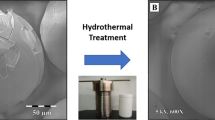
Abstract
In this study, a hydrothermal treatment (HT) at 200 °C was used to deal with the cracking of the external gelation-made thorium oxide (ThO2) microspheres and the effects of HT on the composition, microstructure, and cracking fraction of the microspheres were investigated. The results showed that HT removed the light weight impurities such as ammonium nitrate, degraded polyvinyl alcohol with light weight as well as absorbed and bonded water, thus resulting in a reduction of the weight loss of the gel microsphere by 26%. Owing to the removal of the impurities, HT led to the improvement of the crystallinity of the dried microspheres and the increase of specific surface area and pore size of the dried ones. Further, HT had distinct effect on the heat treatment of the microspheres: (1) For the drying process (240 °C) where an atmosphere of humidified air and wet microspheres were essential, the cracking fraction of the treated microspheres was only one-tenth of that of the original ones due to the removal of the bonded water by the HT. (2) In the case of elevated temperature treatment (650 and 1350 °C), the removal of residual polyvinyl alcohol by thermal oxidation was easy for the treated microspheres without obvious occurrence of cracking, however, it was not the case for the original counterparts. With the aid of HT, high-quality crack-free ThO2 microspheres were available after the heat treatment and would be potentially used as nuclear fuel for a solid-fueled thorium molten salt reactor.
Graphical Abstract

Highlights
-
Intact high-quality external gelation-made ThO2 ceramic microspheres were prepared.
-
PVA was eliminated by hydrothermal degradation and then an easy thermal oxidation process.
-
Hydrothermal-induced changes of the microstructure and composition of the thorium gel microspheres.
-
Hydrothermal treatment reduced the cracking fraction of the gel microspheres from 100 to 8.4%.










Similar content being viewed by others
References
Galperin A, Reichert P, Radkowsky A (1997) Thorium fuel for light water reactors-reducing proliferation potential of nuclear power fuel cycle. Sci Glob Secur 6:265–290. https://doi.org/10.1080/08929889708426440
Delpech S, Merle-Lucotte E, Heuer D, et al. (2009) Reactor physic and reprocessing scheme for innovative molten salt reactor system. J Fluor Chem 130:11–17. https://doi.org/10.1016/j.jfluchem.2008.07.009
Serp J, Allibert M, Beneš O, et al. (2014) The Molten Salt Reactor (MSR) in Generation IV: Overview and perspectives. Prog Nucl Energy 77:308–319. https://doi.org/10.1016/j.pnucene.2014.02.014
Dai Z (2017) Thorium Molten Salt Reactor Nuclear Energy System (TMSR). In: Dolan TJ (ed) Molten Salt React Thorium Energy, 1st ed. Woodhead, London
Zhang D, Liu L, Liu M, et al. (2018) Review of conceptual design and fundamental research of molten salt reactors in China. Int J Energy Res 42:1834–1848. https://doi.org/10.1002/er.3979
Petti DA, Buongiorno J, Maki JT, et al. (2003) Key differences in the fabrication, irradiation and high-temperature accident testing of US and German TRISO-coated particle fuel, and their implications on fuel performance. Nucl Eng Des 222:281–297. https://doi.org/10.1016/S0029-5493(03)00033-5
Powers JJ, Wirth BD (2010) A Review of TRISO fuel performance models. J Nucl Mater 405:74–82. https://doi.org/10.1016/j.jnucmat.2010.07.030
Liu B, Liang T, Tang C (2006) A review of TRISO-coated particle nuclear fuel performance models. Rare Met 25:337–342. https://doi.org/10.1016/S1001-0521(07)60101-6
Vaidya V (2004) Sol-gel process for ceramic nuclear fuels. Trans Indian Ceram Soc 63:163–167. https://doi.org/10.1080/0371750X.2004.11012156
Vaidya V (2008) Status of sol-gel process for nuclear fuels. J Sol-Gel Sci Technol 46:369–381. https://doi.org/10.1007/s10971-008-1725-0
Sood D (2011) The role sol-gel process for nuclear fuels-an overview. J Sol-Gel Sci Technol 59:404–416. https://doi.org/10.1007/s10971-010-2273-y
Zhou X, Ma J, Hao S, et al. (2012) Preparation of ammonium diuranate particles by external gelation process of Uranium in INET. Nucl Eng Des 250:192–196. https://doi.org/10.1016/j.nucengdes.2012.06.001
