Abstract
Mammalian embryo development begins when the fertilizing sperm triggers a series of elevations in the oocyte’s intracellular free Ca2+ concentration. The elevations are the result of repeated release and re-uptake of Ca2+ stored in the smooth endoplasmic reticulum. Ca2+ release is primarily mediated by the phosphoinositide signaling system of the oocyte. The system is stimulated when the sperm causes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG); IP3 then binds its receptor on the surface of the endoplasmic reticulum that induces Ca2+ release. The manner in which the sperm generates IP3, the Ca2+ mobilizing second messenger, has been the subject of extensive research for a long time. The sperm factor hypothesis has eventually gained general acceptance, according to which it is a molecule from the sperm that diffuses into the ooplasm and stimulates the phosphoinositide cascade. Much evidence now indicates that the sperm-derived factor is phospholipase C-zeta (PLCζ) that cleaves PIP2 and generates IP3, eventually leading to oocyte activation. A recent addition to the candidate sperm factor list is the post-acrosomal sheath WW domain-binding protein (PAWP), whose role at fertilization is currently under debate. Ca2+ influx across the plasma membrane is also important as, in the absence of extracellular Ca2+, the oscillations run down prematurely. In pig oocytes, the influx that sustains the oscillations seems to be regulated by the filling status of the stores, whereas in the mouse other mechanisms might be involved. This work summarizes the current understanding of Ca2+ signaling in mammalian oocytes.
Similar content being viewed by others
Introduction
Prior to fertilization, mammalian oocytes are arrested at the metaphase stage of the second meiotic cell division. The meiotic block is characteristic of the entire animal kingdom and, although it may happen at various stages of the cell cycle, its role is to prevent entry into the embryonic cell cycles without the sperm (Dupré et al. 2011). The ovulated oocyte is a highly differentiated cell that, without fertilization, would die within 24–48 h. The fertilizing sperm, however, provides a stimulus that alleviates the meiotic arrest and activates the oocyte’s developmental program. During activation, the content of cortical granules is released into the perivitelline space. This triggers changes in the oocyte’s extracellular matrix, the zona pellucida, to prevent penetration by additional spermatozoa (Jaffe and Gould 1985). The activity of cell cycle regulatory proteins that maintain the arrest, such as cyclin-dependent kinase 1 (Cdk1; a component of the M-phase Promoting Factor) and Mitogen-Activated Protein Kinase (MAPK; part of the Cytostatic Factor) decreases, while that of others, such as the Anaphase Promoting Complex (APC) increases (Whitaker 1996; Nixon et al. 2002). As a result, the cell cycle resumes, meiosis is completed and after formation of the male and female pronuclei, the activated oocyte (now a 1-cell embryo) enters the first mitotic division. Activation is a remarkable process. It allows a differentiated cell to become totipotent and give rise to all the different cell types of a new organism. The transition is triggered by a highly intricate signal transduction mechanism that the sperm stimulates following sperm–oocyte fusion. This review describes the signaling pathway and discusses how it operates in mammalian oocytes to mediate the formation of an embryo, the founder of a new generation.
The rise of calcium
It was Jacques Loeb who first suggested that oocyte activation involves changes in the concentration of ions in the ooplasm (Loeb 1899). His idea was based on the observation that sea urchin eggs started to develop parthenogenetically in the absence of sperm, simply by being bathed in seawater containing increased levels of ions. At a time when embryo development was explained with “vital forces”, not everybody was impressed; The New York Times referred to him simply as “a man of lively imagination”. The notion, however, was so fascinating that even Mark Twain wrote an essay about it titled “Dr. Loeb’s Incredible Discovery”. The calcium ion (Ca2+) was singled out by Lewis Victor Heilbrunn. Although the importance of Ca2+ in the contraction of skeletal muscle was demonstrated earlier (Ringer 1883), it was Heilbrunn who discovered that Ca2+ was the trigger not only for oocyte activation but also a great number of additional biological processes including ciliary movement, neurotransmitter release, increase or decrease in cell respiration and cell aging (Heilbrunn 1937). Considered by many in his time as a ‘calcium maniac’ (Shreeve 1983), Heilbrunn proposed that the breakdown of the nuclear membrane in the oocyte of the ragworm Nereis following fertilization or parthenogenetic activation was due to the release of Ca2+ inside the cell (Heilbrunn and Wilbur 1937). The increase in the free Ca2+ concentration during fertilization was quantitated in the eggs of another marine invertebrate, the sea urchin Arbacia punctulata (Mazia 1937). It was then demonstrated that treating sea urchin eggs with a Ca2+ ionophore that induced the release of Ca2+ from the intracellular stores caused parthenogenetic activation (Steinhardt and Epel 1974). The role of Ca2+ as the trigger of oocyte activation was proved when in medaka oocytes fertilization was shown accompanied by an elevation in the intracellular free Ca2+ concentration (Ridgway et al. 1977) and inhibition of this increase in sea urchin eggs blocked changes associated with activation (Zucker and Steinhardt 1978; Whitaker and Steinhardt 1982). Since these early studies, it has been firmly established that in virtually all animals it is Ca2+ that induces activation of the dormant oocyte. In most species, the sperm triggers a single elevation in the oocyte’s intracellular free Ca2+ concentration. The increase generally originates at the site of sperm entry and travels across the oocyte as a propagating Ca2+ wave (Gilkey et al. 1978). However, in mammals and some other species, including nemertean worms, ascidians, some annelids and arthropods, a series of low-frequency Ca2+ oscillations take place in the ooplasm at fertilization (Stricker 1999; Kashir et al. 2013a). In these cases, the first sperm-induced Ca2+ transient also arises near the site of sperm attachment and propagates as a wave across the entire oocyte. The initiation site of subsequent waves may undergo a shift: in mouse oocytes, it translocates from the point of sperm entry to the vegetal cortex (Deguchi et al. 2000).
Oscillatory Ca2+ signals have physiological advantages over static Ca2+ increases and they affect subsequent development. The repetitive behavior provides a means to deliver prolonged Ca2+ signals to targets without the deleterious effects of sustained Ca2+ elevations. The amplitude, frequency and duration of the sperm-induced Ca2+ signals encode crucial information and have a profound effect on peri-implantation development in addition to effects on the immediate events of oocyte activation (Ozil and Huneau 2001). Although a single increase in the intracellular free Ca2+ concentration can promote parthenogenetic development, freshly ovulated oocytes showed limited cell cycle progression and mRNA recruitment following activation with a single Ca2+ stimulus and only after aging could a single Ca2+ rise stimulate these critical events (Jones 1998; Ozil et al. 2005). By manipulating the number of Ca2+ transients in fertilized mouse oocytes, it was demonstrated that the first few Ca2+ transients were able to induce development to the blastocyst stage but fewer offspring were born from these embryos, indicating that the developmental competence of the blastocysts was reduced. Microarray analysis of global gene expression patterns in these embryos revealed that ∼20 % of the genes were misregulated, particularly those involved in RNA processing, polymerase II transcription, cell cycle and cell adhesion (Ozil et al. 2006).
How does the sperm trigger the Ca2+ rise?
Once it was clarified that oocyte activation is stimulated by Ca2+, the next question that logically occurred was how the sperm triggers the Ca2+ elevation in the ooplasm? This issue, however, remained unresolved for a long time. Multiple hypotheses were proposed to explain the generation of the fertilization Ca2+ signal. The earliest model known as the “Ca2+ bomb hypothesis” postulated that the sperm introduces Ca2+ into the oocyte that sets off a wave of Ca2+-induced Ca2+ release (Fig. 1) (Jaffe 1983). However, the Ca2+ content of the sperm is not sufficient to trigger Ca2+ release and the hypothesis was subsequently modified to suggest that the sperm serves as a Ca2+ conduit, allowing Ca2+ from the extracellular medium to flow into the ooplasm (Jaffe 1991). The Ca2+ is then pumped into the endoplasmic reticulum, which results in the overloading of the stores and the release of luminal Ca2+. Even in this form, the theory did not stand the test of time. The injection of Ca2+ into the ooplasm fails to cause further Ca2+ release in many species, or to trigger repetitive Ca2+ oscillations in mammalian oocytes (Swann and Whitaker 1986; Swann and Ozil 1994). In addition, no local elevation in the cytoplasmic Ca2+ levels has been detected near the site of sperm–oocyte fusion (Jones et al. 1998); as it turned out, a Ca2+ entry takes place after (rather than before) the first Ca2+ transient (McGuinness et al. 1996).
Schematic illustration of various hypotheses explaining the way the fertilizing sperm induces an elevation in the intracellular free Ca2+ concentration of the oocyte. The Ca2+ bomb hypothesis proposes that the sperm delivers a bolus of Ca2+ that causes Ca2+-induced Ca2+ release in the oocyte. According to the Ca2+ conduit model, the sperm facilitates Ca2+ entry from the extracellular medium. The receptor hypothesis suggests that Ca2+ release is induced when the sperm interacts with an oocyte receptor leading to the generation of IP3, the Ca2+ releasing second messenger. Finally, the sperm factor hypothesis claims that a factor from the sperm diffuses into the ooplasm and causes the production of IP3 to mobilize Ca2+
According to the “receptor hypothesis”, the fertilizing sperm induces the Ca2+ oscillations by binding to a receptor on the surface of the oocyte plasma membrane. Just like hormone–receptor binding in somatic cells, the interaction between a sperm ligand and a receptor spanning the oolemma was proposed to activate a signaling pathway that ultimately leads to the release of Ca2+ from the endoplasmic reticulum. This hypothesis was supported by a number of observations and, for many years, it was the dominant model to explain generation of the fertilization Ca2+ signal (Jaffe 1990; Schultz and Kopf 1995). Although numerous publications suggest that oocytes contain a signaling pathway associated with cell surface receptors, there is no evidence that the sperm triggers oocyte activation via these pathways. The ligands and receptors identified so far on the surface of mammalian gametes are involved in the mediation of sperm–oocyte binding and fusion, with no role in stimulating Ca2+ release (Wassarman et al. 2005).
The “sperm factor hypothesis” proposes that oocyte activation is induced by a soluble factor in the sperm that is released into the oocyte at fertilization. In mouse, it was shown that fusion between the sperm and oocyte membranes precedes the first Ca2+ transient by 1–3 min, which is consistent with the notion that the sperm-resident factor needs time to move into the oocyte cytoplasm before it mobilizes Ca2+ from the internal stores (Lawrence et al. 1997). The finding that the injection of a crude extract isolated from the head of mammalian sperm is able to induce repetitive Ca2+ oscillations in mammalian oocytes also supports this hypothesis (Swann 1990; Wu et al. 1997; Machaty et al. 2000). It was also reported that sperm extracts from fish, frogs and chickens caused oscillations in mouse oocytes (Dong et al. 2000; Coward et al. 2003). In addition, oocytes can be activated by intracytoplasmic sperm injection, where membrane interaction between the sperm and oocyte is bypassed during the injection process. This also argues in favor of the proposal that it is a factor in the sperm head that initiates the Ca2+ changes at fertilization.
