
de Boer HC, van Solingen C, Prins J, Duijs JM, Huisman MV, Rabelink TJ, van Zonneveld AJ (2013) Aspirin treatment hampers the use of plasma microRNA-126 as a biomarker for the progression of vascular disease. Eur Heart J 34:3451–3457. https://doi.org/10.1093/eurheartj/eht007
Willeit P, Zampetaki A, Dudek K, Kaudewitz D, King A, Kirkby NS, Crosby-Nwaobi R, Prokopi M, Drozdov I, Langley SR, Sivaprasad S, Markus HS, Mitchell JA, Warner TD, Kiechl S, Mayr M (2013) Circulating microRNAs as novel biomarkers for platelet activation. Circ Res 112:595–600. https://doi.org/10.1161/CIRCRESAHA.111.300539
Kim J, Lee KS, Kim JH, Lee DK, Park M, Choi S, Park W, Kim S, Choi YK, Hwang JY, Choe J, Won MH, Jeoung D, Lee H, Ryoo S, Ha KS, Kwon YG, Kim YM (2017) Aspirin prevents TNF-alpha-induced endothelial cell dysfunction by regulating the NF-kappaB-dependent miR-155/eNOS pathway: role of a miR-155/eNOS axis in preeclampsia. Free Radic Biol Med 104:185–198. https://doi.org/10.1016/j.freeradbiomed.2017.01.010
Chappell LC, Cluver CA, Kingdom J, Tong S (2021) Pre-eclampsia. Lancet 398:341–354. https://doi.org/10.1016/s0140-6736(20)32335-7
Robbins IM, Kawut SM, Yung D, Reilly MP, Lloyd W, Cunningham G, Loscalzo J, Kimmel SE, Christman BW, Barst RJ (2006) A study of aspirin and clopidogrel in idiopathic pulmonary arterial hypertension. Eur Respir J 27:578–584. https://doi.org/10.1183/09031936.06.00095705
Guzik TJ, Touyz RM (2017) Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 70:660–667. https://doi.org/10.1161/hypertensionaha.117.07802
Delaney C, Davizon-Castillo P, Allawzi A, Posey J, Gandjeva A, Neeves K, Tuder RM, Di Paola J, Stenmark KR, Nozik ES (2021) Platelet activation contributes to hypoxia-induced inflammation. Am J Physiol Lung Cell Mol Physiol 320:L413-l421. https://doi.org/10.1152/ajplung.00519.2020
Jia LX, Qi GM, Liu O, Li TT, Yang M, Cui W, Zhang WM, Qi YF, Du J (2013) Inhibition of platelet activation by clopidogrel prevents hypertension-induced cardiac inflammation and fibrosis. Cardiovasc Drugs Ther 27:521–530. https://doi.org/10.1007/s10557-013-6471-z
Pereira NL, Rihal CS, So DYF, Rosenberg Y, Lennon RJ, Mathew V, Goodman SG, Weinshilboum RM, Wang L, Baudhuin LM, Lerman A, Hasan A, Iturriaga E, Fu YP, Geller N, Bailey K, Farkouh ME (2019) Clopidogrel pharmacogenetics. Circ Cardiovasc Interv 12:e007811. https://doi.org/10.1161/circinterventions.119.007811
Wang ZY, Chen M, Zhu LL, Yu LS, Zeng S, Xiang MX, Zhou Q (2015) Pharmacokinetic drug interactions with clopidogrel: updated review and risk management in combination therapy. Ther Clin Risk Manag 11:449–467. https://doi.org/10.2147/tcrm.S80437
Batkai S, Thum T (2012) MicroRNAs in hypertension: mechanisms and therapeutic targets. Curr Hypertens Rep 14:79–87. https://doi.org/10.1007/s11906-011-0235-6
Vandenwijngaert S, Ledsky CD, Agha O, Wu C, Hu D, Bagchi A, Domian IJ, Buys ES, Newton-Cheh C, Bloch DB (2018) MicroRNA-425 and microRNA-155 cooperatively regulate atrial natriuretic peptide expression and cGMP production. PLoS ONE 13:e0196697. https://doi.org/10.1371/journal.pone.0196697
Xu J, Linneman J, Zhong Y, Yin H, Xia Q, Kang K, Gou D (2022) MicroRNAs in pulmonary hypertension, from pathogenesis to diagnosis and treatment. Biomolecules 12:496. https://doi.org/10.3390/biom12040496
Pullamsetti SS, Doebele C, Fischer A, Savai R, Kojonazarov B, Dahal BK, Ghofrani HA, Weissmann N, Grimminger F, Bonauer A, Seeger W, Zeiher AM, Dimmeler S, Schermuly RT (2012) Inhibition of MicroRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med 185:409–419. https://doi.org/10.1164/rccm.201106-1093oc
Guo CJ, Pan Q, Xiong H, Qiao YQ, Bian ZL, Zhong W, Sheng L, Li H, Shen L, Hua J, Ma X (2014) Therapeutic potential of MicroRNA: a new target to treat intrahepatic portal hypertension? Biomed Res Int 2014:1–8. https://doi.org/10.1155/2014/797898
Lee YS, Dutta A (2009) MicroRNAs in cancer. Annu Rev Pathol 4:199–227. https://doi.org/10.1146/annurev.pathol.4.110807.092222
He B, Zhao Z, Cai Q, Zhang Y, Zhang P, Shi S, Xie H, Peng X, Yin W, Tao Y, Wang X (2020) miRNA-based biomarkers, therapies, and resistance in cancer. Int J Biol Sci 16:2628–2647. https://doi.org/10.7150/ijbs.47203
Mirzaei S, Zarrabi A, Asnaf SE, Hashemi F, Zabolian A, Hushmandi K, Raei M, Goharrizi M, Makvandi P, Samarghandian S, Najafi M, Ashrafizadeh M, Aref AR, Hamblin MR (2021) The role of microRNA-338-3p in cancer: growth, invasion, chemoresistance, and mediators. Life Sci 268:119005. https://doi.org/10.1016/j.lfs.2020.119005
Mishra S, Yadav T, Rani V (2016) Exploring miRNA based approaches in cancer diagnostics and therapeutics. Crit Rev Oncol Hematol 98:12–23. https://doi.org/10.1016/j.critrevonc.2015.10.003
Bertoli G, Cava C, Castiglioni I (2015) MicroRNAs: new biomarkers for diagnosis, prognosis, therapy prediction and therapeutic tools for breast cancer. Theranostics 5:1122–1143. https://doi.org/10.7150/thno.11543
Magri F, Vanoli F, Corti S (2018) miRNA in spinal muscular atrophy pathogenesis and therapy. J Cell Mol Med 22:755–767. https://doi.org/10.1111/jcmm.13450
Acknowledgement
Not Applicable.
Funding
Open Access funding provided by the Qatar National Library.
Author information
Authors and Affiliations
Contributions
AHE conceived of the idea. All authors contributed to the writing and reviewing of the manuscript. AHE made the final editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yaacoub, S., Boudaka, A., AlKhatib, A. et al. The pharmaco-epigenetics of hypertension: a focus on microRNA. Mol Cell Biochem (2024). https://doi.org/10.1007/s11010-024-04947-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-024-04947-9