Abstract
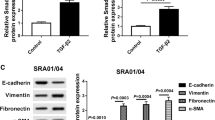
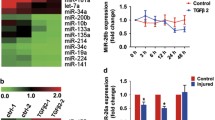
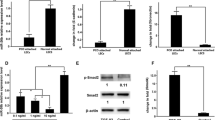
Fibrotic cataract, including anterior subcapsular cataract (ASC) and posterior capsule opacification, always lead to visual impairment. Epithelial–mesenchymal transition (EMT) is a well-known event that causes phenotypic alterations in lens epithelial cells (LECs) during lens fibrosis. Accumulating studies have demonstrated that microRNAs are important regulators of EMT and fibrosis. However, the evidence explaining how microRNAs modulate the behavior and alter the cellular phenotypes of the lens epithelium in fibrotic cataract is insufficient. In this study, we found that hsa-let-7c-3p is downregulated in LECs in human ASC in vivo as well as in TGFβ2-induced EMT in vitro, indicating that hsa-let-7c-3p may participate in modulating the profibrotic processes in the lens. We then demonstrated that overexpression of hsa-let-7c-3p markedly suppressed human LEC proliferation and migration and attenuated TGFβ2-induced EMT and injury-induced ASC in a mouse model. In addition, hsa-let-7c-3p mediated lens fibrosis by directly targeting the CDH11 gene, which encodes cadherin-11 protein, an important mediator in the EMT signaling pathway. It decreased cadherin-11 protein expression at the posttranscriptional level but not at the transcriptional level by binding to a specific site in the 3-untranslated region (3′-UTR) of CDH11 mRNA. Moreover, blockade of cadherin-11 expression with a specific short hairpin RNA reversed TGFβ2-induced EMT in LECs in vitro. Collectively, these data demonstrated that hsa-let-7c-3p plays a clear role in attenuating ASC development and may be a novel candidate therapeutic for halting fibrosis and maintaining vision.






Similar content being viewed by others
Data availability
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Johar K, Vasavada AR, Tatsumi K, Dholakia S, Nihalani B, Rao SS (2007) Anterior capsular plaque in congenital cataract: occurrence, morphology, immunofluorescence, and ultrastructure. Invest Ophthalmol Vis Sci 48:4209–4214. https://doi.org/10.1167/iovs.07-0312
Eldred JA, Dawes LJ, Wormstone IM (2011) The lens as a model for fibrotic disease. Philos T R Soc B 366:1301–1319. https://doi.org/10.1098/rstb.2010.0341
Wang Y, Guo L, Cai S et al (2012) Exome sequencing identifies compound heterozygous mutations in CYP4V2 in a pedigree with retinitis pigmentosa. PLoS ONE 7:e33673. https://doi.org/10.1371/journal.pone.0033673
Andjelic S, Draslar K, Hvala A, Hawlina M (2017) Anterior lens epithelium in cataract patients with retinitis pigmentosa - scanning and transmission electron microscopy study. Acta Ophthalmol 95:e212–e220. https://doi.org/10.1111/aos.13250
Hou M, Bao X, Liu L, Ding Y, Luo F, Wu M (2021) Retinitis pigmentosa-associated anterior subcapsular cataract: morphological features and visual performance. Int Ophthalmol 41:3631–3639. https://doi.org/10.1007/s10792-021-01935-6
Jackson H, Garway-Heath D, Rosen P, Bird AC, Tuft SJ (2001) Outcome of cataract surgery in patients with retinitis pigmentosa. Br J Ophthalmol 85:936–938. https://doi.org/10.1136/bjo.85.8.936
Dikopf MS, Chow CC, Mieler WF, Tu EY (2013) Cataract extraction outcomes and the prevalence of zonular insufficiency in retinitis pigmentosa. Am J Ophthalmol 156:82-88.e2. https://doi.org/10.1016/j.ajo.2013.02.002
Jin-Poi T, Shatriah I, Khairy-Shamel ST, Zunaina E (2013) Rapid anterior capsular contraction after phacoemulsification surgery in a patient with retinitis pigmentosa. Clin Ophthalmol 7:839–842. https://doi.org/10.2147/OPTH.S42122
