Abstract
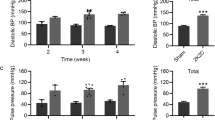
β-Adrenergic blockers are no longer recommended as first-line therapy due to the reduced cardioprotection of traditional β-blockers compared with other antihypertensive drugs. It is unknown whether third-generation β-blockers share the limitations of traditional β-blockers. The aim of the present study was to compare the effects of nebivolol or atenolol on central and peripheral systolic blood pressure (SBP) and its variability and target organ damage (TOD) in N-nitro-L-arginine methyl ester (L-NAME) hypertensive rats. Male Wistar rats were treated with L-NAME for 8 weeks together with oral administration of nebivolol 30 mg/kg (n = 8), atenolol 90 mg/kg (n = 8), or vehicle (n = 8). The control group was composed of vehicle-treated Wistar rats. SBP and its variability, as well as echocardiographic parameters, were assessed during the last 2 weeks of treatment. Tissue levels of tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and transforming growth factor β (TGF-β), and histopathological parameters were evaluated in the left ventricle and aorta. Nebivolol had a greater ability than atenolol to decrease central SBP and mid-term and short-term blood pressure variability (BPV) in L-NAME rats. Echocardiographic analysis showed that nebivolol was more effective than atenolol on E/A wave ratio normalization. Compared with atenolol treatment, nebivolol had a greater protective effect on different TOD markers, inducing a decrease in collagen deposition and a reduction in the proinflammatory cytokines IL-6 and TNF-α in the left ventricle and aorta. Our findings suggest that the adverse hemodynamic profile and the reduced cardiovascular protection reported with traditional β-blockers must not be carried forward to third-generation β-blockers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lewis P. The essential action of propranolol in hypertension. Am J Med. 1976;60:837–52.
James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–20.
Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 2018;36:2284–309.
McCormack T, Boffa RJ, Jones NR, Carville S, McManus RJ. The 2018 ESC/ESH hypertension guideline and the 2019 NICE hypertension guideline, how and why they differ. Eur Heart J. 2019;40:3456–58.
Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Opie LH. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2017;1:CD002003.
Wei J, Galaviz KI, Kowalski AJ, Magee MJ, Haw JS, Narayan KMV, et al. Comparison of cardiovascular events among users of different classes of antihypertension medications: a systematic review and network meta-analysis. JAMA Netw Open. 2020;3:e1921618.
Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOTBPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906.
Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–25.
Höcht C, Bertera FM, Del Mauro JS, Santander Plantamura Y, Taira CA, Polizio AH. What is the real efficacy of beta-blockers for the treatment of essential hypertension? Curr Pharm Des. 2017;23:4658–77.
Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, et al. Effects of beta blockers and calcium-channel blockers on within-individual variability in blood pressure and risk of stroke. Lancet Neurol. 2010;9:469–80.
Fumagalli C, Maurizi N, Marchionni N, Fornasari D. β-blockers: Their new life from hypertension to cancer and migraine. Pharm Res. 2020;151:104587.
Del Pinto R, Ferri C, Parati G. Reduction of blood pressure variability: an additional protective cardiovascular effect of vasodilating beta-blockers? J Hypertens. 2020;38:405–7.
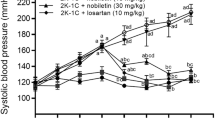
Loutradis C, Bikos A, Raptis V, Afkou Z, Tzanis G, Pyrgidis N, et al. Nebivolol reduces short-term blood pressure variability more potently than irbesartan in patients with intradialytic hypertension. Hypertens Res. 2019;42:1001–10.
Oliver E, Mayor F Jr, D’Ocon P. Beta-blockers: Historical Perspective and Mechanisms of Action. Rev Esp Cardiol. 2019;72:853–62.
Del Mauro JS, Prince PD, Allo MA, Santander Plantamura Y, Morettón MA, González GE, et al. Effects of third-generation β-blockers, atenolol or amlodipine on blood pressure variability and target organ damage in spontaneously hypertensive rats. J Hypertens. 2020;38:536–45.
Bertera FM, Del Mauro JS, Lovera V, Chiappetta D, Polizio AH, Taira CA, et al. Enantioselective pharmacokinetics and cardiovascular effects of nebivolol in L-NAME hypertensive rats. Hypertens Res. 2014;37:194–201.
Del Mauro JS, Prince PD, Donato M, Fernandez Machulsky N, Morettón MA, González GE, et al. Effects of carvedilol or amlodipine on target organ damage in L-NAME hypertensive rats: their relationship with blood pressure variability. J Am Soc Hypertens. 2017;11:227–40.
Varagic J, Ahmad S, Voncannon JL, Moniwa N, Simington SW Jr, Brosnihan BK, et al. Nebivolol reduces cardiac angiotensin II, associated oxidative stress and fibrosis but not arterial pressure in salt-loaded spontaneously hypertensive rats. J Hypertens. 2012;30:1766–74.
