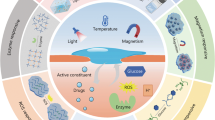
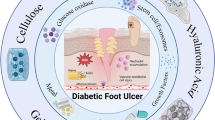
Abstract
Diabetic foot ulcers (DFUs) are one of the most challenging and prevalent refractory wounds associated with diabetes mellitus. It is characterized with long courses, high recurrence and disability rates. Hydrogel-based wound dressings have been demonstrated an effective and promising strategy for treating diabetic wounds. However, the complexity of the pathogenesis and microenvironment in diabetic wounds have restricted both the experts in functional hydrogels and clinicians. The former had no inspiration for clinical demands in the design process, and the latter was confused about clinical use because they knew little about the tremendous potential of the functional hydrogels. Here, important targets for DFUs treatment were listed, and effective products underlying the molecular pathogenesis were suggested for the designer. Then, the application of hydrogels into DFUs were classified in accordance with their functional targets and active ingredients. Hence, it is very convenient for clinicians to make a perfect option for different wounds. Finally, research gaps and future prospects for wound-healing hydrogels were presented. We envision that this review can inspire creativity and innovation in the development of hydrogel-based wound dressings for diabetic foot ulcer treatment.








Similar content being viewed by others
Availability of data and materials
Not applicable.
References
American Diabetes A (2020) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care 43(Suppl 1):S14–S31. https://doi.org/10.2337/dc20-S002
Sun H, Saeedi P, Karuranga S et al (2022) IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 183:109119. https://doi.org/10.1016/j.diabres.2021.109119
Armstrong DG, Swerdlow MA, Armstrong AA et al (2020) Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J Foot Ankle Res 13(1):16. https://doi.org/10.1186/s13047-020-00383-2
Armstrong DG, Boulton AJM, Bus SA (2017) Diabetic foot ulcers and their recurrence. N Engl J Med 376(24):2367–2375. https://doi.org/10.1056/NEJMra1615439
Reardon R, Simring D, Kim B et al (2020) The diabetic foot ulcer. Aust J Gen Pract 49(5):250–255. https://doi.org/10.31128/AJGP-11-19-5161
Huang ZH, Li SQ, Kou Y et al (2019) Risk factors for the recurrence of diabetic foot ulcers among diabetic patients: a meta-analysis. Int Wound J 16(6):1373–1382. https://doi.org/10.1111/iwj.13200
Chamberlain RC, Fleetwood K, Wild SH et al (2022) Foot ulcer and risk of lower limb amputation or death in people with diabetes: a national population-based retrospective cohort study. Diabetes Care 45(1):83–91. https://doi.org/10.2337/dc21-1596
Bandyk DF (2018) The diabetic foot: pathophysiology, evaluation, and treatment. Semin Vasc Surg 31(2–4):43–48. https://doi.org/10.1053/j.semvascsurg.2019.02.001
Francia P, Gualdani E, Policardo L et al (2022) Mortality risk associated with diabetic foot complications in people with or without history of diabetic foot hospitalizations. J Clin Med 11(9):2454. https://doi.org/10.3390/jcm11092454
Dogruel H, Aydemir M, Balci MK (2022) Management of diabetic foot ulcers and the challenging points: an endocrine view. World J Diabetes 13(1):27–36. https://doi.org/10.4239/wjd.v13.i1.27
Schaper NC, van Netten JJ, Apelqvist J et al (2020) Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev 36(Suppl 1):e3266. https://doi.org/10.1002/dmrr.3266
Falanga V (2005) Wound healing and its impairment in the diabetic foot. Lancet 366(9498):1736–1743. https://doi.org/10.1016/S0140-6736(05)67700-8
Ryall C, Duarah S, Chen S et al (2022) Advancements in skin delivery of natural bioactive products for wound management: a brief review of two decades. Pharmaceutics 14(5):1072. https://doi.org/10.3390/pharmaceutics14051072
Ongarora BG (2022) Recent technological advances in the management of chronic wounds: a literature review. Health Sci Rep 5(3):e641. https://doi.org/10.1002/hsr2.641
Hunt TK, Hopf HW (1997) Wound healing and wound infection. What surgeons and anesthesiologists can do. Surg Clin North Am 77(3):587–606. https://doi.org/10.1016/s0039-6109(05)70570-3
Stadelmann WK, Digenis AG, Tobin GR (1998) Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg 176(2A Suppl):26S-38S. https://doi.org/10.1016/s0002-9610(98)00183-4
Undas A, Wiek I, Stepien E et al (2008) Hyperglycemia is associated with enhanced thrombin formation, platelet activation, and fibrin clot resistance to lysis in patients with acute coronary syndrome. Diabetes Care 31(8):1590–1595. https://doi.org/10.2337/dc08-0282
Al SH (2022) Macrophage phenotypes in normal and diabetic wound healing and therapeutic interventions. Cells 11(15):2430. https://doi.org/10.3390/cells11152430
Peng Y, Xiong RP, Zhang ZH et al (2021) Ski promotes proliferation and inhibits apoptosis in fibroblasts under high-glucose conditions via the FoxO1 pathway. Cell Prolif 54(2):e12971. https://doi.org/10.1111/cpr.12971