Masket S, Bostanci Ceran B, Fram NR (2012) Spontaneous dislocation of posterior chamber intraocular lenses (PC IOLs) in patients with retinitis pigmentosa - Case series. Saudi J Ophthalmol 26:61–65. https://doi.org/10.1016/j.sjopt.2011.09.003
Rockey DC, Bell PD, Hill JA (2015) Fibrosis–a common pathway to organ injury and failure. N Engl J Med 373:96. https://doi.org/10.1056/NEJMc1504848
Lee EH, Joo CK (1999) Role of transforming growth factor-β in transdifferentiation and fibrosis of lens epithelial cells. Invest Ophthalmol Vis Sci 40:2025–2032
Saika S, Miyamoto T, Ishida I et al (2002) TGFbeta-Smad signalling in postoperative human lens epithelial cells. Br J Ophthalmol 86:1428–1433. https://doi.org/10.1136/bjo.86.12.1428
Chen K, Rajewsky N (2007) The evolution of gene regulation by transcription factors and microRNAs. Nat Rev Genet 8:93–103. https://doi.org/10.1038/nrg1990
Eulalio A, Huntzinger E, Izaurralde E (2008) Getting to the root of miRNA-mediated gene silencing. Cell 132:9–14. https://doi.org/10.1016/j.cell.2007.12.024
Chen X, Xiao W, Chen W et al (2017) MicroRNA-26a and -26b inhibit lens fibrosis and cataract by negatively regulating Jagged-1/Notch signaling pathway. Cell Death Differ 24:1990. https://doi.org/10.1038/cdd.2017.147
Zhang L, Wang Y, Li W et al (2017) MicroRNA-30a regulation of epithelial-mesenchymal transition in diabetic cataracts through targeting SNAI1. Sci Rep-UK 7:1117. https://doi.org/10.1038/s41598-017-01320-3
Dong N, Tang X, Xu B (2015) miRNA-181a inhibits the proliferation, migration, and epithelial-mesenchymal transition of lens epithelial cells. Invest Ophth Vis Sci 56:993–1001. https://doi.org/10.1167/iovs.14-15860
Wang Y, Li W, Zhang X et al (2013) MicroRNA-204-5p regulates epithelial-to-mesenchymal transition during human posterior capsule opacification by targeting SMAD4. Invest Ophth Vis Sci 54:323–332. https://doi.org/10.1167/iovs.12-10904
Guo M, Su F, Chen Y, Su B (2022) Interfering Hsa_circRNA_0060640 suppresses TGF-β2-induced proliferation, motility and EMT in human lens epithelium cells by targeting miR-214-3p and collagen type I alpha2 chain. Curr Eye Res 47:735–746. https://doi.org/10.1080/02713683.2022.2053724
Li QL, Zhang HY, Qin YJ et al (2016) MicroRNA-34a promoting apoptosis of human lens epithelial cells through down-regulation of B-cell lympho ma-2 and silent information regulator. Int J Ophthalmol-Chi 9:1555–1560. https://doi.org/10.18240/ijo.2016.11.04
Qin Y, Zhao J, Min X et al (2014) MicroRNA-125b inhibits lens epithelial cell apoptosis by targeting p53 in age-related cataract. Biochim Biophys Acta 1842:2439–2447. https://doi.org/10.1016/j.bbadis.2014.10.002
Zhang F, Meng W, Tong B (2016) Down-regulation of microRNA-133b suppresses apoptosis of lens epithelial cell by up-regulating BCL2L2 in age-related cataracts. Med Sci Monit 22:4139–4145. https://doi.org/10.12659/msm.896975
Han HB, Gu J, Zuo HJ et al (2012) Let-7c functions as a metastasis suppressor by targeting MMP11 and PBX3 in colorectal cancer. J Pathol 226:544–555. https://doi.org/10.1002/path.3014
Guo WT, Wang XW, Yan YL et al (2015) Suppression of epithelial-mesenchymal transition and apoptotic pathways by miR-294/302 family synergistically blocks let-7-induced silencing of self-renewal in embryonic stem cells. Cell Death Differ 22:1158–1169. https://doi.org/10.1038/cdd.2014.205
Srivastava SP, Hedayat AF, Kanasaki K, Goodwin JE (2019) microRNA crosstalk influences epithelial-to-mesenchymal, endothelial-to-mesenchymal, and macrophage-to-mesenchymal transitions in the kidney. Front Pharmacol 10:904. https://doi.org/10.3389/fphar.2019.00904