Wang G, Ma J, Gao Y, et al. (2016) Preparation of Ceria-stabilized Zirconia microspheres by external gelation: size control. J Sol-Gel Sci Technol 78:514–522. https://doi.org/10.1007/s10971-016-3997-0
Guogao W, Jingtao M, Yong G, et al. (2016) Precisely controlling preparation of ceria-stabilized Zirconia microspheres of ~100 μm by external gelation. Int J Appl Ceram Technol 13:831–837. https://doi.org/10.1111/ijac.12560
Vaidya V, Mukherjee S, Joshi J, et al. (1987) A study of chemical parameters of the internal gelation based sol-gel process for uranium dioxide. J Nucl Mater 148:324–331. https://doi.org/10.1016/0022-3115(87)90026-2
Hunt RD, Collins JL (2004) Uranium Kernel formation via internal gelation. Radiochim Acta 92:909–915. https://doi.org/10.1524/ract.92.12.909.55110
Schreinemachers C, Bukaemskiy A, Klinkenberg M, et al. (2014) Characterization of Uranium Neodymium oxide microspheres synthesized by internal gelation. Prog Nucl Energy 72:17–21. https://doi.org/10.1016/j.pnucene.2013.07.016
Naefe P, Zimmer E (1979) Preparation of Uranium kernels by an external gelation process. Nucl Technol 42:163–171. https://doi.org/10.13182/NT79-A32147
Tiegs SM, Haas PA, Spence RD (1979) Sphere-Cal Process: Fabrication of Fuel Pellets from Gel Microspheres. Oak Ridge National Laboratory, Oak Ridge, Tennessee
Arima T, Idemitsu K, Yamahira K, et al. (2005) Application of internal gelation to sol–gel synthesis of Ceria-Doped Zirconia microspheres as Nuclear Fuel Analogous Materials. J Alloy Compd 394:271–276. https://doi.org/10.1016/j.jallcom.2004.10.040
Gao Y, Ma J, Zhao X, et al. (2015) The formation of alumina ceramic microspheres by internal gelation process. Key Eng Mater 655:103–107
Sukarsono R, Rachmawati M, Susilowati S, et al. (2018) Effect of sol concentration, aging and drying process on cerium stabilization zirconium gel produced by external gelation. J Phys: Conf Ser 962:012056. https://doi.org/10.1088/1742-6596/962/1/012056
Riyanto S, Mutiara E, Yusnitha E, et al. (2021) R&D on surrogate kernel fabrication in support of Reaktor Daya Eksperimental (RDE) Project. J Phys: Conf Ser 2048:012011. https://doi.org/10.1088/1742-6596/2048/1/012011
Ringel H, Zimmer E (1979) The external gelation of thorium process for preparation of ThO2 and (Th, U) O2 fuel kernels. Nucl Technol 45:287–298. https://doi.org/10.13182/NT79-A32297
Nagarajan K, Vaidya V (2012) Sol-Gel Processes for Nuclear Fuel Fabrication. In: Aparicio M, Jitianu A, Klein L (eds) Advances in Sol-Gel Derived Materials and Technologies. Springer, Boston, MA
Yamagishi S, Takahashi Y (1985) Sol-gel method using carbon tetrachloride as drop-formation medium for producing large Tho2-base microspheres. J Nucl Sci Technol 22:995–1000. https://doi.org/10.1080/18811248.1985.9735755
Huang H, Lin J, Chao Y, et al. (2016) Preparation of Zr-doped ThO2 ceramic microsphere by Sol-Gel method. In: Hong J (ed.) Proceedings of The 20th Pacific Basin Nuclear Conference. Springer, Singapore, https://doi.org/10.1007/978-981-10-2317-0_52
Hunt RD, Montgomery FC, Collins JL (2010) Treatment techniques to prevent cracking of amorphous microspheres made by the internal gelation process. J Nucl Mater 405:160–164. https://doi.org/10.1016/j.jnucmat.2010.08.007
Katalenich JA (2014) Production of Monodisperse, Crack-Free Cerium Oxide Microspheres by Internal Gelation Sol-Gel Methods. University of Michigan, Ann A, Michigan
Katalenich JA (2020) Use of a pressurized water treatment to prevent cracking of internal gelation sol-gel microspheres. J Sol-Gel Sci Technol 94:298–309. https://doi.org/10.1007/s10971-020-05230-1
Wang G, Ma J, Gao Y, et al. (2016) A comparative study of small-size Ceria–Zirconia microspheres fabricated by external and internal gelation. J Sol-Gel Sci Technol 78:673–681. https://doi.org/10.1007/s10971-016-3981-8
Wang F, Yan C, Cao C, et al. (2017) Synthesis of thorium sol for fabricating fuel kernels. Nucl Sci Tech 28:1–6. https://doi.org/10.1007/s41365-017-0243-6
Landers J, Gor GY, Neimark AV (2013) Density functional theory methods for characterization of porous materials. Colloids Surf A 437:3–32. https://doi.org/10.1016/j.colsurfa.2013.01.007