Hu SC, Lan CE (2016) High-glucose environment disturbs the physiologic functions of keratinocytes: focusing on diabetic wound healing. J Dermatol Sci 84(2):121–127. https://doi.org/10.1016/j.jdermsci.2016.07.008
Wei F, Wang A, Wang Q et al (2020) Plasma endothelial cells-derived extracellular vesicles promote wound healing in diabetes through YAP and the PI3K/Akt/mTOR pathway. Aging (Albany NY) 12(12):12002–12018. https://doi.org/10.18632/aging.103366
Nie X, Zhang H, Shi X et al (2020) Asiaticoside nitric oxide gel accelerates diabetic cutaneous ulcers healing by activating Wnt/beta-catenin signaling pathway. Int Immunopharmacol 79:106109. https://doi.org/10.1016/j.intimp.2019.106109
Geng K, Ma X, Jiang Z et al (2023) WDR74 facilitates TGF-beta/Smad pathway activation to promote M2 macrophage polarization and diabetic foot ulcer wound healing in mice. Cell Biol Toxicol 39(4):1577–1591. https://doi.org/10.1007/s10565-022-09748-8
Yang Y, Zhang B, Yang Y et al (2022) FOXM1 accelerates wound healing in diabetic foot ulcer by inducing M2 macrophage polarization through a mechanism involving SEMA3C/NRP2/Hedgehog signaling. Diabetes Res Clin Pract 184:109121. https://doi.org/10.1016/j.diabres.2021.109121
Luanraksa S, Jindatanmanusan P, Boonsiri T et al (2018) An MMP/TIMP ratio scoring system as a potential predictive marker of diabetic foot ulcer healing. J Wound Care 27(12):849–855. https://doi.org/10.12968/jowc.2018.27.12.849
Zubair M, Ahmad J (2019) Role of growth factors and cytokines in diabetic foot ulcer healing: a detailed review. Rev Endocr Metab Disord 20(2):207–217. https://doi.org/10.1007/s11154-019-09492-1
Yang H-L, Tsai Y-C, Korivi M et al (2017) Lucidone promotes the cutaneous wound healing process via activation of the PI3K/AKT. Wnt/β-catenin and NF-κB signaling pathways 1864(1):151–168
Zhao Y, Liu M, Zhang Y et al (2015) Expression changes of Wnt/β-catenin signaling pathway in diabetic ulcer. Chin J Pathophysiol 31(11):2033–2038. https://doi.org/10.3969/j.issn.1000-4718.2015.11.018
Lee EG, Luckett-Chastain LR, Calhoun KN et al (2019) Interleukin 6 function in the skin and isolated keratinocytes is modulated by hyperglycemia. J Immunol Res 3:5087847. https://doi.org/10.1155/2019/5087847
Amin KN, Umapathy D, Anandharaj A et al (2020) miR-23c regulates wound healing by targeting stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) among patients with diabetic foot ulcer. Microvasc Res 127:103924. https://doi.org/10.1016/j.mvr.2019.103924
Liu L, Chen R, Jia Z et al (2022) Downregulation of hsa-miR-203 in peripheral blood and wound margin tissue by negative pressure wound therapy contributes to wound healing of diabetic foot ulcers. Microvasc Res 139:104275. https://doi.org/10.1016/j.mvr.2021.104275
Pichu S, Sathiyamoorthy J, Vimalraj S et al (2017) Impact of lysyl oxidase (G473A) polymorphism on diabetic foot ulcers. Int J Biol Macromol 103:242–247. https://doi.org/10.1016/j.ijbiomac.2017.05.050
Xu Y, Chen H, Fang Y et al (2022) Hydrogel combined with phototherapy in wound healing. Adv Healthc Mater 11(16):e2200494. https://doi.org/10.1002/adhm.202200494
Stan D, Tanase C, Avram M et al (2021) Wound healing applications of creams and “smart” hydrogels. Exp Dermatol 30(9):1218–1232. https://doi.org/10.1111/exd.14396
Gjorevski N, Sachs N, Manfrin A et al (2016) Designer matrices for intestinal stem cell and organoid culture. Nature 539(7630):560–564. https://doi.org/10.1038/nature20168
Zhang W, Zhang Y, Zhang Y et al (2021) Adhesive and tough hydrogels: from structural design to applications. J Mater Chem B 9(30):5954–5966. https://doi.org/10.1039/d1tb01166a
Fuchs S, Shariati K, Ma M (2020) Specialty Tough Hydrogels and Their Biomedical Applications. Adv Healthc Mater 9(2):e1901396. https://doi.org/10.1002/adhm.201901396
Vasile C, Pamfil D, Stoleru E et al (2020) New developments in medical applications of hybrid hydrogels containing natural polymers. Molecules 25(7):1539. https://doi.org/10.3390/molecules25071539
Hotz N, Wilcke L, Weber W (2013) Design, synthesis, and application of stimulus-sensing biohybrid hydrogels. Macromol Rapid Commun 34(20):1594–1610. https://doi.org/10.1002/marc.201300468
Malone-Povolny MJ, Maloney SE, Schoenfisch MH (2019) Nitric Oxide Therapy for Diabetic Wound Healing. Adv Healthc Mater 8(12):e1801210. https://doi.org/10.1002/adhm.201801210
Guo S, Dipietro LA (2010) Factors affecting wound healing. J Dent Res 89(3):219–229. https://doi.org/10.1177/0022034509359125
Bjarnsholt T, Kirketerp-Moller K, Jensen PO et al (2008) Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen 16(1):2–10. https://doi.org/10.1111/j.1524-475X.2007.00283.x
Satitsri S, Muanprasat C (2020) Chitin and chitosan derivatives as biomaterial resources for biological and biomedical applications. Molecules 25(24):5961. https://doi.org/10.3390/molecules25245961