Schneider DJ, Wu M, Le TT et al (2012) Cadherin-11 contributes to pulmonary fibrosis: potential role in TGF-beta production and epithelial to mesenchymal transition. FASEB J 26:503–512. https://doi.org/10.1096/fj.11-186098
Kim NH, Choi SH, Lee TR, Lee CH, Lee AY (2014) Cadherin 11, a miR-675 target, induces N-cadherin expression and epithelial-mesenchymal transition in melasma. J Invest Dermatol 134:2967–2976. https://doi.org/10.1038/jid.2014.257
Takashima Y, Kawaguchi A, Yamanaka R (2019) Promising prognosis marker candidates on the status of epithelial-mesenchymal transition and glioma stem cells in glioblastoma. Cells-Basel 8:1312. https://doi.org/10.3390/cells8111312
Srinivasan Y, Lovicu FJ, Overbeek PA (1998) Lens-specific expression of transforming growth factor beta1 in transgenic mice causes anterior subcapsular cataracts. J Clin Invest 101:625–634. https://doi.org/10.1172/JCI1360
Lovicu FJ, Schulz MW, Hales AM et al (2002) TGFbeta induces morphological and molecular changes similar to human anterior subcapsular cataract. Br J Ophthalmol 86:220–226. https://doi.org/10.1136/bjo.86.2.220
Xiao W, Chen X, Li W et al (2015) Quantitative analysis of injury-induced anterior subcapsular cataract in the mouse: a model of lens epithelial cells proliferation and epithelial-mesenchymal transition. Sci Rep 5:8362. https://doi.org/10.1038/srep08362
Latvala T, Uusitalo M, Puolakkainen P, Kivela T, Tervo T (2000) Immunolocalization of transforming growth factor-beta1 and tenascin in human secondary cataract. Acta Ophthalmol Scand 78:344–347. https://doi.org/10.1034/j.1600-0420.2000.078003344.x
Saika S, Miyamoto T, Kawashima Y et al (2000) Immunolocalization of TGF-beta1, -beta2, and -beta3, and TGF-beta receptors in human lens capsules with lens implants. Graefes Arch Clin Exp Ophthalmol 238:283–293. https://doi.org/10.1007/s004170050354
Saika S, Miyamoto T, Okada Y et al (2000) Transforming growth factor-beta isoform proteins in cell and matrix deposits on intraocular lenses. J Cataract Refract Surg 26:709–715. https://doi.org/10.1016/s0886-3350(99)00402-2
Lovicu FJ, Shin EH, McAvoy JW (2016) Fibrosis in the lens. Sprouty regulation of TGFbeta-signaling prevents lens EMT leading to cataract. Exp Eye Res 142:92–101. https://doi.org/10.1016/j.exer.2015.02.004
Connor TB Jr, Roberts AB, Sporn MB et al (1989) Correlation of fibrosis and transforming growth factor-beta type 2 levels in the eye. J Clin Invest 83:1661–1666. https://doi.org/10.1172/JCI114065
Roush S, Slack FJ (2010) The let-7 family of microRNAs. Trends Cell Biol 18:505–516. https://doi.org/10.1016/j.tcb.2008.07.007
Melton C, Judson RL, Blelloch R (2010) Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature 463:621–626. https://doi.org/10.1038/nature08725
Yu F, Yao H, Zhu P et al (2007) let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell 131:1109–1123. https://doi.org/10.1016/j.cell.2007.10.054
Sampson VB, Rong NH, Han J et al (2007) MicroRNA let-7a down-regulates MYC and reverts MYC-induced growth in Burkitt lymphoma cells. Cancer Res 67:9762–9770. https://doi.org/10.1158/0008-5472.CAN-07-2462
Mayr C, Hemann MT, Bartel DP (2007) Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 315:1576–1579. https://doi.org/10.1126/science.1137999
Johnson SM, Grosshans H, Shingara J et al (2005) RAS is regulated by the let-7 microRNA family. Cell 120:635–647. https://doi.org/10.1016/j.cell.2005.01.014
Kubo E, Hasanova N, Sasaki H, Singh DP (2013) Dynamic and differential regulation in the microRNA expression in the developing and mature cataractous rat lens. J Cell Mol Med 17:1146–1159. https://doi.org/10.1111/jcmm.12094