The signaling pathway
The fertilizing sperm can generate the initial Ca2+ transient even in the absence of extracellular Ca2+; this led to the realization that the origin of Ca2+ that activates the oocyte is intracellular (Gilkey et al. 1978). In many cells, the intracellular stores reside in the smooth endoplasmic reticulum. Ca2+ is loaded into the lumen of the stores by sarcoplasmic/endoplasmic reticulum Ca2+ ATPases (SERCA pumps) and stored while attached to Ca2+-binding proteins (Berridge 2002). During signaling, the stored Ca2+ is released into the cytosol through Ca2+ release channels. Two types of channels, which also function as receptors for their respective Ca2+ mobilizing ligands, are available for Ca2+ release. The inositol 1,4,5-trisphosphate (IP3) receptor is a protein complex of four subunits that surround the channel pore (Mikoshiba 1993). Each subunit can bind one IP3 molecule and binding leads to the release of Ca2+ from the store. The sensitivity of the IP3 receptor to IP3 is biphasic: it is the greatest in the physiological range between 0.5 and 1 μM (Hajnóczky and Thomas 1994). The receptor is also gated by Ca2+. Its cytoplasmic region has at least one binding site for Ca2+ (Mignery and Südhof 1990) and experimental data indicate that the receptor shows biphasic sensitivity to cytoplasmic Ca2+. Thus, regulation of the receptor is complex: it is opened by IP3 but is also desensitized by it, while low and high Ca2+ concentrations make it relatively insensitive to otherwise activating IP3 levels. The other type of Ca2+ release channels/receptors is the ryanodine receptor. It is also a homotetramer of four subunits and its opening is controlled by cyclic adenosine diphosphate ribose (cADPR), by Ca2+ itself and in skeletal muscle by electromechanical coupling to the dihydropiridine receptor located in the plasma membrane (Coronado et al. 1994).
In mammalian oocytes, the generation of the fertilization Ca2+ transients is mediated by the phosphoinositide signaling system. Such a system produces a signal when IP3 binds its receptor, leading to the opening of the channel and the release of Ca2+ into the cytosol. IP3 is produced when phospholipase C (PLC), a cytoplasmic enzyme, cleaves phosphatidylinositol 4,5-bisphosphate (PIP2), a phosphoinositide, into IP3 and diacylglycerol (DAG) (Miyazaki et al. 1993). Currently, there are 13 known mammalian phosphoinositide-specific PLC isozymes; their classification is based on structure and regulation. They include 4 types of PLCβ (PLCbeta), 2 types of PLCγ (PLCgamma), 3 types of PLCδ (PLCdelta), PLCε (PLCepsilon), PLCζ (PLCzeta) and 2 types of PLCη (PLCeta) (Bunney and Katan 2011). All isozymes exhibit characteristic X and Y catalytic domains that together form the active site responsible for cleaving PIP2. They also contain various combinations of regulatory domains that target the enzymes to their respective activators or substrates; these include pleckstrin homology (PH) domains, Src homology 2 (SH2) domains and constant or conserved region 2 (C2) domains. The mechanism of activation varies depending on the specific combination of regulatory domains present in each PLC isozyme. PH domains bind phosphoinositides such as PIP2 and PIP3 and thus they typically serve to target PLC to the plasma membrane where most phosphoinositides are located. In addition, they can also mediate interaction with heterotrimeric G proteins (Camps et al. 1992). SH2 domains interact with receptor tyrosine kinases and also with non-receptor tyrosine kinases such as Src (Weiss 1993). Finally, C2 domains bind Ca2+ and bestow phospholipid-binding properties to the enzyme.
Several lines of evidence indicate the involvement of the phosphoinositide signaling cascade during fertilization. Biochemical analyses in sea urchin and frogs have shown an increased turnover of polyphosphoinositides and an elevation in IP3 levels after gamete interaction (Turner et al. 1984; Snow et al. 1996). In addition, a monoclonal antibody against the IP3 receptor inhibits the sperm-induced Ca2+ transients (Miyazaki et al. 1992), while sustained microinjection of IP3, or adenophostin (an IP3 analogue) can also trigger Ca2+ oscillations in mammalian oocytes (Swann 1994; Jones and Nixon 2000). Further evidence also shows that the PLC inhibitor U73122 blocks activation in the sea urchin and mouse (Dupont et al. 1996; Lee et al. 1998). Finally, in mouse and bovine oocytes, there is a significant down-regulation of the IP3 receptors, i.e., their number decreases markedly at the time of fertilization (Brind et al. 2000; Jellerette et al. 2000). Normally, this occurs only after a substantial rise in the IP3 concentration, implying that at fertilization the sperm stimulates an increase in IP3 levels in the oocyte cytoplasm. Initial investigations were focused on oocyte-resident PLCs and the presence of PLCβ, PLCγ and PLCδ was demonstrated in the female gamete. PLCβ isoforms are generally coupled to membrane receptors via a G protein, whereas γ isoforms are directly linked to receptor tyrosine kinases. Microinjection of a non-hydrolyzable analog of GTP that stimulates G proteins (GTPγS) caused activation in sea urchin eggs (Turner et al. 1986) and induced regenerative Ca2+ rises in some mammalian oocytes (Miyazaki 1988; Swann 1992; Fissore et al. 1995). Also, overexpression of the G protein-coupled muscarinic receptor in frog, mouse and pig oocytes led to activation after exposure of the oocytes to acetylcholine, the receptor’s ligand (Kline et al. 1988; Williams et al. 1992; Machaty et al. 1997). These findings suggested that the pathway that mediated Ca2+ release might include a PLCβ connected to a G protein-coupled receptor.
An alternative signaling mechanism that was implicated by experimental data involved PLCγ and an associated receptor tyrosine kinase. Overexpression and subsequent stimulation of such receptors in frog and mouse oocytes leads to activation (Yim et al. 1994; Mehlmann et al. 1998), which seems to support the theory. However, recombinant SH2 domains of PLCγ block PLCγ activation by the receptor but they cannot inhibit Ca2+ release at fertilization (Mehlmann et al. 1998; Runft et al. 1999), which argues against the involvement of PLCγ in the signaling process at fertilization. In addition, when the phosphoinositide signaling system is artificially activated using GTPγS, or non-hydrolyzable analogs of IP3, the Ca2+ signal that is generated is still a far cry from the low-frequency Ca2+ oscillations associated with mammalian fertilization (Miyazaki et al. 1990; Swann and Ozil 1994; Galione et al. 1994; Machaty et al. 1997). Taken together, these data indicate that the oocytes contain a phosphoinositide signaling pathway; however, the exact mechanism that mediates its activation at fertilization has not been identified by these studies.
Finding PLCζ
As described above, a number of observations supported the idea that the sperm might stimulate the phosphoinositide signaling pathway by introducing a soluble factor into the oocyte after fusion. This led to a quest to identify the oocyte activating factor but the efforts proved futile for a long time. The molecule was shown to be a protein since heat treatment or proteases abolished its Ca2+-inducing activity; it was also believed to have a high molecular mass and be present in cytosolic extracts (Swann 1990). Mammalian sperm extracts showed high PLC enzyme activity in biochemical assays, which suggested that the sperm factor that activates the oocyte might itself be a PLC (Rice et al. 2000). Spermatozoa of mammalian species express several PLC isoforms including PLCβ, -γ and -δ (Fukami 2002); however, when the recombinant forms of these proteins were injected into oocytes, they were unable to induce Ca2+ oscillations at physiological levels (Mehlmann et al. 2001). And because chromatographic fractionation of sperm extracts indicated that none of the known PLC isoforms were present in the fraction that were able to induce regenerative Ca2+ rises (Parrington et al. 2002), the idea came that the sperm factor might be a novel PLC.
The analysis of mouse expressed sequence tag (EST) databases led to the identification and eventual amplification of a new, testis-specific PLC variety, termed PLCζ (Saunders et al. 2002). With its ∼74 kDa molecular weight, it is the smallest known mammalian PLC. Recombinant PLCζ, or its complementary RNA (cRNA), are both able to induce regenerative Ca2+ oscillations in mouse oocytes similar to those found at fertilization (Saunders et al. 2002; Cox et al. 2002; Kouchi et al. 2004). Furthermore, when injected into human and pig oocytes, PLCζ cRNA can stimulate embryo development to the blastocyst stage (Rogers et al. 2004; Yoneda et al. 2006). Immunodepletion with an anti-PLCζ antibody suppressed the extracts’ ability to induce Ca2+ release in mouse oocytes or sea urchin egg homogenates (Saunders et al. 2002). The presence of PLCζ orthologues has been demonstrated in the sperm of other mammalian species, including hamster, pig, horse, monkey and human (reviewed by Nomikos et al. 2013). In mice, the protein is localized in the postacrosomal region of the perinuclear theca, a condensed layer of cytosolic proteins that covers the nucleus (Young et al. 2009); in cattle, it resides in the equatorial region of the sperm head (Yoon and Fissore 2007). This is the localization that is expected from a sperm-resident factor that needs to gain rapid access to the ooplasm after gamete fusion to mobilize Ca2+ (Lawrence et al. 1997). The use of tagged versions of the protein has indicated that approximately 40 fg PLCζ is able to trigger repetitive Ca2+ transients in mouse oocytes and this is the amount estimated to be present in a single sperm (Saunders et al. 2002). Injection of PLCζ into mouse oocytes causes a down-regulation of IP3 receptors similar to that seen at fertilization, indicating that PLCζ generates IP3 in the cytoplasm (Lee et al. 2010). When spermatozoa from transgenic mice showing reduced PLCζ expression were used to fertilize oocytes, the Ca2+ oscillations generated in the ooplasm stopped prematurely (Knott et al. 2005). Although these mice were not completely infertile, they produced markedly reduced litter sizes following mating. Sperm of human patients that failed to activate the oocyte also had deficiencies in their PLCζ: they either showed reduced or complete absence of the enzyme, or possessed deleterious mutations within the catalytic X and Y domains (Yoon et al. 2008; Kashir et al. 2012). The analysis of sperm that is completely devoid of PLCζ would provide invaluable information regarding the role of the protein in Ca2+ signaling at fertilization. Unfortunately, although PLCζ-knockout mice have already been created, they are unable to produce sperm; the germ cells in the testes of such animals develop only up to the round spermatid stage (Ito et al. 2010). It was demonstrated some time ago (Kimura and Yanagimachi 1995) that microinjection of round mouse spermatids is unable to activate oocytes (a potential explanation to this might be that, according to one study, PLCζ expression in mice begins in elongated spermatids only [Yoneda et al. 2006]); hence, these cells cannot be used to determine the importance of PLCζ in fertilization. Nevertheless, despite the absence of this ultimate test, the data listed above strongly argue in favor of the idea that, in mammals, PLCζ has a central role in the generation of the Ca2+ signal to activate the oocyte and stimulate embryo development.