Webber MJ, Pashuck ET (2021) (Macro)molecular self-assembly for hydrogel drug delivery. Adv Drug Deliv Rev 172:275–295. https://doi.org/10.1016/j.addr.2021.01.006
Waters DJ, Engberg K, Parke-Houben R et al (2010) Morphology of photopolymerized end-linked poly(ethylene glycol) hydrogels by small angle X-ray Scattering. Macromolecules 43(16):6861–6870. https://doi.org/10.1021/ma101070s
Ferreira PG, Ferreira VF, da Silva FC et al (2022) Chitosans and nanochitosans: recent advances in skin protection, regeneration, and repair. Pharmaceutics 14(6):1307. https://doi.org/10.3390/pharmaceutics14061307
Ganan M, Carrascosa AV, Martinez-Rodriguez AJ (2009) Antimicrobial activity of chitosan against Campylobacter spp. and other microorganisms and its mechanism of action. J Food Prot 72(8):1735–1738. https://doi.org/10.4315/0362-028x-72.8.1735
Matica MA, Aachmann FL, Tondervik A et al (2019) Chitosan as a wound dressing starting material: antimicrobial properties and mode of action. Int J Mol Sci 20(23):5889. https://doi.org/10.3390/ijms20235889
Raafat D, von Bargen K, Haas A et al (2008) Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol 74(12):3764–3773. https://doi.org/10.1128/AEM.00453-08
Lopez-Moya F, Suarez-Fernandez M, Lopez-Llorca LV (2019) Molecular mechanisms of chitosan interactions with fungi and plants. Int J Mol Sci 20(2):332. https://doi.org/10.3390/ijms20020332
Tan H, Ma R, Lin C et al (2013) Quaternized chitosan as an antimicrobial agent: antimicrobial activity, mechanism of action and biomedical applications in orthopedics. Int J Mol Sci 14(1):1854–1869. https://doi.org/10.3390/ijms14011854
Vishu Kumar AB, Varadaraj MC, Gowda LR et al (2005) Characterization of chito-oligosaccharides prepared by chitosanolysis with the aid of papain and Pronase, and their bactericidal action against Bacillus cereus and Escherichia coli. Biochem J 391(Pt 2):167–175. https://doi.org/10.1042/BJ20050093
Hu C, Long L, Cao J et al (2021) Dual-crosslinked mussel-inspired smart hydrogels with enhanced antibacterial and angiogenic properties for chronic infected diabetic wound treatment via pH-responsive quick cargo release. Chem Eng J 411:128564
He J, Li Z, Wang J et al (2023) Photothermal antibacterial antioxidant conductive self-healing hydrogel with nitric oxide release accelerates diabetic wound healing. Compos Part B Eng 266:110985
Kasparova P, Zmuda M, Vankova E et al (2021) Low-molecular weight chitosan enhances antibacterial effect of antibiotics and permeabilizes cytoplasmic membrane of Staphylococcus epidermidis biofilm cells. Folia Microbiol (Praha) 66(6):983–996. https://doi.org/10.1007/s12223-021-00898-6
Jing YJ, Hao YJ, Qu H et al (2007) Studies on the antibacterial activities and mechanisms of chitosan obtained from cuticles of housefly larvae. Acta Biol Hung 58(1):75–86. https://doi.org/10.1556/ABiol.57.2007.1.7
Younes I, Sellimi S, Rinaudo M et al (2014) Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int J Food Microbiol 185:57–63. https://doi.org/10.1016/j.ijfoodmicro.2014.04.029
Mellegard H, Strand SP, Christensen BE et al (2011) Antibacterial activity of chemically defined chitosans: influence of molecular weight, degree of acetylation and test organism. Int J Food Microbiol 148(1):48–54. https://doi.org/10.1016/j.ijfoodmicro.2011.04.023
Younes I, Rinaudo M (2015) Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 13(3):1133–1174. https://doi.org/10.3390/md13031133
Cele ZED, Somboro AM, Amoako DG et al (2020) Fluorinated Quaternary Chitosan Derivatives: Synthesis, Characterization, Antibacterial Activity, and Killing Kinetics. ACS Omega 5(46):29657–29666. https://doi.org/10.1021/acsomega.0c01355
Hoque J, Adhikary U, Yadav V et al (2016) Chitosan derivatives active against multidrug-resistant bacteria and pathogenic fungi: in vivo evaluation as topical antimicrobials. Mol Pharm 13(10):3578–3589. https://doi.org/10.1021/acs.molpharmaceut.6b00764
Wahid F, Hu XH, Chu LQ et al (2019) Development of bacterial cellulose/chitosan based semi-interpenetrating hydrogels with improved mechanical and antibacterial properties. Int J Biol Macromol 122:380–387. https://doi.org/10.1016/j.ijbiomac.2018.10.105
Omidi S, Kakanejadifard A (2019) Modification of chitosan and chitosan nanoparticle by long chain pyridinium compounds: Synthesis, characterization, antibacterial, and antioxidant activities. Carbohydr Polym 208:477–485. https://doi.org/10.1016/j.carbpol.2018.12.097
Zhao X, Wu H, Guo B et al (2017) Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 122:34–47. https://doi.org/10.1016/j.biomaterials.2017.01.011
Wang M, Yue L, Niazi S et al (2022) Synthesis and characterization of cinnamic acid conjugated N-(2-hydroxy)-propyl-3-trimethylammonium chitosan chloride derivatives: A hybrid flocculant with antibacterial activity. Int J Biol Macromol 206:886–895. https://doi.org/10.1016/j.ijbiomac.2022.03.075
Sivanesan I, Muthu M, Gopal J et al (2021) Nanochitosan: commemorating the metamorphosis of an exoskeletal waste to a versatile nutraceutical. Nanomater (Basel) 11(3):821. https://doi.org/10.3390/nano11030821