Peng Z, Kong L (2007) A thermal degradation mechanism of Polyvinyl Alcohol/Silica nanocomposites. Polym Degrad Stab 92:1061–1071. https://doi.org/10.1016/j.polymdegradstab.2007.02.012
Voorhees KJ, Baugh SF, Stevenson DN (1996) The thermal degradation of Poly (Ethylene Glycol)/Poly (Vinyl Alcohol) binder in alumina ceramics. Thermochim Acta 274:187–207. https://doi.org/10.1016/0040-6031(95)02583-9
Yu Z, Li B, Chu J, et al. (2018) Silica in situ enhanced PVA/Chitosan biodegradable films for food packages. Carbohydr Polym 184:214–220. https://doi.org/10.1016/j.carbpol.2017.12.043
Nakamoto K (1970) Infrared Spectra of Inorganic and Coordination Compounds. Wiley, New York
Le Toullec M, Simmons CJ, Simmons JH (1988) Infrared spectroscopic studies of the hydrolysis reaction during leaching of heavy-metal fluoride glasses. J Am Ceram Soc 71:219–224. https://doi.org/10.1111/j.1151-2916.1988.tb05851.x
Hussein GA, Ismail HM (1995) Texture assessment of thoria as a final decomposition product of hydrated thorium nitrate and oxycarbonate. Colloids Surf, A 99:129–139. https://doi.org/10.1016/0927-7757(95)03089-V
Gong W, Ewing R, Wang L, et al. (1995) Aeschynite and euxenite structure-types as host phases for rare-earths and actinides from Hlw. MRS Online Proc Libr 412:377–384. https://doi.org/10.1557/PROC-412-377
Fei P, Shi Y, Zhou M, et al. (2013) Effects of nano-TiO2 on the properties and structures of starch/Poly (ε-Caprolactone) composites. J Appl Polym Sci 130:4129–4136. https://doi.org/10.1002/app.39695
Wang Y, Zhong M, Chen F, et al. (2009) Visible light photocatalytic activity of TiO2/D-PVA for MO degradation. Appl Catal B 90:249–254. https://doi.org/10.1016/j.apcatb.2009.03.032
Lloyd MH, Bischoff K, Peng K, et al. (1976) Crystal habit and phase attribution of U (VI) oxides in a gelation process. J Inorg Nucl Chem 38:1141–1147
Katalenich JA, Kitchen BB (2021) Effects of pressurized water treatment on internal gelation sol-gel microspheres. J Sol-Gel Sci Technol 98:288–299. https://doi.org/10.1007/s10971-021-05479-0
Kogan A (2000) Direct solar thermal splitting of water and on-site separation of the products—IV. development of porous ceramic membranes for a solar thermal water-splitting reactor. Int J Hydrog Energy 25:1043–1050
Song X, Jiang W, Zhang J (2021) Sodium hypochlorite and Cu-O-Mn/columnar activated carbon catalytic oxidation for treatment of ultra-high concentration polyvinyl alcohol wastewater. Chemosphere 285:131526. https://doi.org/10.1016/j.chemosphere.2021.131526
Lu Y, Kong Q, Jing R, et al. (2013) Solid state oxidation of polyvinyl alcohol by Hydrogen Peroxide-Cu (II). Polym Degrad Stab 98:1103–1109. https://doi.org/10.1016/j.polymdegradstab.2013.03.022
Zhao R, Wang L, Chai Z-F, et al. (2014) Synthesis of ThO2 nanostructures through a hydrothermal approach: influence of Hexamethylenetetramine (HMTA) and Sodium Dodecyl Sulfate (SDS). RSC Adv 4:52209–52214. https://doi.org/10.1039/C4RA07466A
Li S, Li G, Chen Q, et al. (2019) Facile green synthesis of degraded-PVA coated TiO2 nanoparticles with enhanced photocatalytic activity under visible light. J Phys Chem Solids 129:92–98. https://doi.org/10.1016/j.jpcs.2019.01.002
Acknowledgements
This study was supported by the Thorium Molten Salt Reactor Nuclear Energy System under the Strategic Pioneer Sci. & Tech. Project of Chinese Academy of Sciences [Grant Number XDA02030000 and XDA02030200] and the Frontier Science Key Program of the Chinese Academy of Sciences [Grant Number QYZDY-SSW-JSC016].
Author contributions
Conceptualization: PW, JL; Methodology: PW, CY, HH; Formal analysis and investigation: JZ, SX, JC; Writing-original draft preparation: PW; Writing-review and editing: PW, CC; Funding acquisition: JL; Resources: PW, JL; Supervision: JL, CC, ZZ.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, P., Zou, J., Huang, H. et al. Efficient prevention of cracking of external gelation-made thorium oxide microspheres by hydrothermal treatment. J Sol-Gel Sci Technol 105, 709–720 (2023). https://doi.org/10.1007/s10971-023-06046-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-023-06046-5