PLCζ characteristics
The PLCζ orthologues identified in various mammalian species are all similar in size (Swann et al. 2006). Surprisingly, they lack the N-terminal PH domain that is present in other PLC isoforms and instead contain two pairs of EF hand domains, followed by the XY catalytic domain and a C2 domain at the C-terminus. PLCζ is much more potent than other PLC isoforms in generating Ca2+ oscillations; its closest homologue, PLCδ1 triggers oscillations only when it is present in mouse oocytes at concentrations higher than 1 pg (Saunders et al. 2002; Nomikos et al. 2011). As in other isoforms, the XY catalytic domain is responsible for enzymatic activity; a point mutation in this domain causes a loss in the enzyme’s ability to generate IP3 and induce Ca2+ oscillations (Nomikos et al. 2011). The activity is not species specific as cRNA of various mammalian or non-mammalian PLCζ orthologues can cause oscillations in mouse oocytes (Cox et al. 2002; Coward et al. 2005).
The EF hands possess Ca2+ binding residues and provide the enzyme with high Ca2+ sensitivity; deletion or mutation of conserved Ca2+ binding residues in this region abolish Ca2+-induced PLC activity. PLCζ is 100-fold more sensitive to Ca2+ than PLCδ and this is believed to be a major reason why the enzyme is highly effective in oocytes. Even at resting Ca2+ levels, PLCζ shows half maximal activity and, with rising cytosolic Ca2+ concentrations, its enzymatic activity increases markedly (Nomikos et al. 2005). Thus, following gamete fusion, when PLCζ diffuses into the ooplasm, a small increase in cytosolic Ca2+ causes a significant elevation in PLC activity, leading to the generation of large amounts of extra IP3. This creates a positive feedback loop of IP3 production and Ca2+ release in fertilized oocytes, setting the stage for the regenerative Ca2+ signal.
C2 domains generally bind Ca2+ (Nalefski and Falke 1996) and Ca2+ binding to the C2 domain is typically crucial for enzyme activity (Zheng et al. 2000). However, the C2 domain of PLCζ has no predicted Ca2+ binding site. Deletion of this domain does not alter enzyme activity of PLCζ, nevertheless it abolishes its ability to induce Ca2+ oscillations, indicating that the C2 domain is critical for PLCζ function (Nomikos et al. 2005). Another segment, the XY-linker that joins together the X and Y catalytic domains, also has a major impact on PLCζ function. As mentioned before, unlike other isoforms, PLCζ does not have a PH domain that typically functions to bind PIP2 at the plasma membrane. It was proposed, however, that positively charged residues within this region might target the enzyme to PIP2, possibly via electrostatic interactions (Nomikos et al. 2007). A decrease in the net positive charge of the X-Y linker, or the deletion of the entire linker, caused a decline in the enzyme’s ability to bind PIP2 in vitro or to induce Ca2+ oscillations after microinjection into oocytes (Nomikos et al. 2011). There are also distinct variations in the size of the X–Y linker between species. It is the shortest in humans and the longest in the Cynomolgus monkey (Swann et al. 2006); these differences may explain the diverse potency of PLCζ orthologs to generate Ca2+ transients (Saunders et al. 2007). Finally, the X–Y linker also possesses a predicted nuclear localization signal sequence that may be important in the control of PLCζ function (Larman et al. 2004).
PLCζ localization in the sperm
According to the sperm factor hypothesis, a compound diffuses from the sperm into the ooplasm and causes Ca2+ release from the endoplasmic reticulum. The Ca2+ oscillations begin soon after the fusion of the gametes; in the mouse, the elapsed time is approximately 1–3 min (Lawrence et al. 1997; Jones et al. 1998); in the hamster, it is shorter, about 10 s (Miyazaki 1991). During this time (the so-called latent period), the sperm factor is supposed to get into the ooplasm and initiate the mobilization of Ca2+. This means that the factor must reside in the sperm at a location that provides easy access to the ooplasm. It is believed that the most ideal localization for the oocyte-activating factor is the post-acrosomal region of the perinuclear theca, a condensed layer of cytosolic proteins surrounding the nucleus of the sperm (Yanagimachi 1994). Immunofluorescent analysis of mouse spermatozoa determined that PLCζ is localized in the post-acrosomal region of the sperm head (Fujimoto et al. 2004); this region also seems to possess the ability to activate oocytes after intracytoplasmic sperm injection (Kimura et al. 1998; Perry et al. 2000). Importantly, this is the area that is exposed to the oocyte cytoplasm following fusion of the sperm’s equatorial region with the oolemma. In other species, it was found in the equatorial or acrosomal region (Yoon and Fissore 2007; Kashir et al. 2013b), while in equine sperm, PLCζ also resided in the principal piece of the tail (Bedford-Guaus et al. 2011). This latter finding was quite unexpected; however, because microinjection of the equine sperm tail caused Ca2+ oscillations into mouse oocytes (Bedford-Guaus et al. 2011), this further strengthened the idea that PLCζ is the molecule that triggers activation.
Solubility of PLCζ may be another aspect that influences its function as an oocyte-activating factor. Early studies in the hamster and swine determined that cytosolic extracts of the spermatozoa contained the active factor (Swann 1990); later experiments in the mouse, however, asserted that the sperm heads retained the activity after the removal of the soluble cytosolic fraction (Kimura et al. 1998; Perry et al. 2000). This seems to indicate differences among species and also variations in the solubility of PLCζ (solubility here refers to the ability to move via diffusion in the oocyte cytosol, it does not mean that it can be extracted into an aqueous solution). In hamster, the initial Ca2+ transient begins within seconds following gamete fusion (Miyazaki 1991) and much of PLCζ appears to exist in a soluble form in hamster sperm (Swann 1990) facilitating easy access to PIP2 and rapid Ca2+ mobilization in the oocyte cytoplasm. In mice, on the other hand, the Ca2+ oscillations are initiated several minutes after sperm-oocyte fusion and this relatively long latent period may be the consequence of the low solubility of mouse PLCζ that requires a longer time to move into the ooplasm from the sperm head. This idea is supported by the observation that, during isolation, the mouse sperm cytosol does not retain the oocyte-activating factor and more elaborate approaches are necessary for its extraction (Perry et al. 2000). Porcine PLCζ has been found in both soluble and insoluble fractions (Kurokawa et al. 2005). Based on these observations, an idea has been formulated, claiming that soluble PLCζ is located in the equatorial region of the sperm head and, due to its easy access to the ooplasm, it stimulates Ca2+ oscillations rapidly following gamete fusion. In contrast, insoluble PLCζ localizes in the postacrosomal region and mobilizes Ca2+ in a somewhat belated manner, once incorporation of the sperm head in the oocyte cytoplasm is at a more advanced stage (Kashir et al. 2014).
PLCζ action in the oocyte
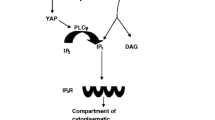
PLC enzymes generate IP3 by cleaving the phospholipid PIP2. Because PIP2 resides solely in biological membranes, one would expect PLCζ to accumulate in the plasma membrane where most of the cells’ PIP2 is located. However, fluorescently tagged mouse PLCζ localized in the cytoplasm instead of below the oolemma (Yu et al. 2012). In addition, there is no decrease in the PIP2 concentration at the plasma membrane in mouse oocytes at fertilization (Halet et al. 2002) and the level of DAG, the other product of PIP2 hydrolysis, does not increase at the plasma membrane during fertilization or after PLCζ injection (Yu et al. 2008). This apparent contradiction is solved in light of the findings that PIP2 in mouse oocytes resides not only in the plasma membrane but also in the membrane of vesicles inside the oocyte cortex (Yu et al. 2012). By means of immunocytochemistry, it was determined that PLCζ localized in the same vesicular structures and after PLCζ injection these vesicles displayed decreased PIP2 levels. Targeting an inositol phosphate phosphatase to the plasma membrane also supported these observations. The expression of such a phosphatase in mouse oocytes reduced the amount of plasma membrane-resident PIP2 and entirely abolished the Ca2+ transients triggered by the microinjection of PLCδ1 without affecting the sperm- or PLCζ-induced Ca2+ oscillations. This also explains previous reports that extracts made of boar sperm were able to generate IP3 most effectively in the subcellular fraction of sea urchin egg homogenates that were rich in yolk vesicles (Rice et al. 2000). This suggests that PLCζ uses a unique signaling cascade to mobilize Ca2+ during fertilization when it hydrolyzes PIP2 in intracellular membranes. The potential mechanism that PLCζ uses to induce Ca2+ release in oocytes is shown in Fig. 2.
Hypothetical mechanism of PLCζ-induced Ca2+ release. Following gamete fusion, PLCζ diffuses into the ooplasm and hydrolyses PIP2 located in the membrane of cytoplasmic vesicles. It is possible that it binds to a yet-to-be-identified protein in the vesicular membrane (symbolized by a red rectangle). The IP3 generated as a result of the hydrolysis moves to the endoplasmic reticulum and induces the release of stored Ca2+. Elevated Ca2+ levels lead to increased PLCζ activity, which, through a positive feedback loop, stimulates further increase of Ca2+ and IP3 (from Swann and Lai 2013, with permission)
In mammalian oocytes, the sperm-induced Ca2+ signal oscillates for an extended period of time. In the mouse, the oscillations cease after about 3–4 h, which coincides with the formation of the male and female pronuclei. The termination of the oscillations was proposed to be due to the sequestration of PLCζ into the forming pronuclei (Marangos et al. 2003) and a number of observations support this notion. It has been shown that, if pronuclear formation is inhibited, the oscillations continue indefinitely. In addition, the oscillations are absent while the pronuclei exist but at the onset of mitosis they resume as the nuclear envelopes break down. Transferring of the male or female pronucleus from fertilized oocytes causes Ca2+ oscillations in the cytoplasm of unfertilized oocytes while pronuclei of parthenogenetically activated oocytes are unable to do so (Kono et al. 1996). Furthermore, immunocytochemical experiments indicate that recombinant mouse PLCζ accumulates in the pronuclei upon the cessation of the oscillations (Larman et al. 2004; Yoda et al. 2004). Positively charged amino acid residues within the X–Y linker region are probably responsible for nuclear localization as mutation of these residues to negatively charged ones results in a loss of the nuclear translocation ability and in the persistence of oscillations after pronuclear formation (Larman et al. 2004). This suggests that nuclear sequestration of PLCζ is the reason for the cessation of the oscillations and it also explains why the oscillations resume at nuclear envelope breakdown, when the first mitotic division begins. Interestingly, nuclear sequestration of PLCζ seems to be characteristic in the mouse only, as bovine, rat and human PLCζ do not accumulate in the pronuclei, even in mouse oocytes following ectopic expression (Ito et al. 2008). Furthermore, rat PLCζ does not accumulate in the pronuclei of rat zygotes but mouse PLCζ does. It has also been reported that, in bovine and rabbit zygotes, the oscillations continue beyond pronucleus formation (Fissore et al. 1992; Fissore and Robl 1993). This indicates that additional factors may also control the Ca2+ signal and currently it is unclear how the Ca2+ oscillations in species other than the mouse are terminated.