Duan S, Wang R (2013) Bimetallic nanostructures with magnetic and noble metals and their physicochemical applications. Prog Nat Sci Mater Int 23(2):113–126. https://doi.org/10.1016/j.pnsc.2013.02.001
Guo Z, Chen Y, Wang Y et al (2020) Advances and challenges in metallic nanomaterial synthesis and antibacterial applications. J Mater Chem B 8(22):4764–4777. https://doi.org/10.1039/d0tb00099j
Hamdan S, Pastar I, Drakulich S et al (2017) Nanotechnology-driven therapeutic interventions in wound healing: potential uses and applications. ACS Cent Sci 3(3):163–175. https://doi.org/10.1021/acscentsci.6b00371
Kedziora A, Speruda M, Krzyzewska E et al (2018) Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int J Mol Sci 19(2):444. https://doi.org/10.3390/ijms19020444
Choudhury H, Pandey M, Lim YQ et al (2020) Silver nanoparticles: Advanced and promising technology in diabetic wound therapy. Mater Sci Eng C Mater Biol Appl 112:110925. https://doi.org/10.1016/j.msec.2020.110925
Burdusel AC, Gherasim O, Grumezescu AM et al (2018) Biomedical applications of silver nanoparticles: an up-to-date overview. Nanomater (Basel) 8(9):681. https://doi.org/10.3390/nano8090681
Das S, Baker AB (2016) Biomaterials and nanotherapeutics for enhancing skin wound healing. Front Bioeng Biotechnol 4:82. https://doi.org/10.3389/fbioe.2016.00082
Bruna T, Maldonado-Bravo F, Jara P et al (2021) Silver nanoparticles and their antibacterial applications. Int J Mol Sci 22(13):7202. https://doi.org/10.3390/ijms22137202
Zhao Y, Li Z, Song S et al (2019) Skin-Inspired Antibacterial Conductive Hydrogels for Epidermal Sensors and Diabetic Foot Wound Dressings. Adv Funct Mater 29(31):1901474. https://doi.org/10.1002/adfm.201901474
Wang P, Jiang S, Li Y et al (2021) Virus-like mesoporous silica-coated plasmonic Ag nanocube with strong bacteria adhesion for diabetic wound ulcer healing. Nanomedicine 34:102381. https://doi.org/10.1016/j.nano.2021.102381
Pham TN, Jiang YS, Su CF et al (2020) In situ formation of silver nanoparticles-contained gelatin-PEG-dopamine hydrogels via enzymatic cross-linking reaction for improved antibacterial activities. Int J Biol Macromol 146:1050–1059. https://doi.org/10.1016/j.ijbiomac.2019.09.230
Laurenti M, Cauda V (2017) ZnO nanostructures for tissue engineering applications. Nanomater (Basel) 7(11):374. https://doi.org/10.3390/nano7110374
Jamnongkan T, Sukumaran SK, Sugimoto M et al (2015) Towards novel wound dressings: antibacterial properties of zinc oxide nanoparticles and electrospun fiber mats of zinc oxide nanoparticle/poly (vinyl alcohol) hybrids. J Polym Eng 35(6):575–586
Ottone C, Rivera VF, Fontana M et al (2014) Ultralong and mesoporous ZnO and γ-Al2O3 oriented nanowires obtained by template-assisted hydrothermal approach. J Mater Sci Technol 30(12):1167–1173
Dumontel B, Canta M, Engelke H et al (2017) Enhanced biostability and cellular uptake of zinc oxide nanocrystals shielded with a phospholipid bilayer. J Mater Chem B 5(44):8799–8813. https://doi.org/10.1039/c7tb02229h
Ahmed S, Chaudhry SA et al (2017) A review on biogenic synthesis of ZnO nanoparticles using plant extracts and microbes: a prospect towards green chemistry. J Photochem Photobiol B 166:272–284. https://doi.org/10.1016/j.jphotobiol.2016.12.011
Hajipour MJ, Fromm KM, Ashkarran AA et al (2012) Antibacterial properties of nanoparticles. Trends Biotechnol 30(10):499–511. https://doi.org/10.1016/j.tibtech.2012.06.004
Chupani L, Zuskova E, Niksirat H et al (2017) Effects of chronic dietary exposure of zinc oxide nanoparticles on the serum protein profile of juvenile common carp (Cyprinus carpio L.). Sci Total Environ 579:1504–1511. https://doi.org/10.1016/j.scitotenv.2016.11.154
Blecher K, Nasir A, Friedman A (2011) The growing role of nanotechnology in combating infectious disease. Virulence 2(5):395–401. https://doi.org/10.4161/viru.2.5.17035
Huh AJ, Kwon YJ (2011) “Nanoantibiotics”: a new paradigm for treating infectious diseases using nanomaterials in the antibiotics resistant era. J Control Release 156(2):128–145. https://doi.org/10.1016/j.jconrel.2011.07.002
Steffy K, Shanthi G, Maroky AS et al (2018) Enhanced antibacterial effects of green synthesized ZnO NPs using Aristolochia indica against multi-drug resistant bacterial pathogens from Diabetic Foot Ulcer. J Infect Public Health 11(4):463–471. https://doi.org/10.1016/j.jiph.2017.10.006
Vinotha V, Iswarya A, Thaya R et al (2019) Synthesis of ZnO nanoparticles using insulin-rich leaf extract: anti-diabetic, antibiofilm and anti-oxidant properties. J Photochem Photobiol B 197:111541. https://doi.org/10.1016/j.jphotobiol.2019.111541
Tavakoli S, Mokhtari H, Kharaziha M et al (2020) A multifunctional nanocomposite spray dressing of Kappa-carrageenan-polydopamine modified ZnO/L-glutamic acid for diabetic wounds. Mater Sci Eng C Mater Biol Appl 111:110837. https://doi.org/10.1016/j.msec.2020.110837