Cosentino F, Bonetti S, Rehorik R, Eto M, Werner-Felmayer G, Volpe M, et al. Nitric-oxide mediated relaxations in salt-induced hypertension: effect of chronic beta1-selective receptor blockade. J Hypertens. 2002;20:421–8.
Wang Y, Zhang F, Liu Y, Yin S, Pang X, Li Z, et al. Nebivolol alleviates aortic remodeling through eNOS upregulation and inhibition of oxidative stress in l-NAME-induced hypertensive rats. Clin Exp Hypertens. 2017;39:628–39.
Xu LP, Shen FM, Shu H, Miao CY, Jiang YY, Su DF. Synergism of atenolol and amlodipine on lowering and stabilizing blood pressure in spontaneously hypertensive rats. Fundam Clin Pharm. 2004;18:33–38.
Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals. Part 2: Blood pressure measurement in experimental animals: a statement for professionals from the subcommittee of professional and public education of the American Heart Association council on high blood pressure research. Hypertension. 2005;45:299–310.
Pladys P, Lahaie I, Cambonie G, Thibault G, Lê NL, Abran D, et al. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res. 2004;55:1042–9.
Langager AM, Hammerberg BE, Rotella DL, Stauss HM. Very low-frequency blood pressure variability depends on voltage-gated L-type Ca2+ channels in conscious rats. Am J Physiol Heart Circ Physiol. 2007;292:H1321–H1327.
Salman IM. Current approaches to quantifying tonic and reflex autonomic outflows controlling cardiovascular function in humans and experimental animals. Curr Hypertens Rep. 2015;17:84.
Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, et al. I. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–67.
González GE, Wilensky L, Cassaglia P, Morales C, Gelpi RJ. Early administration of enalapril prevents diastolic dysfunction and ventricular remodeling in rabbits with myocardial infarction. Cardiovasc Pathol. 2016;25:208–13.
Guerrero E, Voces F, Ardanaz N, Montero MJ, Arévalo M, Sevilla MA. Long-term treatment with nebivolol improves arterial reactivity and reduces ventricular hypertrophy in spontaneously hypertensive rats. J Cardiovasc Pharm. 2003;42:348–55.
Xiong X, You C, Feng QC, Yin T, Chen ZB, Ball P, et al. Pulse width modulation electro-acupuncture on cardiovascular remodelling and plasma nitric oxide in spontaneously hypertensive rats. Evid Based Complement Altern Med. 2011;2011:812160.
Wang DS, Xie HH, Shen FM, Cai GJ, Su DF. Blood pressure variability, cardiac baroreflex sensitivity and organ damage in experimentally hypertensive rats. Clin Exp Pharm Physiol. 2005;32:545–52.
Zambrano LI, Pontes RB, Garcia ML, Nishi EE, Nogueira FN, Higa EMS, et al. Pattern of sympathetic vasomotor activity in a model of hypertension induced by nitric oxide synthase blockade. Physiol Rep. 2019;7:e14183.
Cavalcante GL, Ferreira FN, da Silva MTB, Soriano RN, ALMM Filho, DDR Arcanjo, et al. Acetylcholinesterase inhibition prevents alterations in cardiovascular autonomic control and gastric motility in L-NAME-induced hypertensive rats. Life Sci. 2020;256:117915.
Parati G, Torlasco C, Pengo M, Bilo G, Ochoa JE. Blood pressure variability: its relevance for cardiovascular homeostasis and cardiovascular diseases. Hypertens Res. 2020;43:609–20.
Bouissou-Schurtz C, Lindesay G, Regnault V, Renet S, Safar ME, Molinie V, et al. Development of an experimental model to study the relationship between day-to-day variability in blood pressure and aortic stiffness. Front Physiol. 2015;6:368.
Parati G, Ochoa JE, Lombardi C, Bilo G. Assessment and management of blood-pressure variability. Nat Rev Cardiol. 2013;10:143–55.
Stauss HM. Identification of blood pressure control mechanisms by power spectral analysis. Clin Exp Pharmacol Physiol. 2007;34:362–88.
Takeda K, Nakagawa Y, Hashimoto T, Sakurai H, Imai S. Effects of several beta-blocking agents on the development of hypertension in spontaneously hypertensive rats. Jpn J Pharm. 1979;29:171–8.
Takeda K, Nakagawa Y, Chin WP, Imai S. A comparison of antihypertensive effects of atenolol and propranolol in the spontaneously hypertensive, DOCA/saline hypertensive and renal hypertensive rats. Jpn J Pharm. 1982;32:283–9.