Other proposed sperm factors
A number of additional molecules have also been proposed to serve as a sperm-resident activating factor. The first candidate was “oscillin”, a protein isolated by serial chromatographic purification from hamster sperm. The protein seemed to be an oscillogen, as it co-migrated with the ability of the extract to trigger Ca2+ oscillations in oocytes (Parrington et al. 1996). However, recombinant oscillin did not cause Ca2+ oscillations in oocytes, indicating that it was not the active factor in the sperm (Wolosker et al. 1998). Another candidate sperm factor was tr-kit, a truncated form of the c-kit receptor (Sette et al. 1997). In mouse oocytes, tr-kit induced parthenogenetic activation and it was suggested that it stimulated PLCγ1 through phosphorylation by Fyn, a Src-like kinase (Sette et al. 2002). Nevertheless, although its action is inhibited by a PLCγ SH3 construct, the same construct has no effect on sperm-induced oocyte activation (Mehlmann et al. 1998). In addition, there is no evidence to indicate that tr-kit is able to induce regenerative Ca2+ oscillations, which would be an expectation from a bona fide sperm factor.
The latest addition to the list of potential sperm factors is the postacrosomal sheath WW domain-binding protein (PAWP). WW domains are small functional modules found in many signaling and structural proteins and are known to mediate protein–protein interactions. They are named after their two signature tryptophan (W) residues that play an important role in the domains’ function. Interaction between WW domain-containing proteins and their ligands is important for a great number of cellular events such as transcriptional activation, cell cycle control and ubiquitin ligation (Sudol and Hunter 2000; Macias et al. 2002). WW domains fall into two major groups (Group I and Group II) based on their ligand preferences, PAWP specifically binds to Group I WW domains (Wu et al. 2007a). PAWP expression begins during spermatid elongation in humans, rhesus monkey, mice, cows, pigs and rabbits (Wu et al. 2007a, b; Aarabi et al. 2014a) and the expressed protein resides in the postacrosomal sheath of the perinuclear theca, a localization that allows rapid access to the ooplasm. It has also been demonstrated that, after sperm–oocyte fusion, PAWP is released into the oocyte cytoplasm (Wu et al. 2007a, b). Microinjection of recombinant PAWP into Xenopus oocytes causes Ca2+ release (Aarabi et al. 2010), while in Xenopus, porcine, bovine and macaque oocytes, it was shown to trigger cell cycle progression and pronuclear formation (Wu et al. 2007a). In addition, the recombinant protein, or its cRNA, has been shown to trigger Ca2+ oscillations in mouse and human oocytes. The oscillations are blocked by co-injection of a peptide derived from the WWI domain-binding motif of PAWP that acts as a competitive inhibitor (Aarabi et al. 2014a). This inhibitory peptide is also able to block the Ca2+ transients after intracytoplasmic sperm injection, which implies that PAWP is involved in oocyte activation at fertilization. Correlative analyses in humans and livestock species have shown that inadequate amounts of PAWP in spermatozoa are associated with low fertility, possibly because such sperm are unable to trigger oocyte activation (Aarabi et al. 2014b). It has been suggested that, once PAWP is released into the oocyte, it interacts with Group I WW domain-containing proteins, such as the yes-associated protein (YAP). These proteins are highly expressed in oocytes and possess a SH3 binding motif (Chen and Sudol 1995); they may in turn activate PLCγ noncanonically, via its SH3 domain (Aarabi et al. 2014b). Such activation of PLCγ, i.e., via its SH3 instead of SH2 domain, has been observed in Xenopus oocytes and human neurons (Browaeys-Poly et al. 2007; Reynolds et al. 2008). However, since the microinjection of fusion proteins containing the SH3 domain of PLCγ did not inhibit fertilization in mouse oocytes (Mehlmann et al. 1998), it is still not clear what signaling cascade PAWP might use to trigger Ca2+ oscillations. In addition, the ability of mouse PAWP to trigger repetitive Ca2+ transients could not be verified by others (Nomikos et al. 2014, 2015b). Furthermore, in a most recent study, human PAWP was not able to induce Ca2+ oscillations in mouse oocytes and the PAWP-derived inhibitory peptide was also unable to block sperm-induced Ca2+ oscillations (Nomikos et al. 2015a). Detailed analyses of PAWP structure and function are also missing (Kashir et al. 2015); because of these reasons, the recognition of PAWP as the sperm-derived molecule that causes oocyte activation at fertilization requires further verification.
Ca2+ influx
Once the Ca2+ signal is initiated, in mammalian oocytes it takes the form of repetitive Ca2+ oscillations. It is not completely clear what causes the signal to oscillate; the complex regulation and basic feedback properties of the IP3 receptor are usually listed as the most likely causes (Berridge and Galione 1988). In addition, the oscillations also depend on Ca2+ influx. After each Ca2+ rise, the resting Ca2+ level is rapidly restored. SERCA pumps move Ca2+ back into the endoplasmic reticulum (Kline and Kline 1992) and Ca2+ uptake by mitochondria may also be significant (Eisen and Reynolds 1985). At the same time, plasma membrane Ca2+ ATPase (PMCA) pumps and Na+/Ca2+ exchangers in the plasma membrane are also available for Ca2+ removal (Carroll 2000). In fact, a substantial efflux of Ca2+ was demonstrated in sea urchin eggs, frog and mouse oocytes after Ca2+ release (Steinhardt and Epel 1974; Shapira et al. 1996; Pepperell et al. 1999) and this outward Ca2+ current might be so substantial that the entry of extracellular Ca2+ becomes necessary to compensate for the loss. The need for Ca2+ entry to sustain the oscillations was first demonstrated in hamster oocytes. In these cells, the repetitive hyperpolarizations in the membrane potential (that were caused by a K+ conductance activated by the sperm-induced Ca2+ transients) were reduced in frequency and ultimately stopped upon superfusion with Ca2+-free medium (Igusa and Miyazaki 1983). In a similar manner, the train of Ca2+ spikes in fertilized mouse oocytes slows down or stops if Ca2+ is removed from the extracellular medium (Kline and Kline 1992; Shiina et al. 1993). Additional data supporting the function of a Ca2+ influx mechanism during fertilization were provided by experiments using dithiothreitol (DTT), a sulfhydryl reducing agent. In unfertilized mouse oocytes, DTT is able to stimulate the influx of divalent cations (including Ca2+), whereas in fertilized ones it increases the frequency of the Ca2+ oscillations (Cheek et al. 1993). This also implies that Ca2+ entry has an important role in signaling at fertilization. The link between the Ca2+ influx and the Ca2+ transients has been analyzed in fertilized mouse oocytes. According to the study, the rising phase of each transient is followed by an increase in Ca2+ entry, while the influx weakens (but still persists) between the transients (McGuinness et al. 1996). Results from another report also indicated that, during the initial Ca2+ release in sperm extract-injected mouse oocytes, a Ca2+ influx is generated and persists throughout the oscillations (Mohri et al. 2001). These observations all support the concept that the sperm-induced Ca2+ oscillations are associated with a Ca2+ influx across the plasma membrane and that the extracellular Ca2+ is essential for the refilling of the Ca2+ stores (Miyazaki 1991). They also seem to suggest that the filling status of the stores controls the influx: the release of Ca2+ from the endoplasmic reticulum apparently triggers the Ca2+ entry mechanism. Other potential mechanisms to mediate the Ca2+ influx initially included voltage-operated channels but they were quickly ruled out. Hamster oocytes contain voltage-gated Ca2+ channels (Miyazaki and Igusa 1981); however, as sustained hyperpolarization of the oolemma increases the frequency of the sperm-induced hyperpolarization responses, their involvement is highly questionable (Miyazaki 1991). Voltage-gated Ca2+ channels have also been demonstrated in mouse oocytes (Murnane and De Felice 1993; Day et al. 1995) but because mouse oocytes show only negligible hyperpolarizations during fertilization (Igusa et al. 1983), it is unlikely that such channels mediate the Ca2+ influx.
In many cell types, the Ca2+ signal is biphasic: the release of Ca2+ from the intracellular stores is followed by Ca2+ influx across the plasma membrane. The mechanism is known as store-operated Ca2+ entry and it is a major signaling pathway in non-excitable cells (Putney 1986). In some cases, the extracellular Ca2+ entering the cell serves to keep cytoplasmic Ca2+ levels elevated and thus has a major role in the generation of the Ca2+ signal; in other cases, it helps to maintain the repetitive signal by refilling the intracellular stores (for a review, see Putney and Tomita 2012). In oocytes, it was also found that mobilizing the stored Ca2+ generated a Ca2+ influx across the plasma membrane. In mouse oocytes, depleting the intracellular stores with thapsigargin, an inhibitor of the SERCA pumps, activated a Ca2+ influx (Kline and Kline 1992). Ca2+ is known to slowly leak out of the endoplasmic reticulum via the ‘leak pathway’ and, because the blocked pumps are unable to reload Ca2+, the stores become depleted. The fact that store-depletion triggers a Ca2+ influx, without the activation of the phosphoinositide cascade, indicates that store-operated Ca2+ entry is functional in oocytes and may serve to refill the endoplasmic reticulum. Incubation in the presence of thapsigargin was later found to also stimulate Ca2+ entry in pig and human oocytes (Machaty et al. 2002; Martín-Romero et al. 2008), indicating that the mechanism might have a role in Ca2+ signaling.
Previous research suggested that the Ca2+ entry triggered by the filling status of the Ca2+ store is under the regulation of protein kinase C (PKC). 12-O-tetradecanoyl phorbol acetate (TPA) and phorbol-12-myristate-13-acetate (PMA) are phorbol esters that can very effectively stimulate PKC. When applied to mouse oocytes, they cause low amplitude Ca2+ oscillations and a variety of oocyte activation events downstream of the Ca2+ signal (Cuthbertson and Cobbold 1985; Endo et al. 1986; Colonna et al. 1989; Ducibella et al. 1991). PKC activation also promoted Ca2+ influx and repetitive Ca2+ oscillations (Yu et al. 2008), while constitutively active PKC constructs triggered a persistent elevation in cytosolic Ca2+ levels after the release of Ca2+ from the internal stores (Madgwick et al. 2005). In addition, 1-oleyl-2-acetyl-sn-glycerol (OAG), a synthetic analogue of endogenous diacylglycerol, the physiological activator of PKC, induces activation of mouse oocytes (Colonna et al. 1989). It has also been demonstrated that, in fertilized mouse oocytes, fluorescently labeled PKCs translocate repeatedly to the plasma membrane and the translocations occur in synchrony with the Ca2+ transients and the periodic increases in the rate of Ca2+ influx (Halet et al. 2004). On the other hand, inhibition of PKCs with bisindolylmaleimide I (BIM) blocks thapsigargin-induced Ca2+ influx and terminates the sperm-induced Ca2+ oscillations. It is possible that the Ca2+ entry channel in the plasma membrane or some accessory proteins are phosphorylated by PKC that in turn results in an increase in Ca2+ entry. These data imply that PKC is involved in the regulation of cytoplasmic Ca2+ levels in the oocyte, potentially by controlling a store-operated entry mechanism.