Soenen SJ, Parak WJ, Rejman J et al (2015) (Intra)cellular stability of inorganic nanoparticles: effects on cytotoxicity, particle functionality, and biomedical applications. Chem Rev 115(5):2109–2135. https://doi.org/10.1021/cr400714j
Wynn TA, Barron L (2010) Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis 30(3):245–257. https://doi.org/10.1055/s-0030-1255354
Bratton DL, Henson PM (2011) Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol 32(8):350–357. https://doi.org/10.1016/j.it.2011.04.009
Krzyszczyk P, Schloss R, Palmer A et al (2018) The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol 9:419. https://doi.org/10.3389/fphys.2018.00419
Shen T, Dai K, Yu Y et al (2020) Sulfated chitosan rescues dysfunctional macrophages and accelerates wound healing in diabetic mice. Acta Biomater 117:192–203. https://doi.org/10.1016/j.actbio.2020.09.035
Yang H, Song L, Sun B et al (2021) Modulation of macrophages by a paeoniflorin-loaded hyaluronic acid-based hydrogel promotes diabetic wound healing. Mater Today Bio 12:100139. https://doi.org/10.1016/j.mtbio.2021.100139
Xia H, Dong Z, Tang Q et al (2023) Glycopeptide-based multifunctional hydrogels promote diabetic wound healing through ph regulation of microenvironment. Adv Funct Mater. https://doi.org/10.1002/adfm.202215116
Wong SL, Demers M, Martinod K et al (2015) Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 21(7):815–819. https://doi.org/10.1038/nm.3887
Yuan Y, Fan D, Shen S et al (2022) An M2 macrophage-polarized anti-inflammatory hydrogel combined with mild heat stimulation for regulating chronic inflammation and impaired angiogenesis of diabetic wounds. Chem Eng J 433:133859
Soehnlein O, Steffens S, Hidalgo A et al (2017) Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol 17(4):248–261. https://doi.org/10.1038/nri.2017.10
Karima M, Kantarci A, Ohira T et al (2005) Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: association with periodontitis. J Leukoc Biol 78(4):862–870. https://doi.org/10.1189/jlb.1004583
Kaur T, Dumoga S, Koul V et al (2020) Modulating neutrophil extracellular traps for wound healing. Biomater Sci 8(11):3212–3223. https://doi.org/10.1039/d0bm00355g
Hyun SW, Kim J, Jo K et al (2018) Aster koraiensis extract improves impaired skin wound healing during hyperglycemia. Integr Med Res 7(4):351–357. https://doi.org/10.1016/j.imr.2018.09.001
Li N, Yang L, Pan C et al (2020) Naturally-occurring bacterial cellulose-hyperbranched cationic polysaccharide derivative/MMP-9 siRNA composite dressing for wound healing enhancement in diabetic rats. Acta Biomater 102:298–314. https://doi.org/10.1016/j.actbio.2019.11.005
Zhou W, Duan Z, Zhao J et al (2022) Glucose and MMP-9 dual-responsive hydrogel with temperature sensitive self-adaptive shape and controlled drug release accelerates diabetic wound healing. Bioact Mater 17:1–17. https://doi.org/10.1016/j.bioactmat.2022.01.004
Walton DM, Minton SD, Cook AD (2019) The potential of transdermal nitric oxide treatment for diabetic peripheral neuropathy and diabetic foot ulcers. Diabetes Metab Syndr 13(5):3053–3056. https://doi.org/10.1016/j.dsx.2018.07.003
Ahmed R, Augustine R, Chaudhry M et al (2022) Nitric oxide-releasing biomaterials for promoting wound healing in impaired diabetic wounds: state of the art and recent trends. Biomed Pharmacother 149:112707. https://doi.org/10.1016/j.biopha.2022.112707
Suschek CV, Feibel D, von Kohout M et al (2022) Enhancement of nitric oxide bioavailability by modulation of cutaneous nitric oxide stores. Biomedicines 10(9):2124. https://doi.org/10.3390/biomedicines10092124
Nathan CF, Hibbs JB Jr (1991) Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol 3(1):65–70. https://doi.org/10.1016/0952-7915(91)90079-g
Bredt DS, Snyder SH (1990) Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A 87(2):682–685. https://doi.org/10.1073/pnas.87.2.682
Coneski PN, Schoenfisch MH (2012) Nitric oxide release: part III. Meas report Chem Soc Rev 41(10):3753–3758. https://doi.org/10.1039/c2cs15271a
Neufeld BH, Reynolds MM (2016) Critical nitric oxide concentration for Pseudomonas aeruginosa biofilm reduction on polyurethane substrates. Biointerphases 11(3):031012. https://doi.org/10.1116/1.4962266
Kreuger MR, Tames DR, Mariano M (1998) Expression of NO-synthase in cells of foreign-body and BCG-induced granulomata in mice: influence of L-NAME on the evolution of the lesion. Immunology 95(2):278–282. https://doi.org/10.1046/j.1365-2567.1998.00542.x
Krischel V, Bruch-Gerharz D, Suschek C et al (1998) Biphasic effect of exogenous nitric oxide on proliferation and differentiation in skin derived keratinocytes but not fibroblasts. J Invest Dermatol 111(2):286–291. https://doi.org/10.1046/j.1523-1747.1998.00268.x
Howdieshell TR, Webb WL, Sathyanarayana MD et al (2003) Inhibition of inducible nitric oxide synthase results in reductions in wound vascular endothelial growth factor expression, granulation tissue formation, and local perfusion. Surgery 133(5):528–537. https://doi.org/10.1067/msy.2003.128