Wu C, Lin H, Wang Q et al (2012) Discrepant expression of microRNAs in transparent and cataractous human lenses. Invest Ophthalmol Vis Sci 53:3906–3912. https://doi.org/10.1167/iovs.11-9178
Peng CH, Liu JH, Woung LC et al (2012) MicroRNAs and cataracts: correlation among let-7 expression, age and the severity of lens opacity. Br J Ophthalmol 96:747–751. https://doi.org/10.1136/bjophthalmol-2011-300585
Takeichi M (1990) Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem 59:237–252. https://doi.org/10.1146/annurev.bi.59.070190.001321
Takeichi M (1991) Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251:1451–1455. https://doi.org/10.1126/science.2006419
Pishvaian MJ, Feltes CM, Thompson P et al (1999) Cadherin-11 is expressed in invasive breast cancer cell lines. Can Res 59:947–952
Birtolo C, Pham H, Morvaridi S et al (2017) Cadherin-11 is a cell surface marker up-regulated in activated pancreatic stellate cells and is involved in pancreatic cancer cell migration. Am J Pathol 187:146–155. https://doi.org/10.1016/j.ajpath.2016.09.012
Clendenon SG, Sarmah S, Shah B, Liu Q, Marrs JA (2012) Zebrafish cadherin-11 participates in retinal differentiation and retinotectal axon projection during visual system development. Dev Dyn 241:442–454. https://doi.org/10.1002/dvdy.23729
Li JH, Liu S, Zhou H, Qu LH, Yang JH (2014) starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res 42:D92–D97. https://doi.org/10.1093/nar/gkt1248
Zhao Z, Sun W, Guo Z et al (2020) Mechanisms of lncRNA/microRNA interactions in angiogenesis. Life Sci 254:116900. https://doi.org/10.1016/j.lfs.2019.116900
Wiener D, Schwartz S (2021) The epitranscriptome beyond m6A. Nat Rev Genet 22:119–131. https://doi.org/10.1111/tpj.14193
Lin C, Ma M, Zhang Y et al (2022) The N6-methyladenosine modification of circALG1 promotes the metastasis of colorectal cancer mediated by the miR-342-5p/PGF signalling pathway. Mol Cancer 21:80. https://doi.org/10.1186/s12943-022-01560-6
Khan A, Rehman HU, Habib U, Ijaz U (2022) m6A-finder: detecting m6A methylation sites from RNA transcriptomes using physical and statistical properties based features. Comput Biol Chem 97:107640. https://doi.org/10.1016/j.compbiolchem.2022.107640
Mei Z, Mou Y et al (2023) Emerging mutual regulatory roles between m6A modification and microRNAs. Int J Mol Sci. https://doi.org/10.3390/ijms24010773
Funding
This study was supported by the National Natural Science Foundation of China (No. 81470615, 81770909 and No. 81970783).
Author information
Authors and Affiliations
Contributions
MH, FL and MW performed study concept and design. MH, FL, YD and XB conducted the experiment, provided acquisition and interpretation of data and statistical analysis. MH, FL, YD, XB and MW analyzed data. MH wrote of the paper, all authors critically reviewed, edited and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was also approved by the Institutional Review Board of Zhongshan Ophthalmic Center, Sun Yat-sen University (No. 2016MEKY047). The animal experiment was approved by Animal Use and Care Committee of Zhongshan Ophthalmic Center at Sun Yat-Sen University, Guangzhou.
Consent for publication
The participant has consented to the submission of case report to the journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hou, M., Luo, F., Ding, Y. et al. Let-7c-3p suppresses lens epithelial-mesenchymal transition by inhibiting cadherin-11 expression in fibrotic cataract. Mol Cell Biochem 479, 743–759 (2024). https://doi.org/10.1007/s11010-023-04758-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11010-023-04758-4