Store-operated Ca2+ entry in somatic cells is mediated by the interaction of two proteins, the stromal-interacting molecule (STIM) and Orai proteins. STIM1 and STIM2 are transmembrane proteins that reside in the endoplasmic reticulum (Liou et al. 2005; Roos et al. 2005). With an EF hand located inside the lumen of the store, they are able to sense its Ca2+ content. Upon Ca2+ mobilization, STIM1 forms small clusters (puncta), relocates to regions close to the plasma membrane and activates the Ca2+ entry channels. The channel is formed by Orai proteins (Orai1, Orai2 and Orai3). Orai1, the most potent isoform, resides in the oolemma and, once stimulated by STIM1, it allows Ca2+ in the extracellular medium to flow into the cell (Feske et al. 2006; Vig et al. 2006; Zhang et al. 2006). Both STIM1 and Orai1 have been identified in pig, mouse and frog oocytes (Koh et al. 2007; Gómez-Fernández et al. 2009, 2012; Yu et al. 2009; Wang et al. 2012). In the frog, where the fertilizing sperm triggers a single Ca2+ transient to activate the oocyte, store-operated Ca2+ entry is markedly down-regulated during maturation (Yu et al. 2009). In pig oocytes, the situation seems to be different. Down-regulation of STIM1 using siRNAs leads to a complete elimination of the Ca2+ oscillations associated with fertilization (Lee et al. 2012). Similarly, Orai1 knockdown inhibits store-operated Ca2+ entry and abolishes the sperm-induced Ca2+ transients (Wang et al. 2012), while specific inhibitors of store-operated Ca2+ entry were also effective in disrupting the repetitive Ca2+ signal at fertilization (Wang et al. 2015). This indicates that the Ca2+ influx that sustains the regenerative Ca2+ signal at fertilization is operated by the filling status of the stores and is mediated by STIM1 and Orai1 proteins.
Interestingly, inhibition of store-operated Ca2+ entry in mouse oocytes using pharmacological agents, or by preventing STIM1-Orai1 interaction through the expression of specific protein fragments, does not prevent the sperm-induced Ca2+ oscillations (Miao et al. 2012; Takahashi et al. 2013). This implies that mouse oocytes apply a Ca2+ entry mechanism other than that controlled by the intracellular store to maintain Ca2+ oscillations at fertilization. As mentioned above, the Ca2+ influx in the mouse seems to be under the control of PKC. A candidate channel to provide Ca2+ influx at fertilization is the transient receptor potential (TRP) channel. The TRP protein serves as a Ca2+ channel in a number of cell types and is expressed in various oocytes (Petersen et al. 1995; Machaty et al. 2002). Certain TRP isoforms are known to be regulated by PKC (Hardie 2007), which would account for the stimulatory effect of PKC on the sperm-induced Ca2+ signal. However, recent research has indicated that the TRP channel is not required for normal fertilization (Carvacho et al. 2013). Stimulation of TRPV3 channels leads to Ca2+ entry and subsequent oocyte activation but oocytes collected from transgenic mice that lack TRPV3 channels are able to generate the repetitive Ca2+ spikes characteristic of normal fertilization. This shows that TRPV3 is not essential to sustain the regenerative Ca2+ signal and thus the identity of the Ca2+ entry mechanism that operates in mouse oocytes at fertilization is still unclear.
Future prospects
We have come a long way in the understanding of oocyte activation since Jacques Loeb’s “incredible discovery” and our knowledge regarding the signaling event that marks the formation of a new embryo has increased tremendously. We know that the fertilizing sperm triggers development by inducing an elevation in the oocyte’s cytosolic Ca2+ concentration. The source of Ca2+ is intracellular and, in mammals, the release is mediated by the phosphoinositide signaling system of the oocyte. It is also well accepted that the sperm stimulates this signaling cascade by introducing a soluble factor into the ooplasm after gamete fusion. Several lines of evidence support the idea that this key factor is phospholipase Cζ, a sperm-specific enzyme that, after gamete fusion, cleaves PIP2 and thus generates IP3 to mobilize stored Ca2+. PLCζ is fairly well characterized but we do not know how it finds PIP2 that resides in cytoplasmic vesicles, why it ignores PIP2 in the plasma membrane and whether or not its effect is mediated by a specific protein in the oocyte (Swann and Lai 2013). It is also unclear if it is the only sperm-derived oocyte-activating factor or if it acts in concert with other molecules such as PAWP, the latest addition to the sperm factor candidate list. Finally, the nature of the Ca2+ influx mechanism that is responsible to sustain the low-frequency Ca2+ oscillations also needs further clarification.
A better understanding of the signaling mechanism that operates at fertilization offers major benefits. Proper activation of the oocyte’s developmental program is critical not only during fertilization under normal physiological conditions but also for the success of a number of assisted reproductive technologies. Genetically modified animals have vast potentials and one powerful technology to generate such animals is somatic cell nuclear transfer. Artificial oocyte activation is an integral part of the technology; however, our inability to properly activate the reconstructed oocyte is one of the reasons for the extreme inefficiency of nuclear transfer procedures (Prather 1996). Because we do not completely know the underlying mechanism that mediates Ca2+ signaling in fertilized oocytes, we are not able to artificially induce the repetitive signals. Parthenogenetic activation methods generate Ca2+ changes that do not faithfully recapitulate those occurring after fertilization and this results in poor embryo development. Increasing our knowledge of how the sperm triggers the oscillatory Ca2+ signals will enhance our ability to more precisely control the process of signaling. Also, infertility in humans is a condition affecting more than 70 million (roughly 1 in 7) couples worldwide (Ledger 2008; Ombelet et al. 2008). Although a number of assisted reproductive technologies are available to alleviate the problem, conditions such as severe male factor infertility remain a formidable challenge. Intracytoplasmic sperm injection is a procedure that delivers the sperm directly into the ooplasm and, because it is highly effective in improving fertility rates, its popularity is now on a par with in vitro fertilization (Palermo et al. 2009). However, even this powerful technique can ensure only a clinical pregnancy rate of up to 45 % and the primary reason for the unsuccessful cycles is a failure in oocyte activation. Clinical data indicate that the activation deficiencies are associated with reduced levels of PLCζ (Yoon et al. 2008; Heytens et al. 2009) or PAWP (Aarabi et al. 2014b). PLCζ deficiency has been successfully counteracted with co-injection with mouse PLCζ mRNA (Yoon et al. 2008), while PAWP levels are also believed to have a predictive value in sperm fertilizing ability and, if confirmed to be an oocyte-activating factor, may also have application in the treatment of infertility (Aarabi et al. 2014b). Thus, the injection of a purified, active recombinant protein into the oocyte may have high therapeutic importance. The use of such a protein will also make it possible to assess how the extracellular medium shapes Ca2+ oscillations, as media composition reportedly affects Ca2+ signaling and subsequent embryo development (Dumollard et al. 2006; Banrezes et al. 2011). Characterizing the sperm factor that initiates the Ca2+ signal, along with the entire mechanism that is set in motion by the factor, will ultimately lead to the development of methods to effectively activate oocytes when spermatozoa are unable to do so.
References
Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, Oko R (2014a) Sperm-derived WW domain-binding protein, PAWP, elicits calcium oscillations and oocyte activation in humans and mice. FASEB J 28:4434–4440
Aarabi M, Balakier H, Bashar S, Moskovtsev SI, Sutovsky P, Librach CL, Oko R (2014b) Sperm content of postacrosomal WW binding protein is related to fertilization outcomes in patients undergoing assisted reproductive technology. Fertil Steril 102:440–447
Aarabi M, Qin Z, Xu W, Mewburn J, Oko R (2010) Sperm-borne protein, PAWP, initiates zygotic development in Xenopus laevis by eliciting intracellular calcium release. Mol Reprod Dev 77:249–256
Banrezes B, Sainte-Beuve T, Canon E, Schultz RM, Cancela J, Ozil JP (2011) Adult body weight is programmed by a redox-regulated and energy-dependent process during the pronuclear stage in mouse. PLoS ONE 6, e29388
Bedford-Guaus SJ, McPartlin LA, Xie J, Westmiller SL, Buffone MG, Roberson MS (2011) Molecular cloning and characterization of phospholipase C zeta in equine sperm and testis reveals species-specific differences in expression of catalytically active protein. Biol Reprod 85:78–88
Berridge MJ (2002) The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium 32:235–249
Berridge MJ, Galione A (1988) Cytosolic calcium oscillators. FASEB J 2:3074–3082
Brind S, Swann K, Carroll J (2000) Inositol 1,4,5-trisphosphate receptors are downregulated in mouse oocytes in response to sperm or adenophostin A but not to increases in intracellular Ca2+ or egg activation. Dev Biol 223:251–265
Browaeys-Poly E, Broutin I, Antoine AF, Marin M, Lescuyer A, Vilain JP, Ducruix A, Cailliau K (2007) A non-canonical Grb2-PLC-γ1-Sos cascade triggered by lipovitellin 1, an apolipoprotein B homologue. Cell Signal 19:2540–2548
Bunney TD, Katan M (2011) PLC regulation: emerging pictures for molecular mechanisms. Trends Biochem Sci 36:88–96
Camps M, Carozzi A, Schnabel P, Scheer A, Parker PJ, Gierschik P (1992) Isozyme- selective stimulation of phospholipase C-β2 by G protein βγ-subunits. Nature 360:684–686
Carroll J (2000) Na+-Ca2+ exchange in mouse oocytes: modifications in the regulation of intracellular free Ca2+ during oocyte maturation. J Reprod Fertil 118:337–342