Zhao Y, Luo L, Huang L et al (2022) In situ hydrogel capturing nitric oxide microbubbles accelerates the healing of diabetic foot. J Control Release 350:93–106. https://doi.org/10.1016/j.jconrel.2022.08.018
Tu C, Lu H, Zhou T et al (2022) Promoting the healing of infected diabetic wound by an anti-bacterial and nano-enzyme-containing hydrogel with inflammation-suppressing, ROS-scavenging, oxygen and nitric oxide-generating properties. Biomaterials 286:121597. https://doi.org/10.1016/j.biomaterials
Ando A, Miyamoto M, Saito N et al (2021) Small fibre neuropathy is associated with impaired vascular endothelial function in patients with type 2 diabetes. Front Endocrinol (Lausanne) 12:653277. https://doi.org/10.3389/fendo.2021.653277
Li L, Yang Y, Bai J et al (2022) Impaired vascular endothelial function is associated with peripheral neuropathy in patients with type 2 diabetes. Diabetes Metab Syndr Obes 15:1437–1449. https://doi.org/10.2147/DMSO.S352316
den Dekker A, Davis FM, Kunkel SL et al (2019) Targeting epigenetic mechanisms in diabetic wound healing. Transl Res 204:39–50. https://doi.org/10.1016/j.trsl.2018.10.001
Veves A, Akbari CM, Primavera J et al (1998) Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes 47(3):457–463. https://doi.org/10.2337/diabetes.47.3.457
DiPietro LA (2016) Angiogenesis and wound repair: when enough is enough. J Leukoc Biol 100(5):979–984. https://doi.org/10.1189/jlb.4MR0316-102R
Guan Y, Niu H, Liu Z et al (2021) Sustained oxygenation accelerates diabetic wound healing by promoting epithelialization and angiogenesis and decreasing inflammation. Sci Adv 7(35):eabj0153. https://doi.org/10.1126/sciadv.abj0153
Jee JP, Pangeni R, Jha SK et al (2019) Preparation and in vivo evaluation of a topical hydrogel system incorporating highly skin-permeable growth factors, quercetin, and oxygen carriers for enhanced diabetic wound-healing therapy. Int J Nanomedicine 14:5449–5475. https://doi.org/10.2147/IJN.S213883
Zhu S, Zhao B, Li M et al (2023) Microenvironment responsive nanocomposite hydrogel with NIR photothermal therapy, vascularization and anti-inflammation for diabetic infected wound healing. Bioact Mater 26:306–320
Chen B, Zhang H, Qiu J et al (2022) Mechanical force induced self-assembly of chinese herbal hydrogel with synergistic effects of antibacterial activity and immune regulation for wound healing. Small 18(21):e2201766. https://doi.org/10.1002/smll.202201766
Ning S, Zang J, Zhang B et al (2022) Botanical drugs in traditional chinese medicine with wound healing properties. Front Pharmacol 13:885484. https://doi.org/10.3389/fphar.2022.885484
Li J, Luo J, Chai Y et al (2021) Hypoglycemic effect of Taraxacum officinale root extract and its synergism with Radix Astragali extract. Food Sci Nutr 9(4):2075–2085. https://doi.org/10.1002/fsn3.2176
Luo X, Huang P, Yuan B et al (2016) Astragaloside IV enhances diabetic wound healing involving upregulation of alternatively activated macrophages. Int Immunopharmacol 35:22–28. https://doi.org/10.1016/j.intimp.2016.03.020
Zhao B, Zhang X, Han W et al (2017) Wound healing effect of an Astragalus membranaceus polysaccharide and its mechanism. Mol Med Rep 15(6):4077–4083. https://doi.org/10.3892/mmr.2017.6488
Peng LH, Chen X, Chen L et al (2012) Topical astragaloside IV-releasing hydrogel improves healing of skin wounds in vivo. Biol Pharm Bull 35(6):881–888. https://doi.org/10.1248/bpb.35.881
He X, Wang X, Fang J et al (2017) Bletilla striata: Medicinal uses, phytochemistry and pharmacological activities. J Ethnopharmacol 195:20–38. https://doi.org/10.1016/j.jep.2016.11.026
Zhao Y, Wang Q, Yan S et al (2021) Bletilla striata polysaccharide promotes diabetic wound healing through inhibition of the NLRP3 inflammasome. Front Pharmacol 12:659215. https://doi.org/10.3389/fphar.2021.659215
Zhang P, He L, Zhang J et al (2020) Preparation of novel berberine nano-colloids for improving wound healing of diabetic rats by acting Sirt1/NF-kappaB pathway. Colloids Surf B Biointerfaces 187:110647. https://doi.org/10.1016/j.colsurfb.2019.110647
Xu N, Wang L, Guan J et al (2018) Wound healing effects of a Curcuma zedoaria polysaccharide with platelet-rich plasma exosomes assembled on chitosan/silk hydrogel sponge in a diabetic rat model. Int J Biol Macromol 117:102–107. https://doi.org/10.1016/j.ijbiomac.2018.05.066
Xia S, Weng T, Jin R et al (2022) Curcumin-incorporated 3D bioprinting gelatin methacryloyl hydrogel reduces reactive oxygen species-induced adipose-derived stem cell apoptosis and improves implanting survival in diabetic wounds. Burns Trauma 10:tkac001. https://doi.org/10.1093/burnst/tkac001
Rodriguez-Acosta H, Tapia-Rivera JM, Guerrero-Guzman A et al (2022) Chronic wound healing by controlled release of chitosan hydrogels loaded with silver nanoparticles and calendula extract. J Tissue Viability 31(1):173–179. https://doi.org/10.1016/j.jtv.2021.10.004
