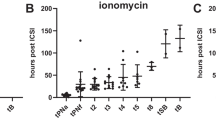
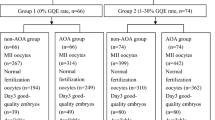
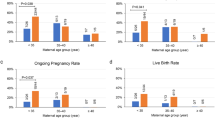
Abstract
Purpose
To investigate whether treatment with commercially available ready-to-use A23187 ionophore (GM508-CultActive) improves embryo development outcome in patients with a history of embryo developmental problems.
Methods
This is a uni-center prospective study in which sibling oocytes of patients with embryos of poor quality on day 5 in the previous cycle were treated or not with CultActive.
Results
Two hundred forty-seven metaphase II (MII) oocytes from 19 cycles performed between 2016 and 2019 were included in the study. After ICSI, the sibling oocytes were assigned to the treatment group or to the control group, following an electronically generated randomization list. A number of 122 MII were treated with CultActive and 125 MII had no treatment and were assigned to the control group. No difference in fertilization rate (p = 0.255) or in the capacity of embryos to reach good quality on day 5 (p = 0.197) was observed between the two groups. The utilization rates defined as the number of embryos transferred or cryopreserved per mature oocyte (p = 0.438) or per fertilized oocytes (p = 0.299) were not significantly different between the treated group and the control group.
Conclusion
The results of the current study do not support the use of CultActive in cases with embryo developmental problems.
Similar content being viewed by others
References
Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney ML, Swann K, Lai FA. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 2002;129(15):3533–44.
Swann K, Larman MG, Saunders CM, Lai FA. The cytosolic sperm factor that triggers Ca2+ oscillations and egg activation in mammals is a novel phospholipase C: PLCzeta. Reproduction. 2004;127:431–9.
Yeste M, Jones C, Amdani SN, Patel S, Coward K. Oocyte activation deficiency: a role for an oocyte contribution? Hum Reprod Update. 2016;22(1):23–47. https://doi.org/10.1093/humupd/dmv040.
Whitaker M. Calcium at fertilization and in early development. Physiol Rev. 2006;86(1):25–88. https://doi.org/10.1152/physrev.00023.2005.
Jones KT. Intracellular calcium in the fertilization and development of mammalian eggs. Clin Exp Pharmacol Physiol. 2007;34(10):1084–9. https://doi.org/10.1111/j.1440-1681.2007.04726.
Schultz RM, Kopf GS. Molecular basis of mammalian egg activation. Curr Top Dev Biol. 1995;30:21–62. https://doi.org/10.1016/s0070-2153(08)60563-3.
Amdani SN, Jones C, Coward K. Phospholipase C zeta (PLCζ): oocyte activation and clinical links to male factor infertility. Adv Biol Regul. 2013;53(3):292–308. https://doi.org/10.1016/j.jbior.2013.07.005.
Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, et al. Human sperm devoid of PLC, zeta 1 fail to induce Ca2+ release and are unable to initiate the first step of embryo development. J Clin Invest. 2008;118:3671–81. https://doi.org/10.1172/JCI36942.
Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, Fissore RA, Hamer R, et al. Reduced amounts and abnormal forms of phospholipase C zeta in spermatozoa from infertile men. Hum Reprod. 2009;24:2417–28. https://doi.org/10.1093/humrep/dep207.
Darwish E, Magdi Y. A preliminary report of successful cleavage after calcium ionophore activation at ICSI in cases with previous arrest at the pronuclear stage. Reprod Biomed Online. 2015;31(6):799–804. https://doi.org/10.1016/j.rbmo.2015.08.012.
Ebner T, Oppelt P, Wöber M, Staples P, Mayer RB, Sonnleitner U, Bulfon-Vogl S, Gruber I, Haid AE, Shebl O. Treatment with Ca2+ ionophore improves embryo development and outcome in cases with previous developmental problems: a prospective multicenter study. Hum Reprod. 2015;30(1):97–102. https://doi.org/10.1093/humrep/deu285.
Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, Fissore R, Madoux S, Ozil JP. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Dev Biol. 2002;250(2):280–91.
Ozil JP, Huneau D. Activation of rabbit oocytes: the impact of the Ca2+ signal regime on development. Development. 2001;128(6):917–28.