Horinouchi T, Morishima S, Tanaka T, Suzuki F, Tanaka Y, Koike K, et al. Different changes of plasma membrane beta-adrenoceptors in rat heart after chronic administration of propranolol, atenolol and bevantolol. Life Sci. 2007;81:399–404.
Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525:263–70.
McEniery CM, Schmitt M, Qasem A, Webb DJ, Avolio AP, Wilkinson IB, et al. Nebivolol increases arterial distensibility in vivo. Hypertension. 2004;44:305–10.
Bordicchia M, Pocognoli A, D’Anzeo M, Siquini W, Minardi D, Muzzonigro G, et al. Nebivolol induces, via β3 adrenergic receptor, lipolysis, uncoupling protein 1, and reduction of lipid droplet size in human adipocytes. J Hypertens. 2014;32:389–96.
Guerrero EI, Ardanaz N, Sevilla MA, Arévalo MA, Montero MJ. Cardiovascular effects of nebivolol in spontaneously hypertensive rats persist after treatment withdrawal. J Hypertens. 2006;24:151–8.
Pouleur AC, Anker S, Brito D, Brosteanu O, Hasenclever D, Casadei B, et al. Rationale and design of a multicentre, randomized, placebo-controlled trial of mirabegron, a Beta3-adrenergic receptor agonist on left ventricular mass and diastolic function in patients with structural heart disease Beta3-left ventricular hypertrophy (Beta3-LVH). ESC Heart Fail. 2018;5:830–41.
Miguel-Carrasco JL, Beaumont J, San José G, Moreno MU, López B, González A, et al. Mechanisms underlying the cardiac antifibrotic effects of losartan metabolites. Sci Rep. 2017;7:41865.
Pacca SR, de Azevedo AP, De Oliveira CF, De Luca IM, De Nucci G, Antunes E. Attenuation of hypertension, cardiomyocyte hypertrophy, and myocardial fibrosis by beta-adrenoceptor blockers in rats under long-term blockade of nitric oxide synthesis. J Cardiovasc Pharm. 2002;39:201–7.
Pires MJ, Rodríguez-Peña AB, Arévalo M, Cenador B, Evangelista S, Esteller A, et al. Long-term nebivolol administration reduces renal fibrosis and prevents endothelial dysfunction in rats with hypertension induced by renal mass reduction. J Hypertens. 2007;25:2486–96.
Ndisang JF, Chibbar R, Lane N. Heme oxygenase suppresses markers of heart failure and ameliorates cardiomyopathy in L-NAME-induced hypertension. Eur J Pharm. 2014;734:23–34.
Ma ZG, Yuan YP, Wu HM, Zhang X, Tang QZ. Cardiac fibrosis: new insights into the pathogenesis. Int J Biol Sci. 2018;14:1645–57.
Meléndez GC, McLarty JL, Levick SP, Du Y, Janicki JS, Brower GL. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010;56:225–31.
Potue P, Wunpathe C, Maneesai P, Kukongviriyapan U, Prachaney P, Pakdeechote P. Nobiletin alleviates vascular alterations through modulation of Nrf-2/HO-1 and MMP pathways in l-NAME induced hypertensive rats. Food Funct. 2019;10:1880–92.
Ceron CS, Rizzi E, Guimarães DA, Martins-Oliveira A, Gerlach RF, Tanus-Santos JE. Nebivolol attenuates prooxidant and profibrotic mechanisms involving TGF-β and MMPs, and decreases vascular remodeling in renovascular hypertension. Free Radic Biol Med. 2013;65:47–56.
Chen R, Sun Y, Cui X, Ji Z, Kong X, Wu S, et al. Autophagy promotes aortic adventitial fibrosis via the IL-6/Jak1 signaling pathway in Takayasu’s arteritis. J Autoimmun. 2019;99:39–47.
Reid JL, Meredith PA. Concentration-effect analysis of antihypertensive drug responses. Hypertension. 1990;16:12–8.
Acknowledgements
This work was supported by grants from Secretaría de Ciencia y Técnica, Universidad de Buenos Aires [Grant Number: 20020150100142BA]. DAC, MD, AHP, GB, GEG, MAM, CAT, AC, and AHP are Career Investigators from Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Argentina.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Del Mauro, J.S., Prince, P.D., Santander Plantamura, Y. et al. Nebivolol is more effective than atenolol for blood pressure variability attenuation and target organ damage prevention in L-NAME hypertensive rats. Hypertens Res 44, 791–802 (2021). https://doi.org/10.1038/s41440-021-00630-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-021-00630-4
Keywords
This article is cited by
-
The pharmaco-epigenetics of hypertension: a focus on microRNA
Molecular and Cellular Biochemistry (2024)
-
Hypoxia-induced miR-210 modulates the inflammatory response and fibrosis upon acute ischemia
Cell Death & Disease (2021)