Gao SQ, Chang C, Li JJ et al (2018) Co-delivery of deferoxamine and hydroxysafflor yellow A to accelerate diabetic wound healing via enhanced angiogenesis. Drug Deliv 25(1):1779–1789. https://doi.org/10.1080/10717544.2018.1513608
Liu J, Qu M, Wang C et al (2022) A dual-cross-linked hydrogel patch for promoting diabetic wound healing. Small 18(17):e2106172. https://doi.org/10.1002/smll.202106172
Wang T, Liao Q, Wu Y et al (2020) A composite hydrogel loading natural polysaccharides derived from Periplaneta americana herbal residue for diabetic wound healing. Int J Biol Macromol 164:3846–3857. https://doi.org/10.1016/j.ijbiomac.2020.08.156
Shukla R, Kashaw SK, Jain AP et al (2016) Fabrication of Apigenin loaded gellan gum-chitosan hydrogels (GGCH-HGs) for effective diabetic wound healing. Int J Biol Macromol 91:1110–1119. https://doi.org/10.1016/j.ijbiomac.2016.06.075
Gan J, Liu C, Li H et al (2019) Accelerated wound healing in diabetes by reprogramming the macrophages with particle-induced clustering of the mannose receptors. Biomaterials 219:119340. https://doi.org/10.1016/j.biomaterials.2019.119340
Veerasubramanian PK, Thangavel P, Kannan R et al (2018) An investigation of konjac glucomannan-keratin hydrogel scaffold loaded with Avena sativa extracts for diabetic wound healing. Colloids Surf B Biointerfaces 165:92–102. https://doi.org/10.1016/j.colsurfb.2018.02.022
Gharaboghaz MNZ, Farahpour MR, Saghaie S (2020) Topical co-administration of Teucrium polium hydroethanolic extract and Aloe vera gel triggered wound healing by accelerating cell proliferation in diabetic mouse model. Biomed Pharmacother 127:110189. https://doi.org/10.1016/j.biopha.2020.110189
Ponrasu T, Veerasubramanian PK, Kannan R et al (2018) Morin incorporated polysaccharide-protein (psyllium-keratin) hydrogel scaffolds accelerate diabetic wound healing in Wistar rats. RSC Adv 8(5):2305–2314. https://doi.org/10.1039/c7ra10334d
Tottoli EM, Dorati R, Genta I et al (2020) Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 12(8):735. https://doi.org/10.3390/pharmaceutics12080735
Zhao L, Niu L, Liang H et al (2017) pH and glucose dual-responsive injectable hydrogels with insulin and fibroblasts as bioactive dressings for diabetic wound healing. ACS Appl Mater Interfaces 9(43):37563–37574. https://doi.org/10.1021/acsami.7b09395
Zhu Y, Zhang J, Song J et al (2019) A multifunctional pro-healing zwitterionic hydrogel for simultaneous optical monitoring of pH and glucose in diabetic wound treatment. Adv Funct Mater 30(6):1905493. https://doi.org/10.1002/adfm.201905493
Dong M, Mao Y, Zhao Z et al (2022) Novel fabrication of antibiotic containing multifunctional silk fibroin injectable hydrogel dressing to enhance bactericidal action and wound healing efficiency on burn wound: in vitro and in vivo evaluations. Int Wound J 19(3):679–691. https://doi.org/10.1111/iwj.13665
Shi M, Zhang H, Song T et al (2019) Sustainable dual release of antibiotic and growth factor from pH-responsive uniform alginate composite microparticles to enhance wound healing. ACS Appl Mater Interfaces 11(25):22730–22744. https://doi.org/10.1021/acsami.9b04750
Liang Y, Zhao X, Hu T et al (2019) Mussel-inspired, antibacterial, conductive, antioxidant, injectable composite hydrogel wound dressing to promote the regeneration of infected skin. J Colloid Interface Sci 556:514–528. https://doi.org/10.1016/j.jcis.2019.08.083
Galkowska H, Wojewodzka U, Olszewski WL (2006) Chemokines, cytokines, and growth factors in keratinocytes and dermal endothelial cells in the margin of chronic diabetic foot ulcers. Wound Repair Regen 14(5):558–565. https://doi.org/10.1111/j.1743-6109.2006.00155.x
Zarei F, Negahdari B, Eatemadi A (2018) Diabetic ulcer regeneration: stem cells, biomaterials, growth factors. Artif Cells Nanomed Biotechnol 46(1):26–32. https://doi.org/10.1080/21691401.2017.1304407
Xiong Y, Chen L, Liu P et al (2022) All-in-One: multifunctional hydrogel accelerates oxidative diabetic wound healing through timed-release of exosome and fibroblast growth factor. Small 18(1):e2104229. https://doi.org/10.1002/smll.202104229
Jeong S, Kim B, Park M et al (2020) Improved diabetic wound healing by EGF encapsulation in gelatin-alginate coacervates. Pharmaceutics 12(4):334. https://doi.org/10.3390/pharmaceutics12040334
Huang L, Shi Y, Li M et al (2021) Plasma exosomes loaded ph-responsive carboxymethylcellulose hydrogel promotes wound repair by activating the vascular endothelial growth factor signaling pathway in type 1 diabetic mice. J Biomed Nanotechnol 17(10):2021–2033. https://doi.org/10.1166/jbn.2021.3165
Banerjee A, Koul V, Bhattacharyya J (2021) Fabrication of in situ layered hydrogel scaffold for the co-delivery of PGDF-BB/Chlorhexidine to regulate proinflammatory cytokines, growth factors, and MMP-9 in a diabetic skin defect albino rat model. Biomacromol 22(5):1885–1900. https://doi.org/10.1021/acs.biomac.0c01709