Malcuit C, Kurokawa M, Fissore RA. Calcium oscillations and mammalian egg activation. J Cell Physiol. 2006;206(3):565–73. https://doi.org/10.1002/jcp.20471.
Machaty Z. Signal transduction in mammalian oocytes during fertilization. Cell Tissue Res. 2016;363(1):169–83. https://doi.org/10.1007/s00441-015-2291-8.
Ozil JP, Banrezes B, Tóth S, Pan H, Schultz RM. Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol. 2006;300(2):534–44. https://doi.org/10.1016/j.ydbio.2006.08.041.
Tóth S, Huneau D, Banrezes B, Ozil JP. Egg activation is the result of calcium signal summation in the mouse. Reproduction. 2006;131(1):27–34. https://doi.org/10.1530/rep.1.00764.
Ferrer-Buitrago M, Bonte D, De Sutter P, Leybaert L, Heindryckx B. Single Ca2+ transients vs oscillatory Ca2+ signaling for assisted oocyte activation: limitations and benefits. Reproduction. 2018;155(2):105–19. https://doi.org/10.1530/REP-17-0098.
Gardner DK, Schoolcraft WB. In-vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: fertility and genetics beyond 1999. Carnforth: Parthenon Press; 1999. p. 378–88.
De Munck N, Santos-Ribeiro S, Mateizel I, Verheyen G. Reduced blastocyst formation in reduced culture volume. J Assist Reprod Genet. 2015;32(9):1365–70. https://doi.org/10.1007/s10815-015-0541-z.
Lv M, Dan Zhang D, He X, Chen B, Li Q, Ding D, Hao Y, Xue R, Ji D, et al. Artificial oocyte activation to improve reproductive outcomes in couples with various causes of infertility: a retrospective cohort study. Reprod Biomed Online. 2020;40(4):501–9. https://doi.org/10.1016/j.rbmo.2020.01.001.
Shebl O, Trautner PS, Enengl S, Reiter E, Allerstorfer C, Rechberger T, Oppelt P, Ebner T. Ionophore application for artificial oocyte activation and its potential effect on morphokinetics: a sibling oocyte study. J Assist Reprod Genet. 2021;38(12):3125–33. https://doi.org/10.1007/s10815-021-02338-3.
Miller N, Biron-Shental T, Sukenik-Halevy R, Klement AH, Sharony R, Berkovitz A. Oocyte activation by calcium ionophore and congenital birth defects: a retrospective cohort study. Fertil Steril. 2016 1;106(3):590–6. https://doi.org/10.1016/j.fertnstert.2016.04.025
Mateizel I, Verheyen G, Van de Velde H, Tournaye H, Belva F. Obstetric and neonatal outcome following ICSI with assisted oocyte activation by calcium ionophore treatment. J Assist Reprod Genet. 2018;35(6):1005–10. https://doi.org/10.1007/s10815-018-1124-6.
Li B, Zhou Y, Yan Z, Li M, Xue S, Cai R, Fu Y, Hong et al. Pregnancy and neonatal outcomes of artificial oocyte activation in patients undergoing frozen-thawed embryo transfer: a 6-year population-based retrospective study. Arch Gynecol Obstet. 2019 Oct;300(4):1083–92. https://doi.org/10.1007/s00404-019-05298-3
Moaz MN, Khattab S, Foutouh IA, Mohsen EA. Chemical activation of oocytes in different types of sperm abnormalities in cases of low or failed fertilization after ICSI: a prospective pilot study. Reprod Biomed Online. 2006;13(6):791–4. https://doi.org/10.1016/s1472-6483(10)61025-5.
Heindryckx B, De Gheselle S, Gerris J, Dhont M, De Sutter P. Efficiency of assisted oocyte activation as a solution for failed intracytoplasmic sperm injection. Reprod Biomed Online. 2008;17(5):662–8. https://doi.org/10.1016/s1472-6483(10)60313-6.
Acknowledgements
The authors wish to thank the clinical embryologists, medical doctors, and laboratory technologists of the Brussels IVF center from Universitair Ziekenhuis Brussel (UZBrussel).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mateizel, I., Santos-Ribeiro, S., Segers, I. et al. Effect of A23187 ionophore treatment on human blastocyst development—a sibling oocyte study. J Assist Reprod Genet 39, 1225–1232 (2022). https://doi.org/10.1007/s10815-022-02467-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02467-3