Carvacho I, Lee HC, Fissore RA (2013) Clapham DE 2013) TRPV3 channels mediate strontium- induced mouse-egg activation. Cell Rep 5:1375–1386
Cheek TR, McGuinness OM, Vincent C, Moreton RB, Berridge MJ, Johnson MH (1993) Fertilisation and thimerosal stimulate similar calcium spiking patterns in mouse oocytes but by separate mechanisms. Development 119:179–189
Chen HI, Sudol M (1995) The WW domain of Yes-associated protein binds a proline-rich ligand that differs from the consensus established for Src homology 3-binding modules. Proc Natl Acad Sci U S A 92:7819–7823
Colonna R, Tatone C, Malgaroli A, Eusebi F, Mangia F (1989) Effects of protein kinase C stimulation and free Ca2+ rise in mammalian egg activation. Gamete Res 24:171–183
Coronado R, Morrissette J, Sukhareva M, Vaughan DM (1994) Structure and function of ryanodine receptors. Am J Physiol 266:C1485–C1504
Coward K, Campos-Mendoza A, Larman M, Hibbitt O, McAndrew B, Bromage N, Parrington J (2003) Teleost fish spermatozoa contain a cytosolic protein factor that induces calcium release in sea urchin egg homogenates and triggers calcium oscillations when injected into mouse oocytes. Biochem Biophys Res Commun 305:299–304
Coward K, Ponting CP, Chang HY, Hibbitt O, Savolainen P, Jones KT, Parrington J (2005) Phospholipase Cζ, the trigger of egg activation in mammals, is present in a non-mammalian species. Reproduction 130:157–163
Cox LJ, Larman MG, Saunders CM, Hashimoto K, Swann K, Lai FA (2002) Sperm phospholipase Cζ from humans and cynomolgus monkeys triggers Ca2+ oscillations, activation and development of mouse oocytes. Reproduction 124:611–623
Cuthbertson KS, Cobbold PH (1985) Phorbol ester and sperm activate mouse oocytes by inducing sustained oscillations in cell Ca2+. Nature 316:541–542
Day M, Johnson MH, Cook D (1995) Changes in inward peak Ca2+ current during progression of the cell cycle in early embryos. Proc Physiol Soc 1995:74
Deguchi R, Shirakawa H, Oda S, Mohri T, Miyazaki S (2000) Spatiotemporal analysis of Ca2+ waves in relation to the sperm entry site and animal-vegetal axis during Ca2+ oscillations in fertilized mouse eggs. Dev Biol 218:299–313
Dong JB, Tang TS, Sun FZ (2000) Xenopus and chicken sperm contain a cytosolic soluble protein factor which can trigger calcium oscillations in mouse eggs. Biochem Biophys Res Commun 268:947–951
Ducibella T, Duffy P, Kurasawa S, Kopf GS, Schultz RM (1991) The cortical reaction and modifications of the zona pellucida are stimulated by protein kinase C agonists in the mouse egg. J Cell Biol 115:461a
Dumollard R, Duchen M, Sardet C (2006) Calcium signals and mitochondria at fertilisation. Semin Cell Dev Biol 17:314–323
Dupont G, McGuinness OM, Johnson MH, Berridge MJ, Borgese F (1996) Phospholipase C in mouse oocytes: characterization of β and γ isoforms and their possible involvement in sperm-induced Ca2+ spiking. Biochem J 316:583–591
Dupré A, Haccard O, Jessus C (2011) Mos in the oocyte: how to use MAPK independently of growth factors and transcription to control meiotic divisions. J Signal Transduct 2011:350412
Eisen A, Reynolds GT (1985) Source and sinks for the calcium released during fertilisation of single sea urchin eggs. J Cell Biol 100:1522–1527
Endo Y, Kopf GS, Schultz RM (1986) Stage-specific changes in protein phosphorylation accompanying meiotic maturation of mouse oocytes and fertilization of mouse eggs. J Exp Zool 239:401–409
Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A (2006) A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441:179–185
Fissore RA, Robl JM (1993) Sperm, inositol trisphosphate, and thimerosal-induced intracellular Ca2+ elevations in rabbit eggs. Dev Biol 159:122–130
Fissore RA, Dobrinsky JR, Balise JJ, Duby RT, Robl JM (1992) Patterns of intracellular Ca2+ concentrations in fertilized bovine eggs. Biol Reprod 47:960–969
Fissore RA, Pinto-Correia C, Robl JM (1995) Inositol trisphosphate-induced calcium release in the generation of calcium oscillations in bovine eggs. Biol Reprod 53:766–774
Fujimoto S, Yoshida N, Fukui T, Amanai M, Isobe T, Itagaki C, Izumi T, Perry AC (2004) Mammalian phospholipase Czeta induces oocyte activation from the sperm perinuclear matrix. Dev Biol 274:370–383
Fukami K (2002) Structure, regulation, and function of phospholipase C isozymes. J Biochem 131:293–299
Galione A, Swann K, Georgiou P, Whitaker M (1994) Regenerative and non-regenerative calcium transients in hamster eggs triggered by inositol 1,4,5-trisphosphate. J Physiol 480:465–474
Gilkey JC, Jaffe LF, Ridgway EB, Reynolds GT (1978) A free calcium wave traverses the activating egg of the medaka, Oryzias latipes. J Cell Biol 76:448–466
Gómez-Fernández C, Pozo-Guisado E, Gañán-Parra M, Perianes MJ, Alvarez IS, Martín- Romero FJ (2009) Relocalization of STIM1 in mouse oocytes at fertilization: early involvement of store-operated calcium entry. Reproduction 138:211–221
Gómez-Fernández C, López-Guerrero AM, Pozo-Guisado E, Álvarez IS, Martín-Romero FJ (2012) Calcium signaling in mouse oocyte maturation: the roles of STIM1, ORAI1 and SOCE. Mol Human Reprod 18:194–203
Hajnóczky G, Thomas AP (1994) The inositol trisphosphate calcium channel is inactivated by inositol trisphosphate. Nature 370:474–477
Halet G, Tunwell R, Balla T, Swann K, Carroll J (2002) The dynamics of plasma membrane PtdIns(4,5)P2 at fertilization of mouse eggs. J Cell Sci 115:2139–2149
Halet G, Tunwell R, Parkinson SJ, Carroll J (2004) Conventional PKCs regulate the temporal pattern of Ca2+ oscillations at fertilization in mouse eggs. J Cell Biol 164:1033–1044
Hardie RC (2007) TRP channels and lipids: from Drosophila to mammalian physiology. J Physiol 578:9–24
Heilbrunn LV (1937) An outline of general physiology, 1st edn. Saunders, Philadelphia
Heilbrunn LV, Wilbur KM (1937) Stimulation and nuclear breakdown in the Nereis egg. Biol Bull 73:557–564
Heytens E, Parrington J, Coward K, Young C, Lambrecht S, Yoon SY, Fissore RA, Hamer R, Deane CM, Ruas M, Grasa P, Soleimani R, Cuvelier CA, Gerris J, Dhont M, Deforce D, Leybaert L, De Sutter P (2009) Reduced amounts and abnormal forms of phospholipase C zeta (PLCzeta) in spermatozoa from infertile men. Hum Reprod 24:2417–2428
Igusa Y, Miyazaki S (1983) Effects of altered extracellular and intracellular calcium concentration on hyperpolarizing responses of the hamster egg. J Physiol 340:611–632
Igusa Y, Miyazaki S, Yamashita N (1983) Periodic hyperpolarizing responses in hamster and mouse eggs fertilized with mouse sperm. J Physiol 340:633–647
Ito M, Nagaoka K, Kuroda K, Kawano N, Yoshida K, Harada Y, Shikano T, Miyado M, Oda S, Toshimori K, Mizukami Y, Murata T, Umezawa A, Miyazaki S, Miyado K (2010) Arrest of spermatogenesis at round spermatids in PLCZ1-deficient mice. In: Abstract at the 11th International Symposium on Spermatology. Okinawa, Japan
Ito M, Shikano T, Kuroda K, Miyazaki S (2008) Relationship between nuclear sequestration of PLCζ and termination of PLCζ-induced Ca2+ oscillations in mouse eggs. Cell Calcium 44:400–410
Jaffe LA (1990) First messengers at fertilization. J Reprod Fertil Suppl 42:107–116
Jaffe LF (1983) Sources of calcium in egg activation: a review and hypothesis. Dev Biol 99:265–276
Jaffe LF (1991) The path of calcium in cytosolic calcium oscillations: a unifying hypothesis. Proc Natl Acad Sci U S A 88:9883–9887
Jellerette T, He CL, Wu H, Parys JB, Fissore RA (2000) Down-regulation of the inositol 1,4,5-trisphosphate receptor in mouse eggs following fertilization or parthenogenetic activation. Dev Biol 223:238–250
Jones KT (1998) Ca2+ oscillations in the activation of the egg and development of the embryo in mammals. Int J Dev Biol 42:1–10
Jones KT, Nixon VL (2000) Sperm-induced Ca2+ oscillations in mouse oocytes and eggs can be mimicked by photolysis of caged inositol 1,4,5-trisphosphate: evidence to support a continuous low level production of inositol 1, 4,5-trisphosphate during mammalian fertilization. Dev Biol 225:1–12
Jones KT, Soeller C, Cannell MB (1998) The passage of Ca2+ and fluorescent markers between the sperm and egg after fusion in the mouse. Development 125:4627–4635
Kashir J, Deguchi R, Jones C, Coward K, Stricker SA (2013a) Comparative biology of sperm actors and fertilization-induced calcium signals across the animal kingdom. Mol Reprod Dev 80:787–815
Kashir J, Jones C, Mounce G, Ramadan WM, Lemmon B, Heindryckx B, de Sutter P, Parrington J, Turner K, Child T, McVeigh E, Coward K (2013b) Variance in total levels of phospholipase C zeta (PLC-ζ) in human sperm may limit the applicability of quantitative immunofluorescent analysis as a diagnostic indicator of oocyte activation capability. Fertil Steril 99:107–117
Kashir J, Konstantinidis M, Jones C, Lemmon B, Lee HC, Hamer R, Heindryckx B, Deane CM, De Sutter P, Fissore RA, Parrington J, Wells D, Coward K (2012) A maternally inherited autosomal point mutation in human phospholipase C zeta (PLCζ) leads to male infertility. Hum Reprod 27:222–231
Kashir J, Nomikos M, Lai FA, Swann K (2014) Sperm-induced Ca2+ release during egg activation in mammals. Biochem Biophys Res Commun 450:1204–1211
Kashir J, Nomikos M, Swann K, Lai FA (2015) PLCζ or PAWP: revisiting the putative mammalian sperm factor that triggers egg activation and embryogenesis. Mol Hum Reprod 21:383–388
Kimura Y, Yanagimachi R (1995) Mouse oocytes injected with testicular spermatozoa or round spermatids can develop into normal offspring. Development 121:2397–2405
Kimura Y, Yanagimachi R, Kuretake S, Bortkiewicz H, Perry AC, Yanagimachi H (1998) Analysis of mouse oocyte activation suggests the involvement of sperm perinuclear material. Biol Reprod 58:1407–1415
Kline D, Kline JT (1992) Thapsigargin activates a calcium influx pathway in the unfertilized mouse egg and suppresses repetitive calcium transients in the fertilized egg. J Biol Chem 267:17624–17630
Kline D, Simoncini L, Mandel G, Maue RA, Kado RT, Jaffe LA (1988) Fertilization events induced by neurotransmitters after injection of mRNA in Xenopus eggs. Science 241:464–467