Yang X, Yang R, Chen M et al (2020) KGF-2 and FGF-21 poloxamer 407 hydrogel coordinates inflammation and proliferation homeostasis to enhance wound repair of scalded skin in diabetic rats. BMJ Open Diabetes Res Care 8(1):e001009. https://doi.org/10.1136/bmjdrc-2019-001009
Lan B, Zhang L, Yang L et al (2021) Sustained delivery of MMP-9 siRNA via thermosensitive hydrogel accelerates diabetic wound healing. J Nanobiotechnology 19(1):130. https://doi.org/10.1186/s12951-021-00869-6
da Silva LP, Santos TC, Rodrigues DB et al (2017) Stem cell-containing hyaluronic acid-based spongy hydrogels for integrated diabetic wound healing. J Invest Dermatol 137(7):1541–1551. https://doi.org/10.1016/j.jid.2017.02.976
Wang H, Sun D, Lin W et al (2023) One-step fabrication of cell sheet-laden hydrogel for accelerated wound healing. Bioact Mater 28:420–431. https://doi.org/10.1016/j.bioactmat.2023.06.005
Wu X, Zhu H, Che J et al (2023) Stem cell niche-inspired microcarriers with ADSCs encapsulation for diabetic wound treatment. Bioact Mater 26:159–168. https://doi.org/10.1016/j.bioactmat.2023.02.031
Shang L, Yu Y, Jiang Y et al (2023) Ultrasound-augmented multienzyme-like nanozyme hydrogel spray for promoting diabetic wound healing. ACS Nano 17(16):15962–15977. https://doi.org/10.1021/acsnano.3c04134
Qi X, Cai E, Xiang Y et al (2023) An immunomodulatory hydrogel by hyperthermia-assisted self-cascade glucose depletion and ROS scavenging for diabetic foot ulcer wound therapeutics. Adv Mater 35(48):e2306632. https://doi.org/10.1002/adma.202306632
Wenlong Li, Haoxiang C, Jingfeng C et al (2023) Poly(pentahydropyrimidine)-based hybrid hydrogel with synergistic antibacterial and pro-angiogenic ability for the therapy of diabetic foot ulcers. Adv Funct Mater 33:2303147. https://doi.org/10.1002/adfm.202303147
Yang JM, Yang JH, Huang HT (2014) Chitosan/polyanion surface modification of styrene-butadiene-styrene block copolymer membrane for wound dressing. Mater Sci Eng C Mater Biol Appl 34:140–148. https://doi.org/10.1016/j.msec.2013.09.001
Cidreira ACM, de Castro KC, Hatami T et al (2021) Cellulose nanocrystals-based materials as hemostatic agents for wound dressings: a review. Biomed Microdevices 23(4):43. https://doi.org/10.1007/s10544-021-00581-0
Varaprasad K, Jayaramudu T, Kanikireddy V et al (2020) Alginate-based composite materials for wound dressing application: A mini review. Carbohydr Polym 236:116025. https://doi.org/10.1016/j.carbpol.2020.116025
Fletcher J (2003) The benefits of using hydrocolloids. Nurs Times 99(21):57
Graca MFP, Miguel SP, Cabral CSD et al (2020) Hyaluronic acid-Based wound dressings: a review. Carbohydr Polym 241:116364. https://doi.org/10.1016/j.carbpol.2020.116364
Chen Y, Wang X, Tao S et al (2023) Research advances in smart responsive-hydrogel dressings with potential clinical diabetic wound healing properties. Mil Med Res 10(1):37. https://doi.org/10.1186/s40779-023-00473-9
Qi X, Xiang Y, Cai E et al (2023) Inorganic–organic hybrid nanomaterials for photothermal antibacterial therapy. Coord Chem Rev 496:215426. https://doi.org/10.1016/j.ccr.2023.215426
Gao L, Li C, Huang W et al (2020) MXene/polymer membranes: synthesis, properties, and emerging applications. Chem Mater 32(5):1703–1747. https://doi.org/10.1021/acs.chemmater.9b04408
Nunan R, Harding KG, Martin P (2014) Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Dis Model Mech 7(11):1205–1213. https://doi.org/10.1242/dmm.016782
Acknowledgements
This study was sponsored by the National Natural Science Fund (NO:81903994), Youth Development of the First Affiliated Hospital of Anhui Medical University (NO:2793), the Key projects of the clinical research of the First Affiliated Hospital of Anhui Medical University (NO: LCYJ2021ZD005), the Peak Discipline Construction Project of School of Public Health, Anhui Medical University (2021, 2022), Anhui Medical University College Students Innovation and Entrepreneurship Project (Provincial Project, 2022).
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows: Jie zhao completed the collection and analysis of relevant literature and the preparation of the first draft of the thesis; Jie liu participated in graphics. Yuxin Hu and Wanxuan Hu participated in the analysis and collation of the literature. Juan Wei, Haisheng Qian and Yexiang Sun are the project creators and principals, and Yexiang Sun critically revised the manuscript for intellectual content, guiding the paper writing. All authors read and agreed to the final text.
Corresponding authors
Ethics declarations
Competing interest
The authors report no declarations of interest.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, J., Liu, J., Hu, Y. et al. Research advances in hydrogel-based wound dressings for diabetic foot ulcer treatment: a review. J Mater Sci 59, 8059–8084 (2024). https://doi.org/10.1007/s10853-024-09493-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-024-09493-9