Knott JG, Kurokawa M, Fissore RA, Schultz RM, Williams CJ (2005) Transgenic RNA interference reveals role for mouse sperm phospholipase Cζ in triggering Ca2+ oscillations during fertilization. Biol Reprod 72:992–996
Koh S, Lee K, Wang C, Cabot RA, Machaty Z (2009) STIM1 regulates store-operated Ca2+ entry in oocytes. Dev Biol 330:368–376
Koh S, Lee K, Wang C, Machaty Z (2007) Characterization of STIM1 gene expression in porcine oocytes. Biol Reprod (Suppl 1) 139
Kono T, Jones KT, Bos-Mikich A, Whittingham DG, Carroll J (1996) A cell cycle-associated change in Ca2+ releasing activity leads to the generation of Ca2+ transients in mouse embryos during the first mitotic division. J Cell Biol 132:915–923
Kouchi Z, Fukami K, Shikano T, Oda S, Nakamura Y, Takenawa T, Miyazaki S (2004) Recombinant phospholipase Cζ has high Ca2+ sensitivity and induces Ca2+ oscillations in mouse eggs. J Biol Chem 279:10408–10412
Kurokawa M, Sato K, Wu H, He C, Malcuit C, Black SJ, Fukami K, Fissore RA (2005) Functional, biochemical, and chromatographic characterization of the complete [Ca2+]i oscillation-inducing activity of porcine sperm. Dev Biol 285:376–392
Larman MG, Saunders CM, Carroll J, Lai FA, Swann K (2004) Cell cycle-dependent Ca2+ oscillations in mouse embryos are regulated by nuclear targeting of PLCζ. J Cell Sci 117:2513–2521
Lawrence Y, Whitaker M, Swann K (1997) Sperm-egg fusion is the prelude to the initial Ca2+ increase at fertilization in the mouse. Development 124:233–241
Ledger WL (2008) Demographics of infertility. Reprod Biomed Online 18(suppl 2):11–14
Lee B, Yoon SY, Malcuit C, Parys JB, Fissore RA (2010) Inositol 1,4,5-trisphosphate receptor 1 degradation in mouse eggs and impact on [Ca2+]i oscillations. J Cell Physiol 222:238–247
Lee K, Wang C, Machaty Z (2012) STIM1 is required for Ca2+ signaling during mammalian fertilization. Dev Biol 367:154–162
Lee SJ, Madden PJ, Shen SS (1998) U73122 blocked the cGMP-induced calcium release in sea urchin eggs. Exp Cell Res 242:328–340
Liou J, Kim ML, Heo WD, Jones JT, Myers JW, Ferrell JE Jr, Meyer T (2005) STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15:1235–1241
Jaffe LA, Gould M (1985) Polyspermy-preventing mechanisms. In: Metz CB, Monry A (eds) Biology of fertilization. Academic, Orlando, pp 223–250
Loeb J (1899) On the nature of the process of fertilization and the artificial production of normal larvae (Plutei) from the unfertilized eggs of sea urchin. Am J Physiol 3:135–138
Machaty Z, Bonk AJ, Kühholzer B, Prather RS (2000) Porcine oocyte activation induced by a cytosolic sperm factor. Mol Reprod Dev 57:290–295
Machaty Z, Mayes MA, Kovács LG, Balatti PA, Kim JH, Prather RS (1997) Activation of porcine oocytes via an exogenously introduced rat muscarinic M1 receptor. Biol Reprod 57:85–91
Machaty Z, Ramsoondar JJ, Bonk AJ, Bondioli KR, Prather RS (2002) Capacitative calcium entry mechanism in porcine oocytes. Biol Reprod 66:667–674
Macias MJ, Wiesner S, Sudol M (2002) WW and SH3 domains, two different scaffolds to recognize proline-rich ligands. FEBS Lett 513:30–37
Madgwick S, Levasseur M, Jones KT (2005) Calmodulin-dependent protein kinase II, and not protein kinase C, is sufficient for triggering cell-cycle resumption in mammalian eggs. J Cell Sci 118:3849–3859
Marangos P, FitzHarris G, Carroll J (2003) Ca2+ oscillations at fertilization in mammals are regulated by the formation of pronuclei. Development 130:1461–1472
Martín-Romero FJ, Ortíz-de-Galisteo JR, Lara-Laranjeira J, Domínguez-Arroyo JA, González-Carrera E, Alvarez IS (2008) Store-operated calcium entry in human oocytes and sensitivity to oxidative stress. Biol Reprod 78:307–315
Mazia D (1937) The release of calcium in Arbacia eggs on fertilization. J Cell Comp Physiol 10:291–304
McGuinness OM, Moreton RB, Johnson MH, Berridge MJ (1996) A direct measurement of increased divalent cation influx in fertilised mouse oocytes. Development 122:2199–2206
Mehlmann LM, Carpenter G, Rhee SG, Jaffe LA (1998) SH2 domain-mediated activation of phospholipase Cγ is not required to initiate Ca2+ release at fertilization of mouse eggs. Dev Biol 203:221–232
Mehlmann LM, Chattopadhyay A, Carpenter G, Jaffe LA (2001) Evidence that phospholipase C from the sperm is not responsible for initiating Ca2+ release at fertilization in mouse eggs. Dev Biol 236:492–501
Miao YL, Stein P, Jefferson WN, Padilla-Banks E, Williams CJ (2012) Calcium influx- mediated signaling is required for complete mouse egg activation. Proc Natl Acad Sci U S A 109:4169–4174
Mignery GA, Südhof TC (1990) The ligand binding site and transduction mechanism in the inositol-1,4,5-triphosphate receptor. EMBO J 9:3893–3898
Mikoshiba K (1993) Inositol 1,4,5-trisphosphate receptor. Trends Pharmacol Sci 14:86–89
Miyazaki S (1988) Inositol 1,4,5-trisphosphate-induced calcium release and guanine nucleotide-binding protein-mediated periodic calcium rises in golden hamster eggs. J Cell Biol 106:345–353
Miyazaki S (1991) Repetitive calcium transients in hamster oocytes. Cell Calcium 12:205–216
Miyazaki S, Igusa Y (1981) Ca-dependent action potential and Ca-induced fertilization potential in golden hamster eggs. In: Ohnishi ST, Endo M (eds) The mechanism of gated calcium transport across biological membranes. Academic, New York, pp pp 305–pp 311
Miyazaki S, Katayama Y, Swann K (1990) Synergistic activation by serotonin and GTP analogue and inhibition by phorbol ester of cyclic Ca2+ rises in hamster eggs. J Physiol 426:209–227
Miyazaki S, Shirakawa H, Nakada K, Honda Y (1993) Essential role of the inositol 1,4,5- trisphosphate receptor/Ca2+ release channel in Ca2+ waves and Ca2+ oscillations at fertilization of mammalian eggs. Dev Biol 158:62–78
Miyazaki S, Yuzaki M, Nakada K, Shirakawa H, Nakanishi S, Nakade S, Mikoshiba K (1992) Block of Ca2+ wave and Ca2+ oscillation by antibody to the inositol 1,4,5-trisphosphate receptor in fertilized hamster eggs. Science 257:251–255
Mohri T, Shirakawa H, Oda S, Sato MS, Mikoshiba K, Miyazaki S (2001) Analysis of Mn2+/Ca2+ influx and release during Ca2+ oscillations in mouse eggs injected with sperm extract. Cell Calcium 29:311–325
Murnane JM, De Felice LJ (1993) Electrical maturation of the murine oocyte: an increase in calcium current coincides with acquisition of meiotic competence. Zygote 1:49–60
Nalefski EA, Falke JJ (1996) The C2 domain calcium-binding motif: Structural and functional diversity. Protein Sci 5:2375–2390
Nixon VL, Levasseur M, McDougall A, Jones KT (2002) Ca2+ oscillations promote APC/C- dependent Cyclin B1 degradation during metaphase arrest and completion of meiosis in fertilizing mouse eggs. Curr Biol 12:746–750
Nomikos M, Blayney LM, Larman MG, Campbell K, Rossbach A, Saunders CM, Swann K, Lai FA (2005) Role of phospholipase C-ζ domains in Ca2+-dependent phosphatidylinositol 4,5-bisphosphate hydrolysis and cytoplasmic Ca2+ oscillations. J Biol Chem 280:31011–31018
Nomikos M, Elgmati K, Theodoridou M, Georgilis A, Gonzalez-Garcia JR, Nounesis G, Swann K, Lai FA (2011). Novel regulation of PLCζ activity via its XY-linker. Biochem J 438:427–432
Nomikos M, Kashir J, Swann K, Lai FA (2013) Sperm PLCζ: from structure to Ca2+ oscillations, egg activation and therapeutic potential. FEBS Lett 587:3609–3616
Nomikos M, Mulgrew-Nesbitt A, Pallavi P, Mihalyne G, Zaitseva I, Swann K, Lai FA, Murray D, McLaughlin S (2007) Binding of phosphoinositide-specific phospholipase C-ζ (PLC-ζ) to phospholipid membranes: potential role of an unstructured cluster of basic residues. J Biol Chem 282:16644–16653
Nomikos M, Sanders JR, Kashir J, Sanusi R, Buntwal L, Love D, Ashley P, Sanders D, Knaggs P, Bunkheila A, Swann K, Lai FA (2015a) Functional disparity between human PAWP and PLCζ in the generation of Ca2+ oscillations for oocyte activation. Mol Hum Reprod Jun 26. pii: gav034. [Epub ahead of print]
Nomikos M, Sanders JR, Theodoridou M, Kashir J, Matthews E, Nounesis G, Lai FA, Swann K (2014) Sperm-specific post-acrosomal WW-domain binding protein (PAWP) does not cause Ca2+ release in mouse oocytes. Mol Hum Reprod 20:938–947
Nomikos M, Swann K, Lai FA (2015b) Is PAWP the "real" sperm factor? Asian J Androl 17:444–446
Ombelet W, Cooke I, Dyer S, Serour G, Devroey P (2008) Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update 14:605–621
Ozil JP, Huneau D (2001) Activation of rabbit oocytes: the impact of the Ca2+ signal regime on development. Development 128:917–928
Ozil JP, Markoulaki S, Toth S, Matson S, Banrezes B, Knott JG, Schultz RM, Huneau D, Ducibella T (2005) Egg activation events are regulated by the duration of a sustained [Ca2+]cyt signal in the mouse. Dev Biol 282:39–54
Ozil JP, Banrezes B, Tóth S, Pan H, Schultz RM (2006) Ca2+ oscillatory pattern in fertilized mouse eggs affects gene expression and development to term. Dev Biol 300:534–544
Palermo GD, Neri QV, Takeuchi T, Rosenwaks Z (2009) ICSI: where we have been and where we are going. Semin Reprod Med 27:191–201
Parrington J, Jones M, Tunwell R, Devader C, Katan M, Swann K (2002) Phospholipase C isoforms in mammalian spermatozoa: potential components of the sperm factor that causes Ca2+ release in eggs. Reproduction 123:31–39
Parrington J, Swann K, Shevchenko VI, Sesay AK, Lai FA (1996) Calcium oscillations in mammalian eggs triggered by a soluble sperm protein. Nature 379:364–368
Pepperell JR, Kommineni K, Buradagunta S, Smith PJ, Keefe DL (1999) Transmembrane regulation of intracellular calcium by a plasma membrane sodium/calcium exchanger in mouse ova. Biol Reprod 60:1137–1143
Perry AC, Wakayama T, Cooke IM, Yanagimachi R (2000) Mammalian oocyte activation by the synergistic action of discrete sperm head components: induction of calcium transients and involvement of proteolysis. Dev Biol 217:386–393
Petersen C, Berridge MJ, Borgese MF, Bennett DL (1995) Putative capacitative calcium entry channels: expression of Drosophila trp and evidence for the existence of vertebrate homologues. Biochem J 311:41–44
Prather RS (1996) Progress in cloning embryos from domesticated livestock. Proc Soc Exp Biol Med 212:38–43
Putney JW Jr (1986) A model for receptor-regulated calcium entry. Cell Calcium 7:1–12
Putney JW, Tomita T (2012) Phospholipase C signaling and calcium influx. Adv Biol Regul 52:152–164
Ridgway EB, Gilkey JC, Jaffe LF (1977) Free calcium increases explosively in activating medaka eggs. Proc Natl Acad Sci U S A 74:623–627
Reynolds CH, Garwood CJ, Wray S, Price C, Kellie S, Perera T, Zvelebil M, Yang A, Sheppard PW, Varndell IM, Hanger DP, Anderton BH (2008) Phosphorylation regulates tau interactions with Src homology 3 domains of phosphatidylinositol 3-kinase, phospholipase Cγ1, Grb2, and Src family kinases. J Biol Chem 283:18177–18186
Rice A, Parrington J, Jones KT, Swann K (2000) Mammalian sperm contain a Ca2+-sensitive phospholipase C activity that can generate InsP3 from PIP2 associated with intracellular organelles. Dev Biol 228:125–135
Ringer S (1883) A further contribution regarding the influence of the different constituents of the blood on the contraction of the heart. J Physiol 4:29–43
Rogers NT, Hobson E, Pickering S, Lai FA, Braude P, Swann K (2004) Phospholipase Cζ causes Ca2+ oscillations and parthenogenetic activation of human oocytes. Reproduction 128:697–702
Roos J, DiGregorio PJ, Yeromin AV, Ohlsen K, Lioudyno M, Zhang S, Safrina O, Kozak JA, Wagner SL, Cahalan MD, Veliçelebi G, Stauderman KA (2005) STIM1, an essential and conserved component of store-operated Ca2+ channel function. J Cell Biol 169:435–445
Runft LL, Watras J, Jaffe LA (1999) Calcium release at fertilization of Xenopus eggs requires type I IP3 receptors, but not SH2 domain-mediated activation of PLCγ or Gq-mediated activation of PLC β. Dev Biol 214:399–411
Saunders CM, Larman MG, Parrington J, Cox LJ, Royse J, Blayney LM, Swann K, Lai FA (2002) PLCζ: a sperm-specific trigger of Ca2+ oscillations in eggs and embryo development. Development 129:3533–3544
Saunders CM, Swann K, Lai FA (2007) PLCζ, a sperm-specific PLC and its potential role in fertilization. Biochem Soc Symp 74:23–36
Schultz RM, Kopf GS (1995) Molecular basis of mammalian egg activation. Curr Top Dev Biol 30:21–62
Sette C, Bevilacqua A, Bianchini A, Mangia F, Geremia R, Rossi P (1997) Parthenogenetic activation of mouse eggs by microinjection of a truncated c-kit tyrosine kinase present in spermatozoa. Development 124:2267–2274
Sette C, Paronetto MP, Barchi M, Bevilacqua A, Geremia R, Rossi P (2002) Tr-kit-induced resumption of the cell cycle in mouse eggs requires activation of a Src-like kinase. EMBO J 21:5386–5395
Shapira H, Lupu-Meiri M, Lipinsky D, Oron Y (1996) Agonist-evoked calcium efflux from a functionally discrete compartment in Xenopus oocytes. Cell Calcium 19:201–210
Shiina Y, Kaneda M, Matsuyama K, Tanaka K, HiroiM DK (1993) Role of the extracellular Ca2+ on the intracellular Ca2+ changes in fertilized and activated mouse oocytes. J Reprod Fertil 97:143–150
Shreeve J (1983) Calcium and The Man. MBL Newslett 8
Snow P, Yim DL, Leibow JD, Saini S, Nuccitelli R (1996) Fertilization stimulates an increase in inositol trisphosphate and inositol lipid levels in Xenopus eggs. Dev Biol 180:108–118
Steinhardt RA, Epel D (1974) Activation of sea-urchin eggs by a calcium ionophore. Proc Natl Acad Sci U S A 71:1915–1919
Stricker SA (1999) Comparative biology of calcium signaling during fertilization and egg activation in animals. Dev Biol 211:157–176
Sudol M, Hunter T (2000) NeW wrinkles for an old domain. Cell 103:1001–1004
Swann K (1990) A cytosolic sperm factor stimulates repetitive calcium increases and mimics fertilization in hamster eggs. Development 110:1295–1302
Swann K (1992) Different triggers for calcium oscillations in mouse eggs involve a ryanodine-sensitive calcium store. Biochem J 287:79–84
Swann K (1994) Ca2+ oscillations and sensitization of Ca2+ release in unfertilized mouse eggs injected with a sperm factor. Cell Calcium 15:331–339
Swann K, Lai FA (2013) PLCζ and the initiation of Ca2+ oscillations in fertilizing mammalian eggs. Cell Calcium 53:55–62
Swann K, Ozil JP (1994) Dynamics of the calcium signal that triggers mammalian egg activation. Int Rev Cytol 152:183–222
Swann K, Whitaker M (1986) The part played by inositol trisphosphate and calcium in the propagation of the fertilization wave in sea urchin eggs. J Cell Biol 103:2333–2342
Swann K, Saunders CM, Rogers NT, Lai FA (2006) PLCζ(zeta): A sperm protein that triggers Ca2+ oscillations and egg activation in mammals. Semin Cell Dev Biol 17:264–273
Takahashi T, Kikuchi T, Kidokoro Y, Shirakawa H (2013) Ca2+ influx-dependent refilling of intracellular Ca2+ stores determines the frequency of Ca2+ oscillations in fertilized mouse eggs. Biochem Biophys Res Commun 430:60–65
Turner PR, Jaffe LA, Fein A (1986) Regulation of cortical vesicle exocytosis in sea urchin eggs by inositol 1,4,5-trisphosphate and a GTP-binding protein. J Cell Biol 102:70–76
Turner PR, Sheetz MP, Jaffe LA (1984) Fertilization increases the polyphosphoinositide content of sea urchin eggs. Nature 310:414–415
Vig M, Peinelt C, Beck A, Koomoa DL, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet JP (2006) CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312:1220–1223
Wang C, Lee K, Gajdócsi E, Bali Papp A, Machaty Z (2012) Orai1 mediates store-operated Ca2+ entry during fertilization in mammalian oocytes. Dev Biol 365:414–423
Wang C, Zhang L, Jaeger LA, Machaty Z (2015) Store-operated Ca2+ entry sustains the fertilization Ca2+ signal in pig eggs. Biol Reprod 93:25. doi:10.1095/biolreprod.114.126151
Wassarman PM, Jovine L, Qi H, Williams Z, Darie C, Litscher ES (2005) Recent aspects of mammalian fertilization research. Mol Cell Endocrinol 234:95–103
Weiss A (1993) T cell antigen receptor signal transduction: a tale of tails and cytoplasmic protein-tyrosine kinases. Cell 73:209–212
Whitaker M (1996) Control of meiotic arrest. Rev Reprod 1:127–135
Whitaker MJ, Steinhardt RA (1982) Ionic regulation of egg activation. Q Rev Biophys 15:593–666
Williams CJ, Schultz RM, Kopf GS (1992) Role of G proteins in mouse egg activation: stimulatory effects of acetylcholine on the ZP2 to ZP2f conversion and pronuclear formation in eggs expressing a functional m1 muscarinic receptor. Dev Biol 151:288–296
Wolosker H, Kline D, Bian Y, Blackshaw S, Cameron AM, Fralich TJ, Schnaar RL, Snyder SH (1998) Molecularly cloned mammalian glucosamine-6-phosphate deaminase localizes to transporting epithelium and lacks oscillin activity. FASEB J 12:91–99
Wu AT, Sutovsky P, Manandhar G, Xu W, Katayama M, Day BN, Park KW, Yi YJ, Xi YW, Prather RS, Oko R (2007a) PAWP, a sperm-specific WW domain-binding protein, promotes meiotic resumption and pronuclear development during fertilization. J Biol Chem 282:12164–12175
Wu AT, Sutovsky P, Xu W, van der Spoel AC, Platt FM, Oko R (2007b) The postacrosomal assembly of sperm head protein, PAWP, is independent of acrosome formation and dependent on microtubular manchette transport. Dev Biol 312:471–483
Wu H, He CL, Fissore RA (1997) Injection of a porcine sperm factor triggers calcium oscillations in mouse oocytes and bovine eggs. Mol Reprod Dev 46:176–189
Yanagimachi R (1994) Mammalian fertilization. In: Knobil E, Neill JD (eds) The physiology of reproduction, 2nd edn. Raven, New York, pp 189–317
Yim DL, Opresko LK, Wiley HS, Nuccitelli R (1994) Highly polarized EGF receptor tyrosine kinase activity initiates egg activation in Xenopus. Dev Biol 162:41–55
Yoda A, Oda S, Shikano T, Kouchi Z, Awaji T, Shirakawa H, Kinoshita K, Miyazaki S (2004) Ca2+ oscillation-inducing phospholipase C zeta expressed in mouse eggs is accumulated to the pronucleus during egg activation. Dev Biol 268:245–257
Yoneda A, Kashima M, Yoshida S, Terada K, Nakagawa S, Sakamoto A, Hayakawa K, Suzuki K, Ueda J, Watanabe T (2006) Molecular cloning, testicular postnatal expression, and oocyte-activating potential of porcine phospholipase Cζ. Reproduction 132:393–401
Yoon SY, Fissore RA (2007) Release of phospholipase C ζ and [Ca2+]i oscillation-inducing activity during mammalian fertilization. Reproduction 134:695–704
Yoon SY, Jellerette T, Salicioni AM, Lee HC, Yoo MS, Coward K, Parrington J, Grow D, Cibelli JB, Visconti PE, Mager J, Fissore RA (2008) Human sperm devoid of PLC, zeta 1 fail to induce Ca2+ release and are unable to initiate the first step of embryo development. J Clin Invest 118:3671–3681
Young C, Grasa P, Coward K, Davis LC, Parrington J (2009) Phospholipase C zeta undergoes dynamic changes in its pattern of localization in sperm during capacitation and the acrosome reaction. Fertil Steril 91:2230–2242
Yu F, Sun L, Machaca K (2009) Orai1 internalization and STIM1 clustering inhibition modulate SOCE inactivation during meiosis. Proc Natl Acad Sci U S A 106:17401–17406
Yu Y, Halet G, Lai FA, Swann K (2008) Regulation of diacylglycerol production and protein kinase C stimulation during sperm- and PLCζ-mediated mouse egg activation. Biol Cell 100:633–643
Yu Y, Nomikos M, Theodoridou M, Nounesis G, Lai FA, Swann K (2012) PLCζ causes Ca2+ oscillations in mouse eggs by targeting intracellular and not plasma membrane PI(4,5)P2. Mol Biol Cell 23:371–380
Zhang SL, Yeromin AV, Zhang XH, Yu Y, Safrina O, Penna A, Roos J, Stauderman KA, Cahalan MD (2006) Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc Natl Acad Sci U S A 103:9357–9362
Zheng L, Krishnamoorthi R, Zolkiewski M, Wang X (2000) Distinct Ca2+ binding properties of novel C2 domains of plant phospholipase Dα and β. J Biol Chem 275:19700–19706
Zucker RS, Steinhardt RA (1978) Prevention of the cortical reaction in fertilized sea urchin eggs by injection of calcium-chelating ligands. Biochim Biophys Acta 541:459–466
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
About this article
Cite this article
Machaty, Z. Signal transduction in mammalian oocytes during fertilization. Cell Tissue Res 363, 169–183 (2016). https://doi.org/10.1007/s00441-015-2291-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2291-8